开发石油天然气是解决能源需求的重要途径之一。CO2驱油技术,是提高传统油藏采收率的有效方法。干燥的CO2是一种非腐蚀性气体,溶于水生成碳酸会引起析氢腐蚀。虽然碳酸是一种弱酸,但是在pH值相同的条件下其总酸度比其他强酸还高,能导致严重的腐蚀。
CO2引起的电化学腐蚀极易引起金属材料的局部点蚀穿孔破坏,在油气工业中CO2局部腐蚀导致的失效十分常见[1~6]。对金属的CO2腐蚀机理和影响因素,已经有较多的研究。Kahyarian等[7]研究了X65钢在饱和CO2酸性盐溶液中(CO2压力为0~1.5 MPa)的电化学腐蚀行为机理,发现阴极反应只是H+的还原,HCO
在CO2环境中金属表面通常形成FeCO3产物层,对金属的腐蚀行为有重要的影响[9~12]。张国安等[9,10,13]研究了碳钢和低合金钢的CO2腐蚀产物,发现不同钢种的腐蚀产物均存在分层。碳钢的内外层腐蚀产物均为FeCO3,含Cr钢的外层腐蚀产物为FeCO3,出现Cr的富集[11,14]。在静态条件下碳钢的耐蚀性较高,而在动态冲刷作用下含Cr钢的耐蚀性更高。这表明,金属在CO2环境中的腐蚀行为,与环境参数、腐蚀产物和腐蚀介质流动等因素密切相关。同时,对电化学反应动力学参数,如交换电流密度、标准电位、活化能以及塔菲尔斜率[15~17],也进行了深入研究。这些研究大都针对动态[9]、超临界[18~21]或常压低压CO2环境[22]。
针对静态环空环境中金属的腐蚀研究比较少,因为油气井中通常用封隔器将环空与外部环境的腐蚀介质隔离,在理论上能避免腐蚀。但是,实际上油管中总会有缺陷。在服役过程中,这些缺陷使油管内的侵蚀性介质(如CO2、H2S等)或地层水(一般矿化度较高、腐蚀性强)向环空渗漏。注入井中大量的CO2渗入环空,使环空液的pH值低至3~4,大幅度降低了油管的耐蚀性。同时,高压CO2的注入使油管的温度降低,也影响油管的腐蚀。由此可见,影响环空环境腐蚀性的因素有一定的波动性。诸多因素间的相互作用,既提高了室内模拟的难度也不利于快速评价油管钢的使役性能。鉴于此,本文基于实际的CO2驱注井环空环境指标和理论计算确定室内模拟环空环境的参数,并进行电化学测试研究环境总压(Ptotal)、CO2分压(
1 实验方法
1.1 实验用材料
实验用J55级油管钢的实测成分列于表1。
表1 实验用J55级油管钢的化学成分
Table 1
| C | Si | Mn | P | S | Cr | Ni | Nb | V | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 0.395 | 0.283 | 1.32 | 0.0419 | 0.0293 | 0.106 | 0.0418 | 0.0158 | 0.0053 | Bal. |
将J55油管钢试样抛光后用4%硝酸酒精溶液(4 mL浓硝酸+ 96 mL无水乙醇)浸蚀,然后用金相显微镜(型号为Axio Scope A1)观察其组织结构。J55油管钢的微观组织为块状珠光体+铁素体,如图1所示。
图1
1.2 搭建室内模拟环空环境
1.2.1 环空模拟液
表2中列出了杏子川采油厂注水井的水质分析数据,对无机盐浓度取上限值,最终确定模拟环空液母液的成分为15 g·L-1 NaCl,10 g·L-1 Na2SO4,1 g·L-1 NaHCO3。
表2 油田水介质的成分
Table 2
| Composition | Value |
|---|---|
| Sulfate / mg·L-1 | 3000~15000 |
| NaHCO3 / mg·L-1 | 150~1000 |
| KCl / mg·L-1 | 12000~15000 |
| NaCl / mg·L-1 | 12000~15000 |
| Inhibitor / mg·L-1 | 50~200 |
| 4.00 |
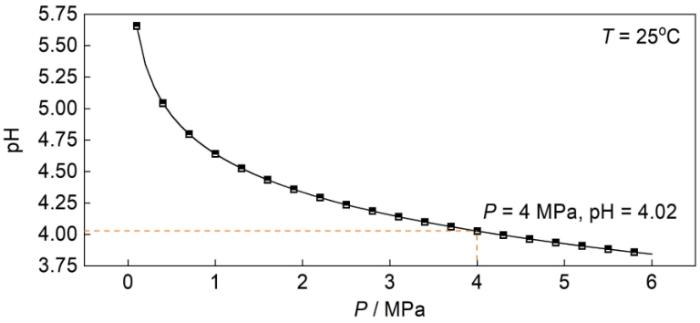
1.2.2 环空环境pH值与CO2分压的关系
其中下标g表示该粒子呈气相;下标aq表示该粒子存在于溶液中;a = mγ为离子的活度,m为涉及的粒子质量摩尔浓度(mol·kg-1),γ为相应的粒子的活度系数;f为气体的逸度(10-1 MPa);P为气体分压(10-1 MPa);φ为逸度系数。
在计算各参数的公式中,TK为温度(K);I为离子强度(mol·L-1);c为离子浓度(mol·L-1);ρw为水的密度(g·cm-3);z为离子价态。式中的参数ai 列于表3。
| Parameters | ρw / g·cm-3 | ||||
|---|---|---|---|---|---|
| a1 | 2.0302 | -7.8294 | -15.707 | -4.098 | 1.8105 × 10-3 |
| a2 | -2.3508 × 10-2 | 8.2473 × 10-3 | 3.0972 × 10-2 | -3245.2 | 138.08 |
| a3 | 2.6144 × 10-5 | -1.0530 × 10-5 | -4.3124 × 10-5 | 2.2362 × 104 | - |
| a4 | - | 4.4527 × 10-4 | 3.8058 × 10-4 | -3.9840 × 107 | - |
| a5 | - | 0.4772 | 1.166 | 13.957 | - |
| a6 | - | -0.118 | -0.3466 | -1262.3 | - |
| a7 | - | - | - | 8.5641 × 105 | - |
根据电中性原理,溶液中的正电荷与负电荷总量相等,即
根据上述反应平衡和计算出的平衡常数,可得一个关于
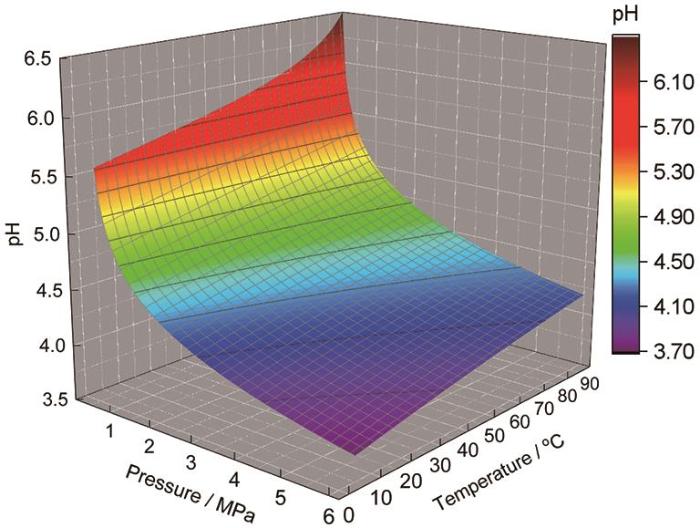
图2
图2
模拟环空环境中pH值的计算结果
Fig.2
Calculated results of pH value for the simulated annulus environment
图3
1.3 高压电化学测试
J55钢电化学测试所用样品的尺寸为10 mm × 10 mm × 2 mm,将其与铜导线焊接后用环氧树脂封装并确保金属与环氧交界处没有气泡。电化学测试前,用砂纸打磨样品的工作表面,最后一道砂纸为2000目。打磨后用去离子水冲洗样品的测试面,放置使其干燥。在电化学测试过程中,J55钢暴露在测试溶液中的面积为1 cm2,如图4所示。
图4
使用PARSTAT 4000型电化学工作站和三电极体系进行电化学测试,铂片电极为辅助电极,Ag/AgCl电极作为参比电极,J55钢样品为研究电极。在高压釜(容积为500 mL)中进行电化学测试。测试时,先将已经预除氧的测试溶液与试样装入高压釜中,将高压釜关闭后向釜中通30 min高纯氮气进一步除去测试溶液中的氧气。随后向高压釜中通入高压氮气和二氧化碳进行加压。加压时,先通入CO2至预定分压,再通入高压氮气至目标环境总压。
高压釜中的压力和温度达到预定值后静置30 min,然后测试开路电位、交流阻抗谱和极化曲线。测试交流阻抗谱时电位为开路电位,扰动电位为10 mV,频率为100 kHz ~10 mHz。使用动电位法(电位从负向正方向扫描)测试J55钢样品的极化曲线,扫描范围为-1.2~-0.2 V(相对于Ag/AgCl电极),电位扫描速度为0.5 mV·s-1。实验结束后,使用EC-Lab 11.16软件拟合J55钢的极化曲线。
1.4 相关性分析方法
常用的相关性分析方法,包括Pearson相关分析、Spearman相关分析和Kendall相关分析等。Pearson相关分析适用于符合二元正态分布数据,Spearman相关分析适用于二元非正态分布数据,Kendall相关分析用于计算分类变量数据。相关性分析的第一步,是用Shapiro-Wilk(SW)法检验数据的正态性。若待分析数据均呈正态分布,则采用Pearson相关分析;否则,采用Spearman相关分析。
相关性分析得到的相关系数(r,-1 ≤ r ≤ 1)和显著性水平(P,双尾检验)用于评判两列数据的相关程度。r < 0表明两列数据的呈负相关,r = 0表明两列数据完全不相关,r > 0表明两列数据的呈正相关。0.75 < |r| ≤ 1,表明两列数据高度相关;0.3 < |r| ≤ 0.75,表明两列数据显著相关;|r| < 0.3,表明两列数据弱相关[27]。P值为所得相关性结果是错误的概率,通常取0.05或0.01;即当P < 0.05或P < 0.01时,相关性分析结果的可信度较高。
2 结果和讨论
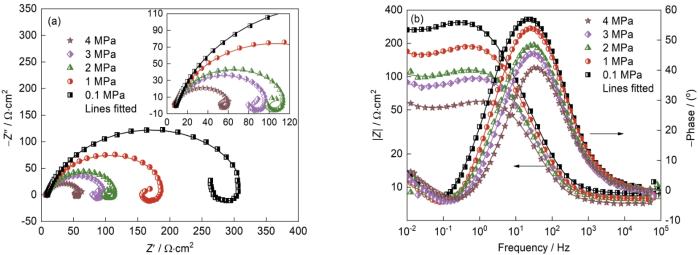
2.1 总压对J55钢电化学行为的影响
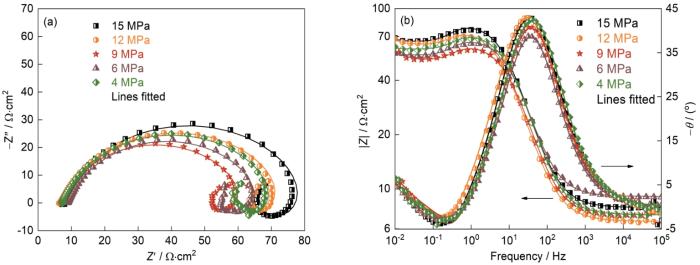
图5
图5
J55钢在不同总压下的Nyquist和Bode图,Nyquist图中的插图为对应的等效电路
Fig.5
Nyquist plots (a) and Bode plots (b) of J55 steel with different total pressure (
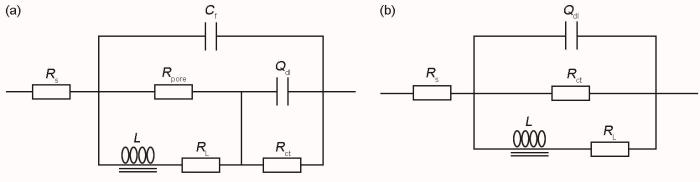
图6
图6
J55钢在不同温度下EIS谱拟合的等效电路
Fig.6
Equivalent circuits for electrochemical impedance spectra with different temperature (a) 25~50oC, (b) 5~15oC
表4 J55钢在不同总压下的电化学阻抗谱拟合数据
Table 4
| Ptotal / MPa | Rs / Ω·cm2 | Qdl × 10-4 / Ω-1·cm-2·s-n | ndl | Rct / Ω·cm2 | RL / Ω·cm2 | L / H∙cm-2 | Cf / F·cm-2 | Rpore / Ω·cm2 |
|---|---|---|---|---|---|---|---|---|
| 15 | 7.86 | 5.38 | 0.814 | 75.18 | 253.2 | 155.8 | 2.298 | 26.17 |
| 12 | 6.60 | 7.49 | 0.796 | 70.08 | 301.4 | 189.4 | 2.03 | 13.66 |
| 9 | 7.10 | 6.23 | 0.812 | 56.67 | 217.9 | 112.3 | 1.349 | 12.77 |
| 6 | 9.08 | 5.35 | 0.819 | 59.40 | 203.5 | 118.3 | 2.705 | 18.62 |
| 4 | 8.51 | 6.50 | 0.784 | 67.92 | 254.7 | 110.1 | 1.95 | 23.12 |
对于不同的Ptotal,J55钢的阻抗谱均呈现出3个时间常数且形状相同,表明Ptotal的变化不影响J55钢的电化学腐蚀机理。阻抗谱高频区域的容抗弧与J55钢的界面双电层电容和电荷转移电阻有关,中频区域的感抗弧与腐蚀过程的中间产物(吸附态Fe(OH),Fe(OH)ads)在J55钢表面的吸附有关[15,28~30],如式(
图7
图7
J55油管钢在不同总压下的动电位极化曲线和拟合结果
Fig.7
Polarization curves (a) and fitting results (b) of J55 steel with different total pressure (
对比图7中不同总压条件下的极化曲线,可见总压的变化主要影响J55钢腐蚀的阴极过程。Ptotal小于9 MPa时,随着Ptotal的提高阴极电流密度明显提高;Ptotal高于9 MPa时,阴极电流密度大幅度减小,表明阴极反应受到抑制。有研究表明,对于一定的CO2分压,环境总压的提高使CO2在水溶液中的溶解度提高[33];环境总压的提高使J55钢表面的双电层变薄,促进Cl-在J55钢表面的吸附和钢材的腐蚀[34]。同时,压力的提高促进保护性腐蚀产物膜的形成,从而降低腐蚀速率[4,35]。因此,压力对腐蚀过程最终的作用是二者的综合结果。本文实验中Ptotal低于9 MPa时Ptotal的提高促进CO2的溶解进而加快阴极去极化过程,最终加速J55钢的溶解,此时总压表现为对腐蚀的促进。Ptotal高于9 MPa后,Ptotal对腐蚀过程的影响是对钢表面腐蚀产物成膜的促进。Ptotal越高则腐蚀产物膜越厚且完整性越高,更好的抑制了钢基体的腐蚀,从而使J55钢的腐蚀速率降低。Zhang等[35,36]研究发现,环境压力的提高促进X70钢在3.5%(质量分数)NaCl水溶液中的初期腐蚀速率,总压与J55钢的腐蚀进程密切相关。在一定的浸泡时间内,总压较低时腐蚀的进程较慢,J55钢处于腐蚀初期,没有生成完整的产物。因此,随着总压的提高腐蚀进程加速;一旦生成产物膜腐蚀即进入中后期,总压使产物膜的增厚加速,从而使腐蚀受到抑制 。
2.2 CO2 分压对J55钢电化学行为的影响
图8
图8
J55油管钢在不同
Fig.8
Nyquist plots (a) and Bode plots (b) of J55 steel with different
表5
J55钢在不同
Table 5
| Rs / Ω·cm2 | Qdl × 10-4 /Ω-1·cm-2·s-n | ndl | Rct / Ω·cm2 | RL / Ω·cm2 | L / H·cm-2 | Cf / F·cm-2 | Rpore / Ω·cm2 | |
|---|---|---|---|---|---|---|---|---|
| 4 | 7.10 | 6.23 | 0.812 | 56.67 | 217.9 | 112.3 | 1.349 | 12.77 |
| 3 | 8.36 | 5.12 | 0.820 | 95.87 | 314.4 | 233 | 1.801 | 12.23 |
| 2 | 7.80 | 5.12 | 0.817 | 115.1 | 449.2 | 451.7 | 1.386 | 10.84 |
| 1 | 7.871 | 3.94 | 0.823 | 197.8 | 620.1 | 640 | 1.284 | 22.69 |
| 0.1 | 6.163 | 2.84 | 0.847 | 268.7 | 933.2 | 1330 | 0.3175 | 25.57 |
图9
图9
J55钢在不同
Fig.9
Polarization curves (a) and fitting results (b) of J55 steel with different
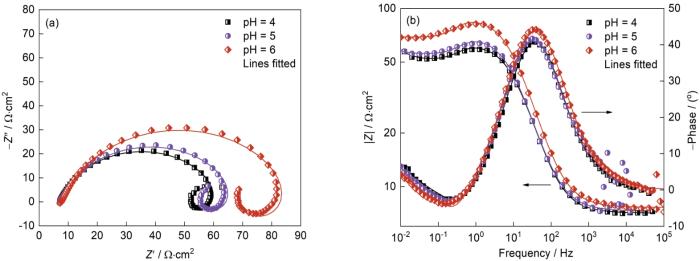
2.3 pH值对J55钢电化学行为的影响
图10
图10
在不同pH值下J55钢的Nyquist和Bode图
Fig.10
Nyquist plots (a) and Bode plots (b) of J55 steel with different pH value (Ptotal = 9 MPa,
表6 J55钢在不同pH下的阻抗谱拟合数据
Table 6
| pH | Rs / Ω·cm2 | Qdl × 10-4 / Ω-1·cm-2·s-n | ndl | Rct / Ω·cm2 | RL / Ω·cm2 | L / H·cm2 | Cf / F·cm2 | Rpore / Ω·cm2 |
|---|---|---|---|---|---|---|---|---|
| 4 | 7.10 | 6.23 | 0.812 | 56.67 | 217.9 | 112.3 | 1.349 | 12.77 |
| 5 | 7.30 | 6.55 | 0.804 | 62.8 | 215 | 103 | 2.32 | 25.3 |
| 6 | 7.65 | 4.94 | 0.807 | 81.5 | 253 | 164 | 3.06 | 32 |
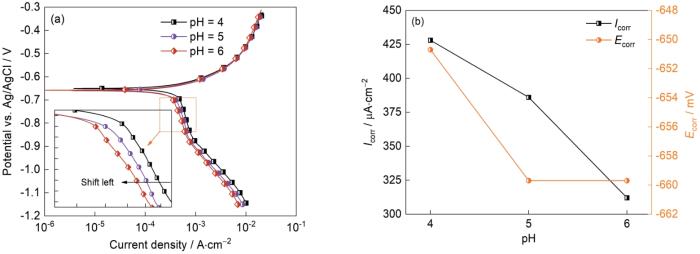
图11给出了初始pH值不同的条件下J55钢的极化曲线和拟合结果,其中的插图为阴极极化曲线的局部放大。可以看出,随着初始pH值的升高阴极电流密度减小。这表明,初始pH值的升高抑制了模拟环境中的阴极析氢反应,从而抑制了J55钢在模拟环空环境中的腐蚀电化学过程,也验证了电化学阻抗谱给出的结果。
图11
图11
J55钢在不同pH值下的动电位极化曲线和拟合结果
Fig.11
Polarization curves (a) and fitting results (b) of J55 steel with different pH value (Ptotal = 9 MPa,
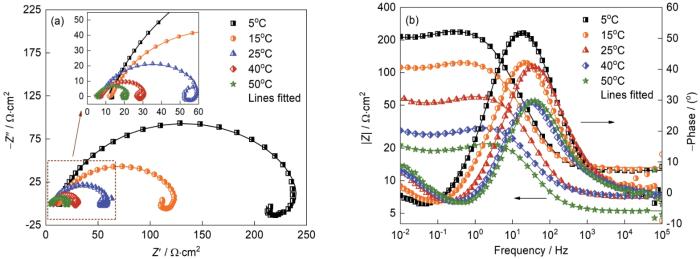
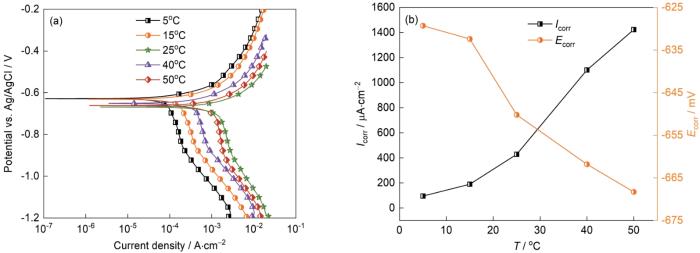
2.4 温度对J55钢电化学行为的影响
图12给出了J55钢在不同温度下(
图12
图12
J55钢在不同温度下的Nyquist和Bode图
Fig.12
Nyquist plots (a) and Bode plots (b) of J55 steel with different temperature (Ptotal = 9 MPa,
表7 J55钢在不同温度下电化学阻抗谱的拟合数据
Table 7
| T / oC | Rs / Ω·cm2 | Qdl × 10-4 / Ω-1·cm-2·s-n | ndl | Rct / Ω·cm2 | RL / Ω·cm2 | L / H·cm2 | Cf / F·cm2 | Rpore / Ω·cm2 |
|---|---|---|---|---|---|---|---|---|
| 5 | 12.4 | 3.67 | 0.83 | 242.8 | 1098 | 1952 | - | - |
| 15 | 13.22 | 4.97 | 0.813 | 116.8 | 562 | 689 | - | - |
| 25 | 7.10 | 6.23 | 0.812 | 56.67 | 217.9 | 112.3 | 1.349 | 12.77 |
| 40 | 7.27 | 13.2 | 0.772 | 26.84 | 74.5 | 19.8 | 3.299 | 2.43 |
| 50 | 5.62 | 12.5 | 0.813 | 18.3 | 53.7 | 12.7 | 3.11 | 7.64 |
在温度的升高过程中,感抗显著减小。其原因是,温度较低时铁的溶解速率较低,中间产物的吸脱附过程较慢(
图13
图13
J55钢在不同温度下的极化曲线和拟合结果
Fig.13
Potentiodynamic polarization curves of J55 steel with different temperatures (a) and fitted results (b) (Ptotal = 9 MPa,
2.5 电化学腐蚀速度与环境因素的相关性
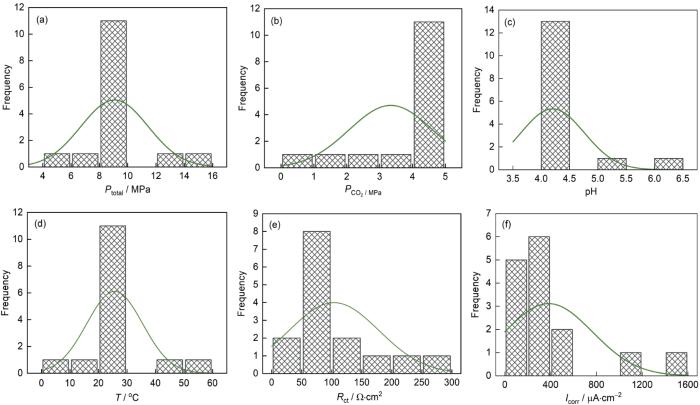
以上实验结果表明,环境总压、CO2分压、pH和温度都不同程度地影响J55钢在模拟环空中的电化学腐蚀行为,但是其腐蚀作用机理不尽相同,因此很难量化对比、判断这些影响因素的主次,加之实际环境中的这些因素存在波动,进一步增大了在室内准确和快速评价油管钢耐蚀性能的时间成本。基于本测试数据(表8),计算了四个因素与J55钢电化学腐蚀速度之间的相关性,以期优化实验参数。
表8 相关性计算的原始数据表
Table 8
| Number | Ptotal / MPa | pH | T / oC | Rct / Ω·cm2 | Icorr / μA·cm-2 | |
|---|---|---|---|---|---|---|
| PP1 | 9 | 4 | 4 | 25 | 56.67 | 428.00 |
| PP2 | 9 | 3 | 4 | 25 | 95.87 | 201.00 |
| PP3 | 9 | 2 | 4 | 25 | 115.1 | 116.00 |
| PP4 | 9 | 1 | 4 | 25 | 197.8 | 88.00 |
| PP5 | 9 | 0.1 | 4 | 25 | 268.7 | 32.00 |
| pH1 | 9 | 4 | 4 | 25 | 56.67 | 428.00 |
| pH2 | 9 | 4 | 5 | 25 | 62.8 | 386.00 |
| pH3 | 9 | 4 | 6 | 25 | 81.5 | 312.00 |
| T1 | 9 | 4 | 4 | 5 | 242.8 | 95.10 |
| T2 | 9 | 4 | 4 | 15 | 116.8 | 189.80 |
| T3 | 9 | 4 | 4 | 25 | 56.67 | 428.00 |
| T4 | 9 | 4 | 4 | 40 | 26.84 | 1101.30 |
| T5 | 9 | 4 | 4 | 50 | 18.3 | 1422.90 |
| PT1 | 15 | 4 | 4 | 25 | 75.18 | 236.21 |
| PT2 | 12 | 4 | 4 | 25 | 70.08 | 313.92 |
| PT3 | 9 | 4 | 4 | 25 | 56.67 | 428.00 |
| PT4 | 6 | 4 | 4 | 25 | 59.40 | 418.17 |
| PT5 | 4 | 4 | 4 | 25 | 67.92 | 395.23 |
2.5.1 数据的正态检验
表9 表7中数据的正态性检验
Table 9
| Shapiro-Wilk | ||
|---|---|---|
| Statistic | Sig. (P value) | |
| Ptotal / MPa | 0.741 | 0.001 |
| 0.599 | 0.000 | |
| pH | 0.421 | 0.000 |
| T / oC | 0.736 | 0.001 |
| Rct / Ω·cm2 | 0.837 | 0.011 |
| Icorr / μA·cm-2 | 0.742 | 0.001 |
图14
图14
环境因素与J55钢腐蚀动力学参数的频率直方图
Fig.14
Frequency histogram of environmental parameters and corrosion kinetics parameters
2.5.2 Spearman相关分析
使用IBM SPSS Statistics分析了数据的Spearman相关,结果列于表10。可以看出,J55钢的腐蚀动力学参数Icorr和Rct的相关系数为-0.998,表明二者之间存在高度负相关,且接近完全负相关。即测试时可以只选择其中一个参数作为特征参数。J55钢的腐蚀动力学参数(Icorr和Rct)与环境因素中的CO2分压和温度均显著相关,其中Icorr与温度和CO2分压显著正相关,Rct与二者是显著负相关。J55钢的动力学参数与环境总压的相关性极低,因为Spearman相关分析的前置条件是两个变量之间存在单调关系,但是J55钢的动力学参数并不是随着总压的变化单调变化。
表10 Spearman等级相关分析结果
Table 10
| Ptotal | pH | T | Rct | Icorr | |||
|---|---|---|---|---|---|---|---|
| Ptotal | r | ||||||
| P | - | ||||||
| r | -0.623* | 0.692** | |||||
| P | - | ||||||
| pH | r | ||||||
| P | - | ||||||
| T | r | -0.692** | 0.633* | ||||
| P | - | ||||||
| Rct | r | -0.623* | -0.692** | -0.986** | |||
| P | - | ||||||
| Icorr | r | 0.692** | 0.633* | -0.986** | |||
| P | - |
3 结论
(1) 随着环境总压(4~15 MPa)的提高J55钢的腐蚀速率先提高后降低;CO2分压(0.1~4 MPa)和温度(5~50℃)的提高都促进J55钢的腐蚀;初始pH值对油管钢腐蚀速率的影响较小。
(2) CO2分压和温度是影响J55钢腐蚀速度的关键因素。CO2分压和温度与Rct的Spearman相关系数分别为-0.623和-0.692,显著性水平分别为0.013 (< 0.05)和0.004 (< 0.01),都属于显著相关。
参考文献
A review of the mechanisms, reaction products and parameters affecting uranium corrosion in wa-ter
[J].
Study on CO2 immiscible flooding technology in low permeability reservoir of Daqing Oilfield
[D].
大庆油田低渗透油藏CO2非混相驱油技术研究
[D].
Corrosion and electrochemical behavior of N80 steel in high temperature and high pressure CO2/O2 environment
[D].
N80钢高温高压CO2/O2腐蚀及电化学行为研究
[D].
Effect of CO2 partial pressure on the stress corrosion cracking behavior of N80 tubing steel in the annulus environment of CO2 injection well
[J].
CO2分压对N80油管钢在CO2驱注井环空环境中应力腐蚀行为的影响
[J].
Effect of imidazoline corrosion inhibitor on stress corrosion behavior of N80 tubing steel in simulated annulus environment
[J].
模拟环空环境下咪唑啉缓蚀剂对N80油管钢应力腐蚀行为的影响
[J].
Effect of service environmental parameters on electrochemical corrosion behavior of L80 casing steel
[J].
On the mechanism of carbon dioxide corrosion of mild steel: Experimental investigation and mathematical modeling at elevated pressures and non-ideal solutions
[J].
Modelling of CO2 corrosion and FeCO3 formation in NaCl solutions
[J].
Corrosion behaviour of N80 carbon steel in formation water under dynamic supercritical CO2 condition
[J].
Morphology and microstructure of CO2 corrosion scales
[J].
CO2腐蚀产物膜的微观形貌和结构特征
[J].研究了碳钢、低Cr钢在不同条件下形成CO2腐蚀产物膜的微观形貌、成分和结构特征. 结果表明, N80(碳钢)、1Cr-L80形成的腐蚀产物膜主要由FeCO3晶体堆垛而成, 而3Cr-L80、5Cr-L80的表层则形成大面积的含Cr化合物, 主要为Cr(OH)3和Cr2O3. 这些Cr的化合物脱水后比较疏松, 容易形成龟裂. 在静态条件下, 碳钢和含Cr钢的腐蚀产物膜均具有分层结构, 碳钢外层与内层腐蚀产物膜的成分变化不大, 主要为Fe、Ca碳酸复盐. 含Cr钢的内层腐蚀产物膜中出现Cr元素的富集, 外层主要为Fe、Ca碳酸复盐. 随着Cr含量的增加, 材料的腐蚀产物膜向非晶态转变. 在静态条件下, 低Cr钢比碳钢的腐蚀速率稍大; 在动态(1.5m/s)条件下, 含Cr钢的腐蚀速率明显低于碳钢, 而且腐蚀速率随着Cr含量的增加而下降.
Comparison of corrosion behaviour for X-65 carbon steel in supercritical CO2-saturated water and water-saturated/unsaturated supercritical CO2
[J].
Passivation of X65 (UNS K03014) carbon steel in NaHCO3 solution in a CO2 environment
[J].
Effect of pH on the corrosion and electrochemical behavior of 3Cr steel in CO2 saturated NaCl solution
[J].
3Cr低合金钢在含饱和CO2的NaCl溶液中的腐蚀电化学行为
[J].研究了含饱和CO2的NaCl溶液pH值对3Cr低合金钢腐蚀及其电化学行为的影响。结果表明: 当NaCl溶液的pH值较低(2, 3.9)时, 腐蚀产物膜为单层结构, 呈龟裂状; 当pH值较高(6.5)时, 腐蚀产物具有三层结构, 外层腐蚀产物为颗粒状, 内层仍呈龟裂状。NaCl溶液的pH值对3Cr低合金钢的腐蚀电化学行为也有显著影响。 NaCl溶液的pH值升高能改变电极过程中的主要阴极反应, 使腐蚀电位逐渐负移, 且电荷转移电阻的增大使腐蚀电流密度减小。
Influence of Cr and Ni elements on the electrochemical and early corrosion behavior of FeMnAlC low-density steel
[J].
A mechanistic model for carbon dioxide corrosion of mild steel in the presence of protective iron carbonate films—part 1: theory and verification
[J].
A Mechanistic Model for carbon dioxide corrosion of mild steel in the presence of protective iron carbonate films—part 2: a numerical experiment
[J].
Development mechanism of internal local corrosion of X80 pipeline steel
[J].The occurrence and development mechanism of internal local corrosion has always been a controversial topic, and especially under flow conditions. In this paper, an improved high shear force loop was experimentally used, and local flow field is induced by simulating corrosion defects on the surface of X80 pipeline steel specimens. The characteristics of corrosion products deposited on the surface of specimens in CO2-saturated NACE solution were investigated by means of electrochemical impedance spectroscopy (EIS), scanning electron microscopy (SEM), X-ray diffraction (XRD), and energy dispersive spectrometry (EDS). The 3D micromorphology of the corrosion test surface after remove the corrosion scale used to measure the size of localized corrosion pit. Under the influence of local defects, the wall shear stress (WSS) and turbulent kinetic energy of local flow fields enhanced significantly, and pressure fluctuations in local flow field were induced. The results showed that the characteristics of surface corrosion products varied with flow velocity. The corrosion scales formed in various regions of specimens with defects exhibited different surface micro-morphologies and chemical compositions. Overall, these data offer new perspectives for better understanding the mechanisms behind local corrosion.
Formation mechanism and protective property of corrosion product scale on X70 steel under supercritical CO2 environment
[J].
Corrosion behavior of deep water oil production tubing material under supercritical CO2 environment: part 1—effect of pressure and temperature
[J].
Exploring the influence of flue gas impurities on the electrochemical corrosion mechanism of X80 steel in a supercritical CO2-saturated aqueous environment
[J].
Electrochemical, mechanical, and tribological properties of corrosion product scales formed on X65 steel under CO2 supercritical pressure environments
[J].
Effect of defect on corrosion behavior of electroless Ni-P coating in CO2-saturated NaCl solution
[J].
Simplified calculation of CaCO3 saturation at high temperatures and pressures in brine solutions
[J].
Real-time observation of carbonic acid formation in aqueous solution
[J].Despite the widespread importance of aqueous bicarbonate chemistry, its conjugate acid, carbonic acid, has remained uncharacterized in solution. Here we report the generation of deuterated carbonic acid in deuterium oxide solution by ultrafast protonation of bicarbonate and its persistence for nanoseconds. We follow the reaction dynamics upon photoexcitation of a photoacid by monitoring infrared-active marker modes with femtosecond time resolution. By fitting a kinetic model to the experimental data, we directly obtain the on-contact proton-transfer rate to bicarbonate, previously inaccessible with the use of indirect methods. A Marcus free-energy correlation supports an associated pKa (Ka is the acid dissociation constant) of 3.45 +/- 0.15, which is substantially lower than the value of 6.35 that is commonly assumed on the basis of the overall carbon dioxide-to-bicarbonate equilibrium. This result should spur further exploration of acid-base reactivity in carbon dioxide-rich aqueous environments such as those anticipated under sequestration schemes.
Thermodynamics of electrolytes. I. Theoretical basis and general equations
[J].
Thermodynamics of electrolytes. II. Activity and osmotic coefficients for strong electrolytes with one or both ions univalent
[J].
New insights on the role of CO2 in the mechanism of carbon steel corrosion
[J].
Reaction model for iron dissolution studied by electrode impedance: I. Experimental results and reaction model
[J].
The role of chloride and sulphate anions in the iron dissolution mechanism studied by impedance measurements
[J].
Electrochemical corrosion behavior of carbon steel under dynamic high pressure H2S/CO2 environment
[J].
The determination of film resistance by means of the frequency at the maximum phase angle and the estimation of the degradation of the coating film
[J].
Thermodynamic models for accurate calculations of densities of brines and solubilities of gas mixtures in brines
[D].
混合气体—卤水体系的密度和气体溶解度计算模型
[D].
The role of hydrostatic pressure on the metal corrosion in simulated deep-sea environments—a review
[J].
Hydrostatic pressure effects on corrosion behavior of X70 pipeline steel in a simulated deep-sea environment
[J].
Degradation in pitting resistance of 316L stainless steel under hydrostatic pressure
[J].