将负载缓蚀剂的微纳米容器添加到涂层中,可使其具有自修复性能。但是,这类涂层缓蚀剂的释放不可控和缺乏环境适应性。在理想情况下,金属表面涂层的微损伤使金属表面暴露,缓蚀剂经纳米容器孔道(介孔或者微孔)释放到金属表面形成保护膜可隔绝腐蚀性物质[17,18]。但是金属设备的服役环境复杂,涂层的微损伤使微纳米载体中的缓蚀剂在损伤处金属未暴露时就提前释放,当涂层受损至金属表面时剩余的缓蚀剂已不足以防护裸露金属基体。因此,在微纳米容器设置各种“开关”控制缓蚀剂的释放,例如对温度、pH值和压力等敏感的开关,其中pH值响应开关最为典型[19,20]。在酸性条件下环境友好的壳聚糖(CS)中的氨基被质子化,产生溶胀释放出纳米容器内的试剂,成为pH值响应开关。Xie等[21]基于埃洛石纳米管将壳聚糖改性制备壳聚糖纳米复合膜,提高了壳聚糖在不同pH值条件下的溶胀率。Hessam等[22]制备了交联的壳聚糖核壳纳米载体,提高了纳米载体的负载能力和所装载试剂在酸性条件下的释放效率。但是,传统的壳聚糖“开关”受到环境刺激响应后只“开”不“关”。CS中丰富的氨基使纳米载体在腐蚀环境下带正电荷,带正电荷的纳米载体与环境中某些离子产生的强相互作用影响CS“开关”的溶胀与收缩[23]。因此,缓蚀剂释放形成保护膜后,微纳米容器担载的缓蚀剂还会持续释放,缓蚀剂过度释放使自修复涂层的防护效果降低。本文选择具有中空结构的埃洛石纳米管作为载体,选择MBT作为封装缓蚀剂,将埃洛石纳米管碱刻蚀(HNTs)扩大其孔径以提高埃洛石纳米管对MBT的装载量,将壳聚糖和聚乙二醇相结合制备壳聚糖和聚乙二醇共聚物(CP),作为封装缓蚀剂释放“开关”并将其包覆在HNTs表面,研究微纳米容器对MBT的控制释放机理并建立碱刻蚀埃洛石纳米管装载MBT并包覆CP(CP-HNTs-MBT)的模型。
1 实验方法
1.1 实验用材料
实验用材料有:2-巯基苯并噻唑(MBT,分析纯)、氢氧化钠(NaOH,纯度98%);埃洛石纳米管(HNT);壳聚糖(CS)、甲醛、乙酸、Span 80、NaCl、无水乙醇(分析纯);聚二甲基硅氧烷(PDMS)、2,4,6,8-四乙烯基-2,4,6,8-四甲基环四硅氧烷(固化剂,TEETMCT);Cu电极(99.99%,10 mm × 10 mm × 10 mm)。
1.2 样品的制备
HNTs的制备:先配制100 mL浓度为5 mol/L的氢氧化钠溶液。将3 g HTN放入烧杯中,加入氢氧化钠溶液后搅拌使两者充分反应。将含反应生成物的溶液在50 ℃超声处理1 h后冷却至室温,然后用离心机离心分离出产物并用蒸馏水充分洗涤直至溶液为中性。将分离出的固体放入温度为105 ℃鼓风干燥箱中加热,直至固体的质量恒定。得到的固体即为HNTs。
HNTs-MBT的制备:向烧杯中加入1 g充分研磨的HNTs,再加入40 mL MBT的质量分数为40 mg/mL的乙醇溶液,然后将混合溶液磁力搅拌48 h。将混合溶液放入真空干燥箱中抽真空1 h,然后将其在常压下静置30 min以使HNTs充分装载MBT。重复3次抽真空-常压静置操作。最后,用无水乙醇将含有固体的混合溶液离心洗涤3次。将所得固体放入80 ℃的鼓风干燥箱中干燥24 h,即得到装载MBT的HNTs纳米管。
CP-HNTs-MBT 的制备:合成壳聚糖-聚乙二醇共聚物(CP)包覆HNTs-MBT表面,其步骤在图1中给出。(1)将200 mg三聚磷酸钠溶于200 mL蒸馏水中并进行磁力搅拌,得到1 mg/mL的三聚磷酸钠溶液(TPP)。(2)将0.4 g壳聚糖溶于400 mL 0.175%醋酸水溶液(每100 mL中含有0.7 mL醋酸),得到1 mg/mL的壳聚糖醋酸水溶液。将壳聚糖醋酸水溶液加热到50 ℃,磁力搅拌2 h直至气泡完全消除。(3)将200 mL壳聚糖醋酸水溶液在室温下缓慢加入2 g聚乙二醇并磁力搅拌30 min。(4)将0.2 g HNTs-MBT加入步骤(3)所得到的溶液中,在室温下磁力搅拌30 min。(5)向步骤(4)的溶液中滴加80 mL 步骤(1)中得到的TPP溶液,并在室温下磁力搅拌30 min。将所得混合溶液用蒸馏水洗涤3次,将所得固体放入真空干燥箱室温干燥48 h,得到CP-HNTs-MBT。
图1
空白涂层电极和自修复涂层电极的制备:依次用粗糙度为240#、600#和1200#的砂纸磨砂Cu基体的表面,然后用去离子水和无水乙醇充分清洗。Cu基体的打磨暴露面积为1 cm2,将其余表面用环氧树脂密封。按照10∶1的比例将PDMS涂层主剂(PDMS)、固化剂(TEETMCT)混合并搅拌均匀。以15%(质量分数)的标准在涂层中加入CP-HNTs-MBT并充分搅拌混合得到浆料。用旋涂仪(Laurell匀胶旋涂仪WS-650-23B)将浆料旋涂到Cu基体(电极)的裸露表面并在室温下静置24 h,然后在40 ℃鼓风干燥箱中固化48 h。固化后即得CP-HNTs-MBT涂层电极。用YZT-1300C涂层测厚仪CP-HNTs-MBT涂层的厚度,将其控制在(90 ± 5) µm。
用相同的方法制备不含CP-HNTs-MBT的涂层,将其命名为空白涂层。
1.3 结构和性能表征
用傅立叶变换红外吸收光谱(FTIR,Nicolet IS10,Thermo Fisher Scientific Inc,USA)表征HNTs、HNTs-MBT和CP-HNTs-MBT的化学官能团,光谱范围500∼4000 cm-1,用KBr粉末压制技术制备测试用样品,扫描速率为4 cm-1。用NETZSCH-Gerätebau GmbH的Netzsch STA449C仪器进行热重分析(TGA),温度区间为30~800 ℃,气氛为空气,升温速度为10 ℃/min。用扫描电子显微镜(SEM,JSM-7500F;JEOL)观察纳米米容器以及涂层表面的划痕的微观形貌结构。进行能谱分析(EDS)表征Cu电极表面涂层愈合前后划痕的成分。用CS350恒电位仪在25 ℃测试CP-HNTs-MBT的电化学阻抗谱(EIS)以表征其防腐性能,同时测试空白涂层。腐蚀环境是3.5%(质量分数)的NaCl溶液,使用Cu/涂层电极(工作电极)、Ag/AgCl(饱和KCl)电极(参比电极)和尺寸为2 cm × 2 cm × 1 mm的铂箔(对电极),组成的三电极系统。测试频率为10-2~105 Hz,交流电压幅值为10 mV,测试周期持续30 d。分析和综合评估最具代表性的数据点(0 d、4 d、8 d、22 d、30 d)以测试CP-HNTs-MBT涂层和空白涂层的阻抗随腐蚀天数的变化。用PAR Model 370电化学工作站,进行扫描Kelvin探针(SKP)(区域覆盖划痕2 mm × 2 mm,扫描步长100 µm)分析CP-HNTs-MBT涂层划痕愈合前后的电压的变化。在面积为1 cm2的涂层表面划出长度为7.00 mm,宽0.25 mm模拟缺陷。使用在3.5%NaCl溶液中浸泡不同周期(0 d、4 d、6 d和8 d)后的涂层试样,在空气中测试缺陷处金属基体与涂层界面处的Volta电位分布。所有SKP测量均在开路电位和室温25 ℃下进行,每次测试时试样的安装方向和扫描位置相同。SVET用来体现试样表面的局部电流的大小及分布,电流由试样表面电势差求出,用XMU-BY-LG 扫描电化学工作站进行SVET测试,所用腐蚀液体为3.5%NaCl溶液,环境温度为25 ℃,测试的电极也是在3.5%NaCl溶液中浸泡了0 d、4 d、6 d、8 d面积为1 cm2涂层表面的模拟缺陷(长度为7.00 mm,宽0.25 mm)。将浸泡不同周期的电极固定在电解池中(每次的位置相同,用水平器将样品调平),探针与试样的距离为100 µm (将探针调至需要扫描的区域中心),然后连接电极并倒入电解液。将试样进行恒电流极化,扫描区域为2 mm × 2 mm,步长为100 µm。用Sufer软件分析测试数据以立体分布图的形式直观地显示出腐蚀电流。
在测试MBT的释放性能前先测试相应物质的浓度-吸光度标准曲线,测试波长范围为200~400 nm,MBT在pH = 5、pH = 7的水溶液中的紫外光谱吸收峰的位置为319 nm,实验涉及的溶液,用盐酸调节其pH值。
将5 mg的MBT溶于500 mL pH = 5、pH = 7的水溶液中,然后分别转移到1000 mL容量瓶中,定容和摇匀后得到浓度为5 mg/L的MBT标准溶液。分别将10.0、20.0、40.0、60.0、80.0、100 mL的标准溶液置于100 mL容量瓶中。将前5种溶液稀释定容,得到浓度为0.5、1.0、2.0、3.0、4.0 、5.0 mg/L的MBT标准溶液。用紫外-可见光分光光度计(UV3600,日本岛津)检测上述两种pH溶液的吸光度。用软件OriginPro绘制散点图并拟合数据,得到MBT在pH = 5、pH = 7的水溶液中的浓度-吸光度标准曲线。
将0.04 g CP-HNTs-MBT粉末加到400 mL蒸馏水中,超声震荡2 h使CP-HNTs-MBT内的缓蚀剂充分释放,用紫外-可见光分光光度计测试高速离心后的清液,根据pH = 7的标准浓度曲线计算MBT的浓度,得到MBT的最大装载量。
将0.04 g CP-HNTs-MBT粉末分别加到pH = 5、7的400 mL水溶液中,测试复合缓蚀剂在不同pH环境下的释放情况。分别在0 h、1 h、3 h、7 h、15 h、31 h、63 h、127 h、191 h、240 h时间点取5 mL上清液检测紫外吸光度,同时补充5 mL相应pH的溶液以保持原溶液总量不变。以测得的吸光度为准根据标准浓度曲线计算不同释放时间的MBT浓度值,得到不同释放时间的释放量(百分比),并得到MBT在pH = 5和pH = 7溶液中MBT浓度的UV光谱。
2 结果和讨论
2.1 HNT、HNTs、MBT和HNTs-MBT的组分
图2给出了HNT、HNTs、MBT和HNTs-MBT的傅立叶变换红外吸收光谱以表征其组分。在HNT和HNTs的内表面都存在-OH,因此在其谱中的3700 cm-1和3621 cm-1处都出现了弹性振动吸收峰[24],在1000 cm-1处出现了HNT和HNTs内表面Al-OH键的变形振动吸收峰[25]。在MBT傅立叶红外光谱中1321 cm-1处的吸收峰与MBT结构中的S-C=N反对称伸缩振动相关,1245 cm-1处的吸收峰与MBT的C-N振动对应[26],而752 cm-1处的吸收峰对应MBT的邻位取代苯环振动,与MBT分子的化学结构相关[27]。在HNTs-MBT的傅立叶红外光谱中不仅出现了HNTs和HNT的特征峰,还出现了MBT的特征峰。这表明,在HNTs-MBT中有MBT成分,证明MBT已经成功地包覆在HNTs,且没有生成新物质而只是一种物理包覆。
图2
图2
HNT、HNTs、MBT和HNTs-MBT的傅立叶红外光谱
Fig.2
Fourier transform infrared spectroscopy of HNT, HNTs, MBT and HNTs-MBT
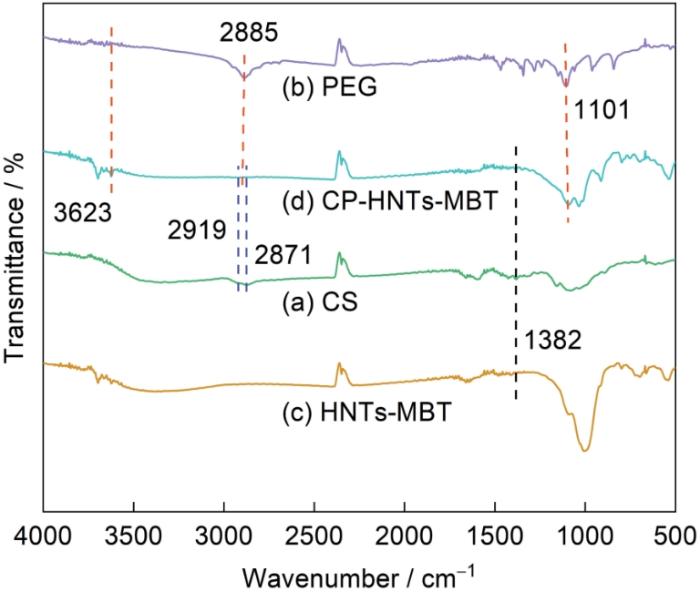
图3给出的CS、PEG、CP-HNTs-MBT和HNTs-MBT的傅立叶红外光谱,可用以分析CS和PEG的共聚物是否成功包覆在HNTs-MBT的表面。在图3a、d中2919 cm-1和2871 cm-1处出现的吸收峰,是脂肪族C-H的伸缩振动峰[28];在图3a、c、d中1382 cm-1处出现的是N-H的弯曲振动峰[29];在图3b、d中3623 cm-1处出现的是PEG中-OH的伸缩振动峰。此峰略宽,可归结为分子间氢键的作用[30]。在2885 cm-1和1101 cm-1处,分别产生了PEG的C-H弯曲振动峰和C-O-C伸缩振动峰[31]。对比图3c和d可见,在CP-HNTs-MBT的谱中同时出现了CS和PEG的吸收峰。这表明,CS和PEG的共聚物已经成功地包覆在HNTs-MBT表面。
图3
图3
CS、PEG、CP-HNTs-MBT和HNTs-MBT的傅立叶红外光谱
Fig.3
Fourier transform infrared spectroscopy of CS, PEG, HNTs-MBT and CP- HNTs-MBT
2.2 HNTs对MBT的装载能力和CS在HNTs-MTB表面的包覆量
图4
图4
HNTs、MBT、HNTs-MBT和CP-HNTs-MBT的TGA分析
Fig.4
TGA of HNTs, MBT, HNTs-MBT and CP-HNTs-MBT
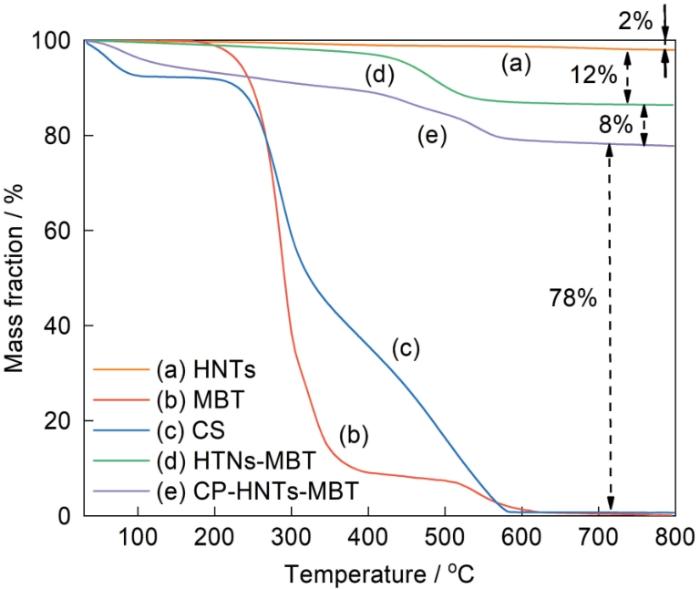
从30 ℃开始CS的质量持续减少,高于200 ℃质量减少的速率提高,到大约570 ℃质量减少到零。在100~200 ℃的质量损失,可归结于水的蒸发。CP-HNTs-MBT的质量损失率约为22%,由此计算出HNTs-MBT与CP-HNTs-MBT的质量差约为8%,表明CS已经成功地包覆在HNTs-MBT表面。
2.3 HNT 、HNTs、HNTs-MBT、CP-HNTs-MBT微观形貌
图5
图5
HNT 、HNTs、HNTs-MBT、CP-HNTs-MBT的SEM照片以及HNTs和CP-HNTs-MBT的TEM照片
Fig.5
SEM images of HNT (a), HNTs (b), HNTs-MBT (c), CP-HNTs-MBT (d) and TEM images of HNTs (e), CP-HNTs-MBT (f)
2.4 CP-HNTs-MBT涂层的阻抗模量
图6 给出了CP-HNTs-MBT涂层和空白涂层上的划痕在3.5%NaCl溶液中的阻抗模量。为了评估CP-HNTs-MBT涂层的自修复能力,进行了EIS测试。在添加CP-HNTs-MBT的涂层电极(对照组)和空白涂层电极(空白组)上制造划痕,每隔2 d取一次测量数据,频率f = 0.01 Hz。从图6a可见,添加CP-HNTs-MBT的涂层电极在30 d中|Z|0.01出现两次峰值,对应的天数分别为8 d和24 d,且8 d的峰值高于24 d的峰值。其原因是,MBT的2次释放使|Z|0.01增大,但MBT的量有限,第1次释放后MBT的减少使第2次释放形成的保护膜阻抗小于第1次释放形成的保护膜阻抗,因此8 d的峰值比24 d的大。0 d、18 d、30 d的|Z|0.01很低,其中18 d的|Z|0.01值最低。0 d MBT未释放;18 d、30 d,保护膜因溶液浸泡受损而使划痕处的阻抗减小。
图6
图6
CP-HNTs-MBT涂层和空白涂层上的划痕在3.5% NaCl溶液中的阻抗模量(f = 0.01 Hz)
Fig.6
Impedance modulus of scratches on CP-HNTs-MBT-coated (a) and blank-coated (b) surfaces in a 3.5% NaCl (mass fraction) solution (f = 0.01 Hz)
从图6b可见,随着测试天数的增加空白涂层电极的|Z|0.01值呈下降的趋势,表明其防腐蚀能力不断降低。其原因是,在划痕的间隙中NaCl溶液与Cu基体接触发生化学腐蚀反应,Cu作为阳极溶解时在附近产生H+而加剧了腐蚀。因此,随着测试天数的增加划痕处的阻抗值不断减小。
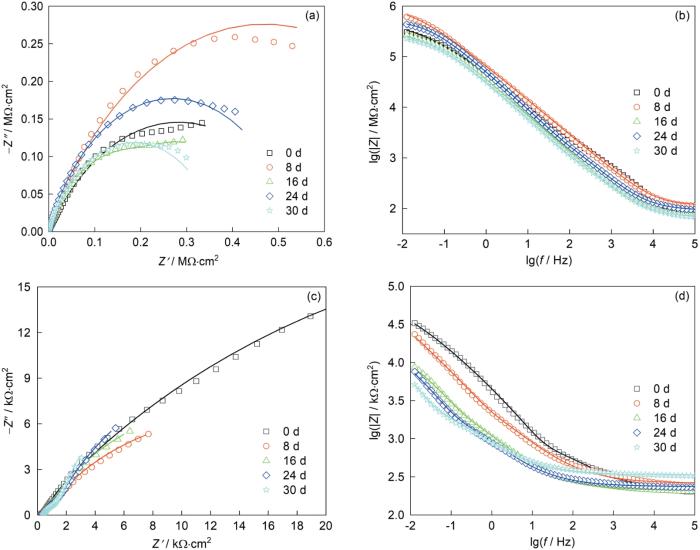
图7给出了添加CP-HNTs-MBT的涂层电极和空白涂层电极的Nyquist和Bode阻抗图。从图7a可见,开始阶段Nyquist容抗弧半径较小,随着测试天数的增加容抗弧半径呈现“增大—减小—增大—减小”的趋势。其原因是,在初始阶段溶液易通过划痕通道与Cu基体表面接触,使容抗弧的半径小。Cu作为阳极易腐蚀产生的H+使局部的pH值减小,使CP共聚物在酸性条件下溶胀。随着测试天数的增加HNTs中的MBT被释放,保护膜的形成使容抗弧半径迅速增大,8 d时达到最大值。保护膜阻止溶液与Cu基体接触,腐蚀受阻使局部H+的浓度降低,pH值增大使CP共聚物收缩和MBT停止释放。但是,随着测试天数的继续增加保护膜遭受破坏而使容抗弧半径减小。保护膜损坏到一定程度,划痕处重复发生MBT释放和保护膜形成的过程。在24 d容抗弧半径再次达到峰值,但是其峰值小于8 d时的峰值。其原因是,MBT的第2次释放量小于第1次,因此生成的保护膜的防腐性能也弱于第1次。这表明,添加CP-HNTs-MBT的涂层不仅具有自修复能力,还能通过局部pH值的变化控制MBT至少2次释放而实现循环自修复。
图7
图7
在3.5% NaCl溶液中浸泡0、8、16、24和30 d后CP-HNTs-MBT涂层和空白涂层上划痕的Nyquist和Bode图
Fig.7
After immersing in a 3.5% NaCl solution for 0, 8, 16, 24, and 30 d, Nyquist (a, c) and Bode (b, d) plots of scratches on copper coated with CP-HNTs-MBT (a, b) and blank-coated surfaces (c, d)
图7c、d分别给出了空白涂层电极的Nyquist和Bode阻抗图。与添加CP-HNTs-MBT的涂层电极相对应的天数相比,空白涂层的容抗弧半径不断减小,特别是从1 d到5 d迅速减小。这表明,空白涂层受损后没有自修复能力,不能阻止或延缓NaCl溶液对Cu基体的腐蚀。
图8
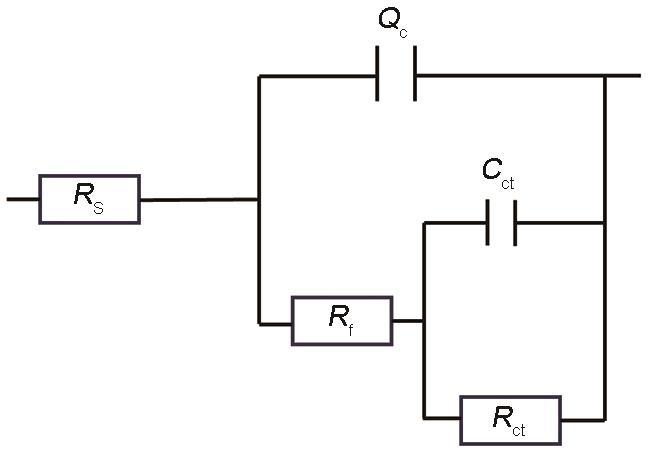
表1 在3.5% NaCl溶液中浸泡0 d,8 d,16 d,24 d,30 d后CP-HNTs-MBT涂层的拟合阻抗参数
Table 1
| t / d | Rs / Ω·cm2 | Y0-Qc / F·cm-2s n | n | Rf / Ω·cm2 | Cct / F·cm-2 | Rct / Ω·cm2 | Rt / Ω·cm2 | K / % |
|---|---|---|---|---|---|---|---|---|
| 0 | 76.36 | 5.871 × 10-6 | 0.6035 | 3.330 × 103 | 4.219 × 10-8 | 5.663 × 105 | 5.696 × 105 | 4.929 |
| 8 | 99.82 | 4.704 × 10-6 | 0.6752 | 3.232 × 105 | 3.915 × 10-8 | 9.402 × 105 | 1.263 × 106 | 4.665 |
| 16 | 84.42 | 5.531 × 10-6 | 0.7284 | 5.964 × 103 | 1.038 × 10-5 | 1.130 × 105 | 1.190 × 105 | 3.765 |
| 24 | 91.5 | 4.767 × 10-6 | 0.7328 | 9.727 × 104 | 1.826 × 10-7 | 5.308 × 105 | 6.281 × 105 | 3.000 |
| 30 | 91.25 | 5.590 × 10-6 | 0.7167 | 6.547 × 103 | 2.015 × 10-7 | 3.655 × 105 | 3.720 × 105 | 3.608 |
表2 在3.5% NaCl溶液中浸泡0 d,8 d,16 d,24 d,30 d后空白涂层的拟合阻抗参数
Table 2
| t / d | Rs / Ω·cm2 | Y0-Qc / F·cm-2s n | n | Rf / Ω·cm2 | Cct / F·cm-2 | Rct / Ω·cm2 | Rt / Ω·cm2 | K / % |
|---|---|---|---|---|---|---|---|---|
| 0 | 2.009 × 102 | 7.707 × 10-5 | 0.5114 | 1.38 × 103 | 4.963 × 10-6 | 7.875 × 104 | 8.013 × 104 | 1.118 |
| 8 | 2.058 × 102 | 3.133 × 10-4 | 0.5278 | 7.377 × 102 | 1.015 × 10-4 | 2.167 × 104 | 2.241 × 104 | 1.500 |
| 16 | 2.664 × 102 | 4.185 × 10-4 | 0.5307 | 5.672 × 102 | 3.311 × 10-4 | 6.730 × 103 | 7.297 × 103 | 1.039 |
| 24 | 3.630 × 102 | 5.704 × 10-4 | 0.5358 | 4.005 × 102 | 9.430 × 10-4 | 5.406 × 103 | 5.806 × 103 | 1.008 |
| 30 | 3.330 × 102 | 5.448 × 10-4 | 0.5225 | 3.668 × 102 | 8.151 × 10-4 | 1.609 × 103 | 1.976 × 103 | 1.022 |
从表1可见,在30 d的浸泡过程中Rf和Rct呈现出“上升—下降—上升—下降”的趋势,Rct与Rf的和Rt从整体上体现了保护膜防腐蚀性能的变化。在0 d涂层被划伤而防腐蚀性能较差,溶液进入划痕与Cu基体直接接触,缓蚀剂没有及时释放和保护膜未生成,因此Rt值较小;随着浸泡天数的增加MBT不断释放,生成保护膜使Rt逐渐增大;涂层浸泡至8 d时Rt达到最大值,划痕处涂层的防腐蚀性能最佳,生成的保护膜能有效阻止腐蚀的进行,MBT也停止释放;到16 d时Rt值降低,因为长期浸泡在NaCl溶液中保护膜逐渐失效,防腐蚀性能降低。到24 d时Rt值又一次增大并在30 d时再次减小,说明在16 d到24 d之间MBT再次释放使划痕处再次生成保护膜。从0 d到30 d共出现2次Rt峰值,表明该涂层至少有2次的循环修复性能。
表2列出的空白涂层拟合结果表明,Rf和Rct都随着浸泡时间的延长不断减小,Rct与Rf的和Rt也不断减小,且在0 d~8 d间减小得最快。这些结果表明,空白涂层没有自修复性能。
2.5 从受损到修复CP-HNTs-MBT涂层电位差的变化
用Kelvin探针验证添加CP-HNTs-MBT涂层的自修复效果,用SKP检测电位差(检测天数为0,4,6,8 d,到8 d第一次保护膜形成时截止),结果如图9所示。图9反映了CP-HNTs-MBT涂层从开始受损(0 d)到修复(8 d)的电位差变化。图9a中出现一个长而深的电势谷(对应在划痕处),因为涂层刚开始受损产生划痕,Cu基体裸露使Cu表面的电位比涂层表面的电位低,最低到-530 mV[35]。从图9b、c可见,随着天数的增加划痕处的电位上升。8 d时划痕处自修复涂层释放的MBT与Cu基体表面紧密结合形成保护膜,隔绝了外部溶液与空气,电势谷消失,如图9d所示。SKP测试结果与EIS和SEM数据分析结论吻合,表明CP-HNTs-MBT涂层具有良好的自修复性能。
图9
图9
CP-HNTs-MBT划痕涂层在3.5% NaCl溶液中浸泡0 d、4 d、6 d和8 d后的SKP电位分布
Fig.9
SKP test of coating scratch area with CP-HNTs-MBT immersed in 3.5% NaCl solution for 0 d (a), 4 d (b), 6 d (c), 8 d (d)
2.6 CP-HNTs-MBT涂层的自修复性能
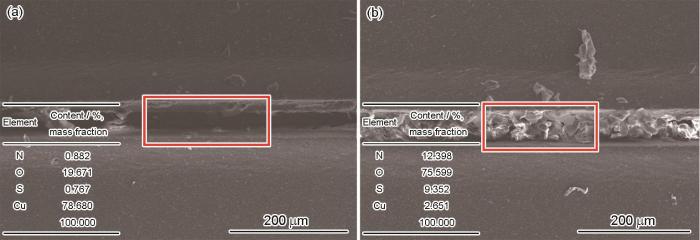
将EDS与SEM相结合,测定微观区域元素的含量和观察微观形貌进一步表征CP-HNTs-MBT涂层的自修复性能。图10a、b给出了0 d和8 d的测量结果(质量分数)。图10a中的划痕较宽,中间区域为暴露的Cu基体表面。所选区域Cu的含量为78.680%,N的含量为0.882%,O的含量为19.671%,S的含量为0.767%。Cu的含量较高的原因是,0 d时划痕底部的Cu表面暴露与NaCl溶液接触。随着CP-HNTs-MBT纳米管释放MBT生成保护膜Cu的表面逐渐被覆盖,MBT的含量大幅度降低。如图10b所示,划痕被MBT填充,生成的保护膜表面较粗糙。在所选区域各元素含量分别为;N为12.398%,O为75.599%,S为9.352%,Cu为2.651%。这表明,CP-HNTs-MBT涂层的自修复性能较高,MBT生成的保护膜很好地覆盖在Cu基体表面。
图10
图10
浸入3.5% NaCl溶液中的含CP-HNTs-MBT涂层上的划痕在0 d和8 d后的SEM图像和EDS数据
Fig.10
SEM image and EDS data of coating scratch area with CP-HNTs-MBT immersed in 3.5% NaCl solution for 0 d (a) and 8 d (b)
2.7 MBT的释放阻止Cu的腐蚀
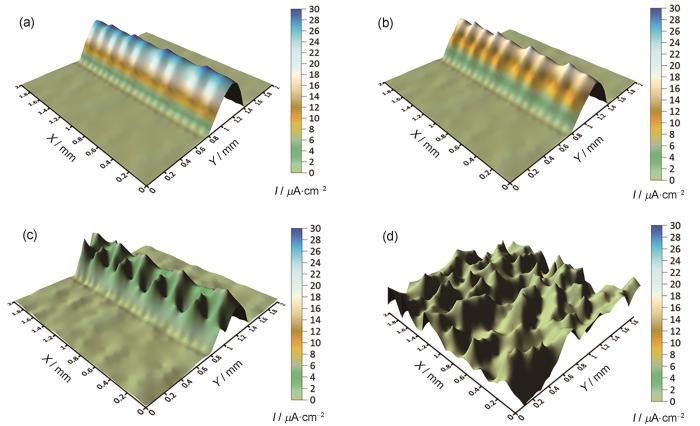
如图11所示的SVET测试结果,在0 d时,电极工作表面划痕处的Cu与溶液接触,既没有缓蚀剂也没有腐蚀产物的影响,腐蚀较快,最高电流密度约为30 µA/cm2,导致观测区域电流密度较大。但到4 d时,观测区域的最高腐蚀电流密度下降至18 µA/cm2,原因是MBT的释放以及腐蚀产物的生成保护了电极工作表面。
图11
图11
CP-HNTs-MBT划痕涂层在3.5% NaCl溶液中浸泡0 d、4 d、6 d和8 d后的SVET电流密度分布
Fig.11
SVET test of coating scratch area with CP-HNTs-MBT immersed in 3.5% NaCl solution for 0 d (a), 4 d (b), 6 d (c), 8 d (d)
到6 d最高电流密度约为6 µA/cm2,而到8 d几乎所有观测区域的腐蚀电流密度都为0,表明MBT的释放阻止了Cu的腐蚀。
2.8 MBT的释放性能
图12
图12
pH = 5和pH = 7的MBT标准浓度曲线
Fig.12
MBT standard concentration curve of (a) pH = 5 and (b) pH = 7
图13
图13
在pH值为5、7条件下MBT释放量与时间的关系
Fig.13
Relationship between MBT release and time under the condition of pH = 5 and pH = 7
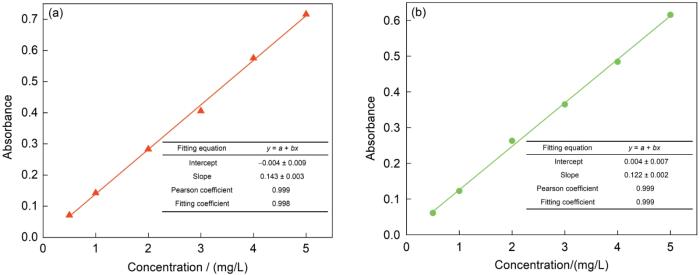
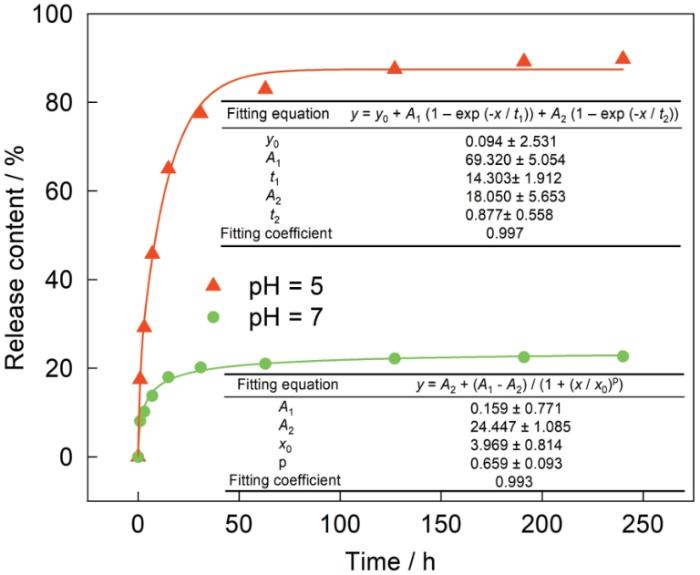
可以看出,在释放初期MBT 的释放速率较高,随着释放时间的延长MBT的释放速率逐渐降低,直到最大释放量。在pH = 5条件下,MBT释放的前 31 h内释放量达到约75 %,此后MBT的释放速率逐渐降低,127 h后释放速率的变化极小。在释放测试的240 h内,MBT的累积释放量约为87%。在pH值=7条件下MBT的释放量始终较小,累积释放量约为20%。在理想情况下,CP-HNTs-MBT在中性溶液中不释放MBT。
图14给出了在pH = 5、7条件下不同释放时间MBT的UV浓度光谱。以319 nm的波长为准,测试MBT在不同释放时间的吸收度。pH = 5时最大吸收度约为1.5,最小约为0.3。吸收度较大的变化,表明MBT的释放量较多。在pH = 7条件下最大吸收度约为0.33,最小约为0.13,变化很小。这表明,MBT的释放量很小。
图14
图14
pH = 5、pH = 7的MBT在不同释放时间的UV光谱
Fig.14
UV spectra of (a) pH = 5 and (b) pH = 7 MBT at different release times
2.9 CP-HNTs-MBT涂层的自修复机理
图15给出了CP-HNTs-MBT涂层受损后的自修复机理。涂层表面划痕处的Cu基体表面与溶液相接触,发生化学腐蚀产生Cu+、CuO和H+而使局部呈现酸性[36]。在酸性环境中CP纳米胶束中CS的氨基被质子化,由-NH2变成-NH
图15
图15
腐蚀机理和自修复机理示意图
Fig.15
Schematic diagram of corrosion mechanism and self-healing mechanism
如果保护膜因溶液浸泡或外部破坏而失效,划痕处将重复上述自修复过程。但是,随着循环修复次数的增加,自修复效果则因HNTs中MBT的减少而越来越差(图6a)。同时,涂层未完全受损(金属表面未暴露)时,划痕处局部pH没有改变,缓蚀剂不会提前释放,于是便实现了缓蚀剂的可控释放和防止错误释放。与传统的微纳米防腐涂层相比,CP-HNTs-MBT涂层的释放时机更加精确和服役期限更长。
3 结论
(1) 用碱刻蚀扩大埃洛石纳米管的管径,可提高其装载缓蚀剂的能力。
(2) 具有pH值响应特性的CP与HNTs相结合,可消除无机纳米载体的团聚和解决缓蚀剂释放时机错误、过量释放和不能多次释放。
参考文献
Functional and smart coatings for corrosion protection: A review of recent advances
[J].
Corrosion behavior of epoxy/zinc duplex coated rebar embedded in concrete in ocean environment
[J].
Carbon dots as new eco-friendly and effective corrosion inhibitor
[J].
Corrosion protection of steel with DMEA-based organic inhibitor
[J].
Pyridine-based functionalized graphene oxides as a new class of corrosion inhibitors for mild steel: an experimental and DFT approach
[J].
Corrosion behavior of epoxy/zinc duplex coated rebar embedded in concrete in ocean environment
[J].
Experimental and analytical study on bond strength of normal uncoated and epoxy-coated reinforcing bars
[J].
Smart lignin-based polyurethane conjugated with corrosion inhibitor as bio-based anticorrosive sublayer coating
[J].
Improvement in corrosion protective performance of polyacrylate coating based on corrosion inhibitors microcapsules
[J].
Halloysite clay nanotubes for controlled release of protective agents
[J].Halloysite is a naturally occurring clay mineral with submicron sized hollow cylindrical morphology. Halloysite morphology, structure and properties were characterized by using SEM, TEM, XRD, FT-IR spectroscopy, surface electrokinetic (zeta) potential and nitrogen adsorption isotherms. Comparison of the halloysite structure with imogolite was also provided. Halloysite toxicological studies revealed that it is environmentally friendly and biocompatible material. Due to its unique tubular shape and availability in thousands of tons halloysite has potential to be applied as nanocontainers for encapsulation of chemically and biologically active agents such as medicines, pharmaceuticals, antiseptics, corrosion inhibitors, antifouling agents, and doped with them plastics producing smart polymeric nanocomposites with improved mechanical strength. Finally possibility to synthesize metal nanorods within the halloysite lumen was demonstrated.
Enhanced efficiency of antiseptics with sustained release from clay nanotubes
[J].
Sustained release of antibacterial agents from doped halloysite nanotubes
[J].
The application of halloysite tubule nanoclay in drug delivery
[J].Natural and biocompatible clay nanotubes are among the best inorganic materials for drug nanoformulations. These halloysite tubes with SiO2 on the outermost surface have diameter of ca. 50 nm, length around 1 micrometer and may be loaded with drugs at 10-30 wt. %. Narrow tube openings allow for controllable sustained drug release for hours, days or even weeks.Physical-chemical properties of these nanotubes are described followed by examples of drug-loading capabilities, release characteristics, and control of duration of release through the end tube capping with polymers. Development of halloysite-polymer composites such as tissue scaffolds and bone cement/dentist resin formulations with enhanced mechanical properties and extension of the drug release to 2-3 weeks are described. Examples of the compression properties of halloysite in tablets and capsules are also shown.We expect that clay nanotubes will be used primarily for non-injectable drug formulations, such as topical and oral dosage forms, cosmetics, as well as for composite materials with enhanced therapeutic effects. These include tissue scaffolds, bone cement and dentist resins with sustained release of antimicrobial and cell growth-promoting medicines (including proteins and DNA) as well as other formulations such as compounds for antiseptic treatment of hospitals.
Inhibition of the initial stages of corrosion by 2-mercaptobenzothiazole adsorption and the effects of interfacial oxides on copper in neutral chloride conditions
[J].
Advancement in corrosion resistance of AA 2024-T3 through sol-gel coatings including nanocontainers
[J].
Corrosion of cemented carbide grades in petrochemical slurries. Part I - Electrochemical adsorption of CN¯, SCN¯ and MBT: A study based on in situ SFG
[J].
Multifunctional konjac glucomannan/xanthan gum self-healing coating for bananas preservation
[J].
Self-healing coatings for large-scale damages via ultrahigh load capacity of healing agents in short kapok microtubules
[J].
“Smart” micro/nano container-based self-healing coatings on magnesium alloys: A review
[J].
Active anticorrosion coatings with halloysite nanocontainers
[J].
Chitosan nanocomposite films based on halloysite nanotubes modification for potential biomedical applications
[J].Chitosan is attracting increasing attention for biomedical applications because of its biocompatibility. In the present study, raw halloysite nanotubes (RHNTs) were functionalised with (3-aminopropyl) triethoxysilane (APTS) and then a sequence of novel chitosan biofilms were prepared by adding amino-modified halloysite nanotubes (HNTs-NH) as a reinforcing material and ethylene glycol diglycidyl ether (EGDE) as a cross-linking agent. The reaction between the APTS and the RHNTs was demonstrated through characterisation of the HNTs-NH. Fourier transform infra-red spectroscopy (FTIR) and X-ray diffraction (XRD) results confirmed the interaction of HNTs-NH with chitosan and EGDE. Scanning electron microscopy (SEM) showed a transformation of the surface morphology of the chitosan films. Measurement of the mechanical and thermal properties showed that the nanocomposite films exhibited substantial improvements in tensile strength, elongation at break and thermal stability compared with those of the pure chitosan films. However, the swelling rate of the nanocomposite films decreased upon incorporation of the HNTs-NH and EGDE. In addition, the water vapour transmission rate (WVTR) of the nanocomposite films was also improved. Given the aforementioned results, chitosan nanocomposites are promising biomedical materials.Copyright © 2019 Elsevier B.V. All rights reserved.
Highly efficient sunitinib release from pH-responsive mHPMC@Chitosan core-shell nanoparticles
[J].
Potential non-releasing bacteria-triggered structure reversible nanomicelles with antibacterial properties
[J].
Preparation and corrosion resistance study of a pH-responsive plasma electrolytic oxidation coating modified by halloysite nanotubes
[J].
Chitosan-halloysite hybrid-nanotubes: Horseradish peroxidase immobilization and applications in phenol removal
[J].
A novel method for preparation of 8-hydroxyquinoline functionalized mesoporous silica: Aluminum complexes and photoluminescence studies
[J].
Chitosan derivatives as green corrosion inhibitors for P110 steel in a carbon dioxide environment
[J].
Ag-lignin hybrid nanoparticles for high-performance solar absorption in photothermal antibacterial chitosan films
[J].
Impact of hybrid aluminum oxide/titanium dioxide nanoparticles on the structural, optical, and electrical properties of polyvinyl alcohol/ polyethylene glycol nanocomposites for flexible optoelectronic devices
[J].
Evaluation of the toxicity and cisplatin drug release ability of nanocomposites of polyethylene glycol and carbon nanotubes functionalized with Zn2+ions
[J].
Self-healing properties of protective coatings containing isophorone diisocyanate microcapsules on carbon steel surfaces
[J].
Electrodeposited polypyrrole coatings on mild steel: Modeling the EIS data with a new equivalent circuit and the influence of scan rate and cycle number on the corrosion protection
[J].
High-resolution Kelvin probe microscopy in corrosion science: Scanning Kelvin probe force microscopy (SKPFM) versus classical scanning Kelvin probe (SKP)
[J].
Corrosion protection performance of a coating with 2-aminino-5-mercato-1,3,4-thiadizole-loaded hollow mesoporous silica on copper
[J].
Surface-enhanced infrared absorption spectroscopy study of anticorrosion behavior of 2-Mercaptobenzothiazole on copper
[J].
Redox and pH dual-responsive PEG and chitosan-conjugated hollow mesoporous silica for controlled drug release
[J].