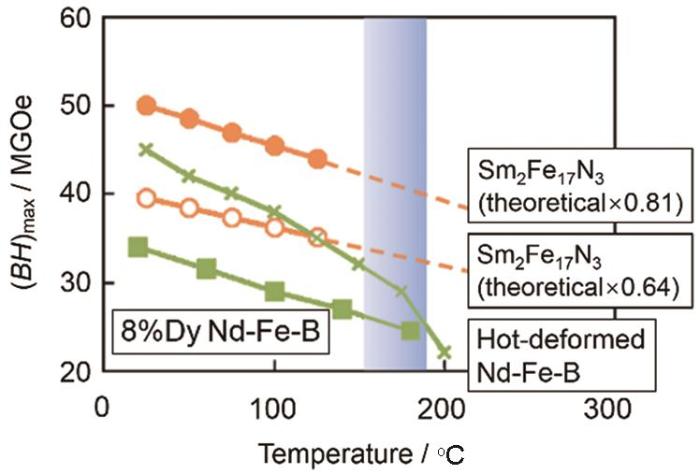
Sm2Fe17金属间化合物具有Th2Zn17结构,其中原子间距较小的Fe-Fe哑铃对产生负交换耦合,使其居里温度(TC)较低且产生易基面磁化[12]。N原子嵌入Sm2Fe17的9e间隙形成Sm2Fe17N3,增大了Fe-Fe哑铃对间距使其交换作用增强并使Sm的4f壳层电场梯度增大,从而提高了居里温度TC[13,14]、增大了各向异性常数K1[15],使Sm2Fe17中生成易c轴硬磁相[16]。Sm2Fe17N3的TC为476℃,K1为8.6 MJ·m-3,饱和磁化强度Ms为160 emu·g-1,理论最大磁能积(BH)max=1/4μ0Ms2=475 kJ·m-3[16~19]。如表1所示,Sm2Fe17N3具有比Nd2Fe14B优良的内禀磁性能。如图1所示,在150℃~200℃温度区间,Sm2Fe17N3磁体的(BH)max只有理论值的64%,但是比Dy含量为8%的Nd2Fe14B[20]高。虽然Sm2Fe17N3具有优异的内禀磁性能,但是N元素在Sm2Fe17母相中扩散缓慢。为了提高渗氮效率,须减小氮原子在Sm2Fe17颗粒中的扩散距离和增大母相颗粒与氮气的接触面积。Sm2Fe17N3的矫顽力类型属于形核型[21,22],磁化率高,畴壁易移动形成反向磁畴。因此,制备临单畴尺寸0.30 μm的颗粒以消除其中的畴壁,,可提高其Hcj[23~25]。这意味着,无论是提高氮化效率还是提升Hcj,细化颗粒都是有效的途径。
表1 Nd2Fe14B和Sm2Fe17N3的磁性能[16~19]
Table 1
| Quantity | Density/kg·m-3 | TC/℃ | Ms/emu·g-1 | Ha/T | K1/MJ·m-3 | (BH)max/kJ·m-3 |
|---|---|---|---|---|---|---|
| Nd2Fe14B | 7760 | 312 | 165 | 7.6 | 4.9 | 515 |
| Sm2Fe17N3 | 7680 | 476 | 160 | 14.6 | 8.6 | 475 |
图1
为了开发高性能的Sm2Fe17N3磁粉:一是探索高效、低成本粉末微细化工艺,实现高Ha;二是消除颗粒细化引起的表面氧化以提高剩磁Mr和(BH)max。目前商用Sm2Fe17N x 粉体的Hcj为12~18 kOe,(BH)max低于35 MGOe,仍有很大的提高空间。本文详细介绍制备高性能Sm2Fe17N x 粉体的球磨破碎和机械合金化,以及甩带和薄带连铸、还原扩散法、氮化处理、表面改性等工艺,并提出需要解决的关键问题和展望未来的进展。
1 高性能Sm2Fe17N x 粉体的制备
1.1 球磨破碎与机械合金化
1.1.1 球磨
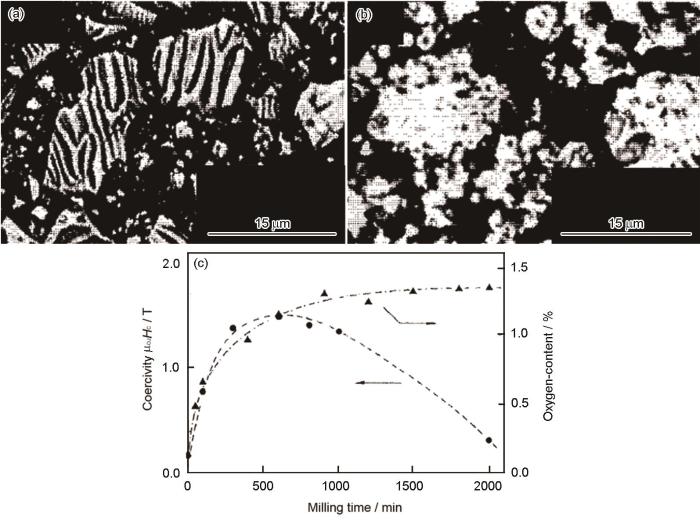
在开发Sm2Fe17N x 初期采用熔炼→均匀化热处理→球磨→二次热处理→氮化流程[32~34],制备出的Sm2Fe17N x 颗粒粗大,且含有大量α-Fe软磁相。其原因是:熔炼的铸锭中Sm、Fe严重偏析,使Sm∶Fe比例偏离生成母相Sm2Fe17的区间;球磨破坏了Sm2Fe17的结构导致非晶化并析出α-Fe[33,34]。对Sm2Fe17N x 进行二次球磨,可减小颗粒尺寸和提高其Hcj[35~37]。如图2所示[35],在粗颗粒氮化后的Sm2Fe17N x 中有明显的畴壁和棱角,二次球磨后颗粒尺寸减小且畴壁消失,颗粒是单畴颗粒的复合体。Kobayashi[36]等认为,尺寸减小到理论单畴尺寸(约0.3 μm)[37]的颗粒其Hcj与颗粒尺寸遵循1/D规律,D为Sm2Fe17N x 颗粒的直径。但是,二次球磨易提高颗粒的含氧量,使Hcj下降(图2c)[35]。
图2
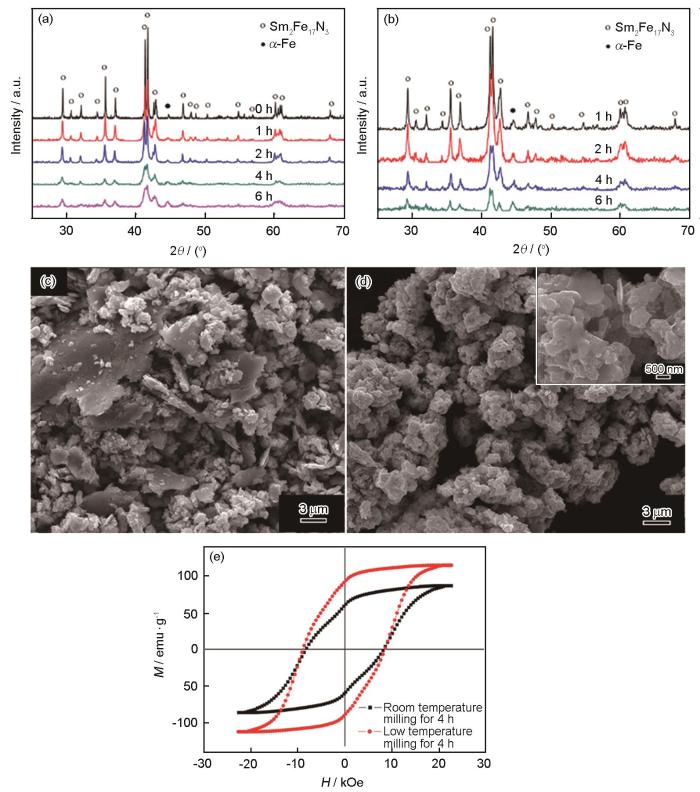
球磨时加入溶媒能防止颗粒团聚,提高细化颗粒的效率。在溶媒中加入适量的油酸等表面活性剂可使颗粒扁平化,提高c轴取向率[38~40]。Zhang等[38]在加有油酸的2-甲基戊烷中分别在室温和0ºC对Sm2Fe17N2.8球磨4 h,如图3所示,随着球磨时间的延长Sm2Fe17N2.8都发生分解,在低温下得到的样品颗粒棱角更少,其Mr为110 emu·g-1高于室温样品的85 emu·g-1,Hcj均为13 kOe。Li等[39,40]认为,球磨时Sm2Fe17N2.8表面氧化导致脱氮分解,颗粒表面的氧化速度随球磨机中温度的升高而提高。Hosokawa等[41]在低氧环境下采用气流磨破碎30 μm的Sm2Fe17N3粉体并控制气压以控制其粒径,发现随着颗粒尺寸从3.0 μm减小至1.1 μm,Hcj从7.3 kOe增加至14.1 kOe,Mr从158 emu·g-1减小至132 emu·g-1,Mr/Ms从0.96减小至0.87;颗粒粒径为1.3 μm时Hcj为12.7 kOe,Mr为147 emu·g-1,(BH)max高达43.6 MGOe。
图3
1.1.2 机械合金化
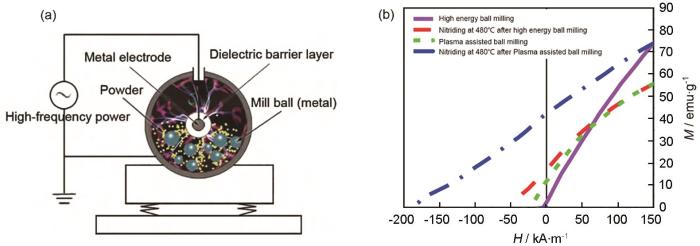
吕曼祺等[42]将Sm和Fe单质粉末在Ar气中振动球磨5 h,得到含有α-Fe的非晶SmFe合金粉末。在800ºC对其进行10 min的均匀化热处理,得到含有微量软磁相的Sm2Fe17粉体。这种机械合金化Sm2Fe17粉体极易自燃。Kou等[43]采用同样的流程制备的Sm2Fe17N x,其Hcj达到31 kOe。Xu等[44]证实:以Fe和Sm的单质金属为原料无法用高能球磨完成机械合金化得到Sm2Fe17,但是结合等离子放电(图4),在球磨速度、时间、原料质量/磨球质量分别为1300 r·min-1、6 h、1∶100的条件下能跨越生成Sm2Fe17的能量壁垒,得到Sm2Fe17+Sm2Fe17N x +α-Fe的复合相,再将其在480℃氮化8 h得到Sm2Fe17N x +α-Fe。虽然最终产物的Hcj和Mr仅为2.5 kOe和45 emu·g-1,但是仍为机械合金化制备Sm2Fe17N x 开辟了新路径。
图4
1.2 薄带连铸与甩带
1.2.1 薄带连铸
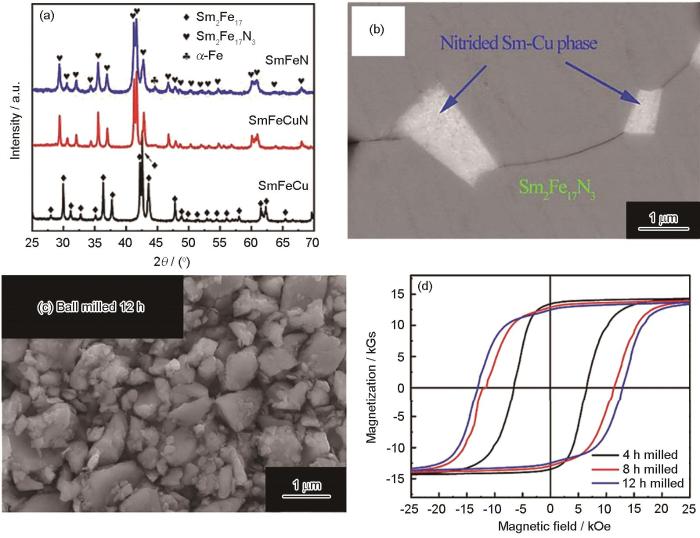
Ma等[45]采用薄带连铸技术制备出厚度为0.8 mm、宽度为1~2 cm的SmFe合金薄带,将其在1000℃均匀化处理后再在惰性气体气氛下破碎成200 μm的粗粉,在437~537℃氮化10~24 h后得到Sm2Fe17N3粗粉,最后将其在含有油酸和硅烷偶联剂的正庚烷中球磨破碎,得到长径为2~6 μm、厚度为100 nm的扁平状粉末。油酸能促进球磨过程中粉体的细化和扁平化,硅烷偶联剂能在颗粒表面形成包覆层从而减少氧化,最终产物的Mr为155 emu·g-1,Hcj为13 kOe。虽然有研究[26]认为直接采用薄带连铸无需均匀化处理也能得到纯相的Sm2Fe17,但是实际上即使在薄带连铸后进行较短时间的均匀化处理也难以得到纯相Sm2Fe17。为解决这一问题,Lu等[46]在SmFe合金中加入原子比为3%的Sm-Cu并建立SmCu+SmCu2+Sm2Fe17的相平衡关系,抑制了软磁相SmFe2和α-Fe的析出,结果如图5所示。
图5
图5
用薄带连铸结合球磨破碎技术制备Sm2(FeCu)17N x
Fig.5
Preparation of Sm2(FeCu)17N x by strip casting method and ball mill crushing technology (a) XRD of SmFeCu, SmFeCuN, SmFeN; (b) Backscattering image of SmCu phase in SmFeCuN; (c) SEM image of SmFeCuN powder and (d) Ob-taining the hysteresis loop of SmFeCuN powder with different milling time[46]
1.2.2 甩带
与薄带连铸相比,甩带能提高合金熔融体的冷却速度,获得更薄的合金带。Pinkerton等[47]用甩带法(35 m·s-1)制备出宽度为1 mm、厚度为30 μm的纳米晶薄带,其晶粒尺寸约为40 nm。将纳米晶薄带球磨破碎至25 μm的粗粉后在700℃真空热处理1 h,然后再进行氮化可使其Hcj提高至22.3 kOe。但是,真空热处理不能提高更大颗粒尺寸(约45 μm)粉体的磁性能。Coey等[19]指出,用甩带法(40 m·s-1)制备的纳米晶Sm2Fe17N3的无序化程度更高,使其单轴各向异性降低,而纳米化使交换耦合作用和晶界钉扎作用增强,能在一定程度上提高剩磁。因此,甩带/薄带联铸结合球磨的流程,更适合制备各向同性的Sm2Fe17N3。基于此,本文认为球磨后真空热处理的作用应该是修复球磨导致的颗粒表面的无序化,阻止单轴各向异性的减弱。但是颗粒尺寸较大时其无序化表面的体积占比很低,作用不显著。
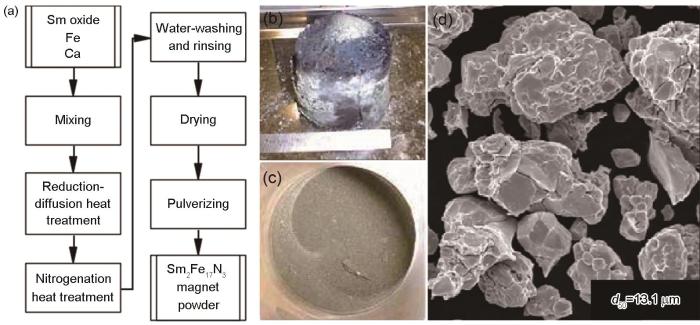
1.3 还原扩散法
用还原扩散法制备Sm2Fe17N3,是基于Ca热还原反应,将Sm的氧化物在Ca的熔点以上还原为单质Sm伴随原子扩散生成Sm2Fe17,再将其氮化得到Sm2Fe17N x。
图6
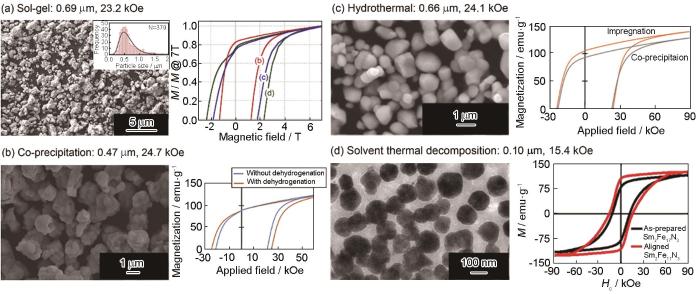
实际上还原扩散法可使用氧化铁等作为前驱体,结合湿化学法能制备出亚微米甚至是纳米级的前驱体以减小最终产物的颗粒尺寸。例如,FeOOH[53]、SmFe(CN)6[53]、Fe2O3[53,54]、Fe3O4[55,56]、Fe@Sm2O3[57]以及复合凝胶[29]和共沉淀复合物[10,58]等。前驱体为高价铁化合物时需要预先将其还原为单质Fe,以减少后续还原扩散阶段Ca的投入量。将溶胶凝胶法与还原扩散法结合能制备出粒径为0.69 μm的Sm2Fe17N3,Hcj达23.2 kOe[29](图7a);将共沉淀法与还原扩散法结合能制备出粒径为0.47 μm的Sm2Fe17N3,Hcj达到24.7 kOe[10](图7b)。Zheng等[57]用超声波热喷雾分解法,以H2为携带气体,在900℃从FeCl3/SmCl3=2.4∶17的溶液出发一步制备出0.49 μm的Fe@Sm2O3球形前驱体,还原扩散处理后得到的Sm2Fe17N3的粒径为0.616 μm,Hcj为14.7 kOe,Mr约为100 emu·g-1。
图7
图7
分别用溶胶凝胶法[29]、共沉淀法[10]、水热法[54]和溶剂热解法[56]制备前驱体,再进行还原扩散后的Sm2Fe17N x 的SEM照片和磁化曲线
Fig.7
Precursors were prepared by sol-gel method[29] (a), coprecipitation method[10] (b), hydrothermal method[54] (c) and solvopyrolysis method[56], SEM image and magnetization curve of Sm2Fe17N x obtained after reduction and diffusion treatment (d)
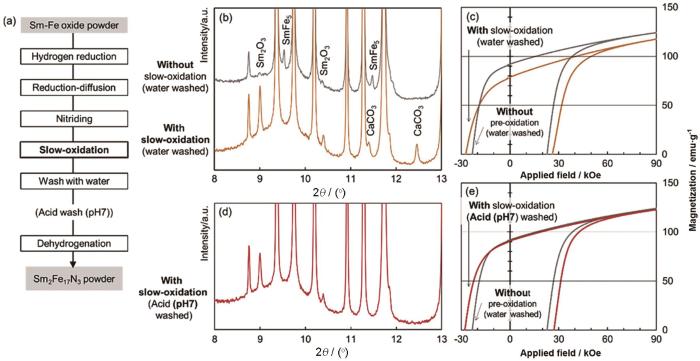
还原扩散处理,会残留大量金属Ca及其副产物CaO。水洗这些残留物会在水中生成的溶解度很低的Ca(OH)2,而长时间水洗易使颗粒表面腐蚀氧化生成Sm2O3和亚稳相SmFe5,尤其是亚微米级细小颗粒氧化尤为显著。有报道证实,用甲醇与氯化铵溶液替代纯水清洗可减轻金属元素的溶解[56]。目前普遍使用pH=5的醋酸水溶液以清除富Sm相从而提高剩磁[10,11,53,54,58],但是不可避免损伤颗粒表面。Okada等[11]在20℃、Ar+10% O2(Volume fraction)条件下进行氧化处理将Sm2Fe17N3颗粒表面的SmFe5转化成氧化物,再用pH=7的醋酸溶液去除颗粒表面的氧化物,得到的产物其Hcj高达28.1 kOe,Mr约为90 emu·g-1,如图8所示。在用NH3/H2氮化和水洗去除金属Ca(产生H2和放热)的过程中会生成Sm2Fe17N x H y (H含量:0.76~1.5 mg/cm3),在Ar气氛下进行脱氢处理能修复其矫顽力[10]。虽然这些实验室水洗工艺的改良修复了磁性能,但操作较为复杂,使处理成本提高。
图8
1.4 氮化
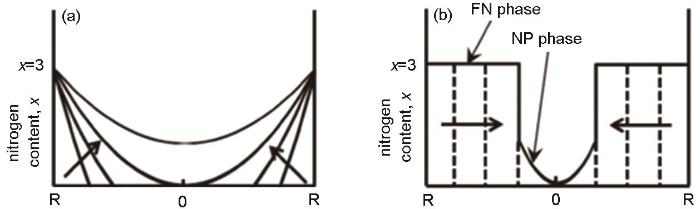
在氮化的初始阶段,先是N2在颗粒表面解离成N原子[61],随后N原子在晶界及微裂纹处富集。这表明,N原子在晶界处的扩散速度比其在晶粒内部扩散速度更高[18]。当x≤2.3时(113)、(300)和(024)晶面的XRD衍射峰明显分裂,表明颗粒内部贫氮相Sm2Fe17N x (x<3)和Sm2Fe17N3相共存[18,42,59],元素分析[59]和热磁分析[61]也支持上述结论。同时,在氮化过程中Sm2Fe17N3相的衍射峰先宽化后尖锐,表明氮原子向颗粒内部扩散伴随着Sm2Fe17N3晶粒的长大[42,59]。因此,当N2在颗粒表面完成分解产生活性N原子后,后续氮化的律速阶段是贫氮相Sm2Fe17N x (x<3)向Sm2Fe17N3相的转移,而非N元素由颗粒外向颗粒内进行梯度扩散,如图9所示[62]。基于上述氮化机制,Onoue等[59]提出,可在氮化时加磁场,因为贫氮相向Sm2Fe17N3相转化时两相磁矩差逐渐变负、系统磁化逐渐降低,从而加快Sm2Fe17N3相生成。
图9
图9
氮原子扩散机制和由Sm2Fe17N x (NP, x<3)向Sm2Fe17N3(FN)相转变引起的晶粒生长机制下的氮元素分布示意图[62]
Fig.9
Schematic diagrams of nitrogen distribution profiles for single nitrogen diffusion process (a) and grain growth pro-cess of the fully-nitride phase of Sm2Fe17N3 (FN) from the ni-trogen-poor phase of Sm2Fe17N x (NP, x<3)[62] (b)
提高N2气氛压力能有效缩短氮化时间,并一定程度抑制α-Fe相的析出[59]。例如,对尺寸为32~53 μm的颗粒进行氮化,在温度为733 K、压力为6 MPa条件下,氮含量达到x=3的时间为48 h,而压力为0.1 MPa时所需时间为72 h。随着氮化时间的增加颗粒表层Sm2Fe17N x 的N含量达到x=3后不会继续增加,而是由颗粒外向颗粒内逐渐增加Sm2Fe17N3的层厚度[62]。这表明,氮化后进行均匀化处理能平衡氮原子在颗粒内的分布,从而提高磁性能[61];减小母相颗粒粒径,即缩短扩散距离,也能加速N原子的扩散。但是实验结果表明,以N2气为氮源时Sm2Fe17N x 中的x只能无限接近于3。
Fukuda等[18]提出,使用NH3/H2混合气体氮化能提高氮化效率。在NH3/H2=35∶65、氮化温度为465℃的条件下对粒径为20~160 μm的Sm2Fe17颗粒进行氮化,得到Sm2Fe17N3的时间仅为2 h;当NH3/H2=40∶60时Sm2Fe17N x 的x能达到6.6,但是x>3时将引发Sm2Fe17N x 的非晶化,使其矫顽力、剩磁和居里温度都大幅降低。NH3分解得到N原子和H2,2NH3⇌3H2+2N,因此提高混合气体中的H2比例可抑制NH3的分解。这意味着,在纯NH3中比在NH3/H2混合气体中氮化速度更高。但是Coey等[17]的实验结果表明,无论采用纯NH3或纯N2进行氮化其结果基本相同,而且在长时间的NH3氮化中没有发现Sm2Fe17N x 中氮含量x超过4。据此可以推测,在氮化初始阶段H2的存在可促进氮化的进行,这或许与表面氧化层还原为金属作为氨气裂解的催化剂或是氢脆导致N原子扩散通道增加有关。
1.5 表面改性
减小Sm2Fe17N3颗粒尺寸虽然有助于提高颗粒矫顽力,但是颗粒表面氧化的加剧使剩磁和矫顽力降低,退磁曲线的方形度变差。Matsuura等[63]将Sm-Fe、Sm2O3、Mn2O3粉末球磨复合,还原扩散处理后得到核壳结构的Sm2Fe17N x -core/Sm2(FeMn)17N x -shell颗粒。具有和不具有Sm2(FeMn)17N x 的样品在Ar气、300℃下进行1.5 h热处理,发现Hcj分别降低了35%和52%。这表明,包覆Sm2(FeMn)17N x 壳层增强了热稳定性[64],如图10a~d所示。Yamaguchi等[65,66]用磁控溅射在Sm2Fe17N x 表面包覆金属Zn纳米层,在低氧环境下热处理后样品的矫顽力反而呈现增加趋势,如图10e所示。实际上,热处理氧化Sm2Fe17N x 颗粒在其表面析出的富Fe相能与Zn反应形成非磁性FeZn相,从而减少软磁相并隔绝Sm2Fe17N x 晶粒[67]。表2列出了用各种手段制备的Sm2Fe17N x 的物理和磁性能。
图10
图10
Sm2Fe17N x /Sm2(Fe,Mn)17N x 核壳粉的制备流程图和示意图、Mn扩散Sm-Fe粉末的截面扫描电镜和元素映射图、Mn扩散Sm-Fe-N核壳粉、无Mn扩散Sm-Fe-N粉热处理前后的退磁曲线[63]以及锌包覆Sm2Fe17N3粉体矫顽力随锌层平均厚度的增加速率[66]
Fig.10
Flow chart and schematic of the preparation of the Sm2Fe17N x /Sm2(Fe,Mn)17N x core-shell powder (a); Cross-sectional SEM and elemental mapping images of Mn-diffused Sm-Fe powder (b); demagnetization curves before and after heat treatment for Mn-diffused Sm-Fe-N core-shell powder (c) and Mn-free Sm-Fe-N powder[63] (d); Rate of increase in coercivity of Zn-coated Sm2Fe17N3 powders as function of average thickness of Zn layer[66] (e)
表2 用不同方法制备Sm2Fe17N x 的氮化条件和性能
Table 2
| Preparation method | Nitridation | Morphology | Impurity | Properties | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
Temperature /K | Time /h | Atmosphere | Length /μm | Ms /emu·g-1 | Mr /emu·g-1 | Hcj /kOe | |||
| Jet-milling technique | / | / | / | 1.30 | / | 163 | 147 | 12.7 | [39] |
| Strip casting technique | 700~800 | 10~24 | N2 | 0.80 | α-Fe | 160 | 155 | 13.0 | [44] |
| Strip casting technique | 693 | 35 | N2 | 0.76 | / | 14.2 (kGs) | 12.5 (kGs) | 13.0 | [45] |
Reduction-diffusion process (Polymerized complex) | 703 | 10 | N2 | 0.69 | / | / | / | 23.2 | [27] |
Reduction-diffusion process (Co-precipitation) | 693 | / | NH3/H2 (1:2 Vol/Vol) | 0.90 | / | 125 | 90 | 28.1 | [11] |
Reduction-diffusion process (Hydrothermal) | 693 | 1 | NH3/H2 (1:2 Vol/Vol) | 0.66 | / | 140 | 105 | 24.1 | [53] |
Reduction-diffusion process (Thermal decomposition) | 873 | / | Melamine (C3H6N6) | 0.10 | / | 128 | / | 15.4 | [55] |
2 总结和展望
(1) 传统的甩带或薄带联铸结合破碎技术的工艺流程,难以将Sm2Fe17N3颗粒粒径减小到1 μm以下并抑制氧化,而且颗粒粒径分布不均、颗粒多棱角局部退磁场大。
(2) 还原扩散法结合湿化学法直接用亚微米级甚至是纳米级铁化合物作为前驱体,能制备出矫顽力达23~28 kOe的临单畴尺寸的Sm2Fe17N3粉体。用溶胶凝胶法、高温水热处理以及热分解法等制备前驱体难以规模化应用。Sm2Fe17N x 永磁体的制备技术及其机制,仍有大量待解决的问题。
参考文献
Perspective and prospects for rare earth permanent magnets
[J].
Prospect for HRE-free high coercivity Nd-Fe-B permanent magnets
[J].
Permanent magnets: history, current research, and outlook
[J].
Magnetic anisotropy—How much is enough for a permanent magnet?
[J].
Magnetic properties of the MnBi intermetallic compound
[J].
Electronic structure and magnetocrystalline anisotropy energy of MnAl
[J].
SmFe12 and SmFe12N x films fabricated by sputtering
[J].
Anisotropic Sm2Fe17N3 sintered magnets without coercivity deterioration
[J].
High coercivity anisotropic Sm2Fe17N3 powders
[J].
Preparation of submicron-sized Sm2Fe17N3 fine powder with high coercivity by reduction-diffusion process
[J].
Synthesis of Sm2Fe17N3 powder having a new level of high coercivity by preventing decrease of coercivity in washing step of reduction-diffusion process
[J].
Magnetic transition and anomalous thermal expansion in R2Fe17 compounds
[J].
Negative exchange interactions and Curie temperatures for Sm2Fe17 and Sm2Fe17N y
[J].
Valence electron structure analysis of Curie temperature and magnetic properties of Sm2Fe17N3
[J].
Sm2Fe17N3 居里温度及磁性能的价电子结构分析
[J].
Magnetocrystalline anisotropy of 3d-4f intermetallics: Breakdown of the linear theory
[J].
Gas phase interstitial modification of rare-earth intermetallics
[J].
Improved magnetic properties by treatment of iron-based rare earth intermetallic compounds in anmonia
[J].
Effect of nitrogen content on magnetic properties of Sm2Fe17N x (0<x<6)
[J].
Sm-Fe-N revisited; remanence enhancement in melt-spun nitroquench material
[J].Following its discovery in the aftermath of Nd2Fe14B, Sm2Fe17N3 seemed to offer intrinsic magnetic properties that were superior or comparable to those of its famous predecessor. But the promise of the new material to challenge Nd2Fe14B was not realized, mainly because the 2:17 nitride powder, prepared by a low-temperature gas-phase interstitial modification process, was unstable at the temperatures needed to process dense sintered magnets. Here we discuss the magnetic properties of Nitroquench, a melt-spun Sm-Fe-N material, which offers superior corrosion resistance and thermal stability compared to melt-spun Nd-Fe-B. The powder, with a crystallite size of approximately 30 nm is in the form of flakes 15-18 mu m thick and about 100 mu m in diameter. Room-temperature coercivity is 690 kAm(-1) after saturation in 14 T, with a remanence of 92 Am(2)kg(-1) and an extrapolated saturation magnetization of 160 Am(2)kg(-1). The remanence enhancement is reflected in a preferred orientation seen in Fe-57 Mossbauer spectra of magnetized isotropic powder, which exhibits different relative intensities of the Delta M = 0 absorption lines according to the direction of the field used to saturate the magnetization. When measured in zero internal field, the remanence ratio M-r/M-s is 64%. The remanence enhancement is attributed to a nanocrystallite size that is not very much greater than the exchange length. The maximum energy product for the powder, assuming full density, is 162 kJm(-3). Nitroquench powder may be used to produce bonded magnets with an energy product > 100 kJm(-3).
New material for permanent magnets on a base of Nd and Fe
[J].
Preparation of anisotropic Sm2Fe17N x magnetic materials by strip casting technique
[J].
Magnetization reversal process of Zn-bonded anisotropic Sm-Fe-N permanent magnets
[J].
Nitrogenation effect of Sm2Fe17 alloys prepared by strip casting technique
[J].
Dependence of coercivity on particle size in Sm2Fe17N3 powders
[J].
Effect of milling on the magnetic and microstructural properties of Sm2Fe17N x permanent magnets
[J].
Microstructural changes in high-coercivity Zn-bonded Sm-Fe-N magnets
[J].
Study on bulk Sm2Fe17N x sintered magnets prepared by spark plasma sintering
[J].
Structure and magnetic properties of Sm2Fe17N x sintering magnets prepared by spark plasma sintering
[J].
High coercivity Sm2Fe17N3 submicron size powder prepared by polymerized-complex and reduction-diffusion process
[J].
Role of surface iron oxides in coercivity deterioration of Sm2Fe17N3 magnet associated with low temperature sintering
[J].Sm2Fe17N3 powders were analyzed by X-ray photoelectron spectroscopy to elucidate the role of surface Fe oxides in the significant deterioration of coercivity observed when Sm2Fe17N3 is sintered at relatively low temperatures. It was demonstrated that the Fe oxides on the powder surface completely disappear after heat treatment at 500 degrees C in Ar. This suggests that the chemical reaction which causes the coercivity deterioration involves consumption of the Fe oxides. A reaction model that postulates a redox reaction between the Fe oxides and Sm2Fe17N3 to produce soft-magnetic alpha-Fe consistently explains both the coercivity drop and the consumption of the Fe oxides. The conventional model of simple Sm2Fe17N3 decomposition, on the other hand, cannot rationally explain the disappearance of the Fe oxides. It is therefore reasonable to consider the redox reaction to be the primary mechanism of coercivity deterioration, in which the Fe oxides play a role as one of the major reactants.
Microstructural behavior on particle surfaces and interfaces in Sm2Fe17N3 powder compacts during low-temperature sintering
[J].
High coercivity in Sm2Fe17N x magnets
[J].
Preparation and magnetic properties of Sm2Fe17N x compound
[J].
Structure and magnetic properties of anisotropic Sm2Fe17N x powders
[J].
Effect of milling on the magnetic and microstructural properties of Sm2Fe17N x permanent magnets
[J].
Dependence of coercivity on particle size in Sm2Fe17N3 powders
[J].
Investigation of the domain structure of Sm2Fe17N x intermetallic nitrides
[J].
Sm2Fe17N x nanoflakes prepared by surfactant assisted cryomilling
[J].
The structure and magnetic properties of Sm-Fe-N powders prepared by ball milling at low temperature
[J].
Fabrication of remarkably magnetic-property-enhanced anisotropic Sm2Fe17N x nanoflakes fabricated by surfactant assisted ball milling at low temperature
[J].
Influences of microstructure on macroscopic crystallinity and magnetic properties of Sm-Fe-N fine powder produced by jet-milling
[J].
Preparation of Sm-Fe alloys by mechanical alloying and its crystal-lization and nitriding processes
[J].
用机械合金化法制备Sm-Fe合金及其晶化与氮化过程探讨
[J].研究了用机械合金化方法制备的Sm_2Fe_(17)合金的晶化与氮化过程。结果表明:机械合金化的Sm-Fe合金是Sm-Fe非晶相与晶态α-Fe的混合物,其成分是不均匀的,Fe在非晶颗粒外层含量较少,晶化过程包含Fe原子的长程扩散和固相反应,在适当的晶化条件下,晶化后全部转化为Sm_2Fe_(17)相。晶化过程中,可能出现六方结构的Th_2Ni_(17)型Sm_2Fe_(17)亚稳相。氮化过程由孕育期、Sm_2Fe_(17)相与其氮化物相共存、最终全部转化为氮化物相三阶段组成;在氮化过程中有十分细小的少量α-Fe析出。
Coercivity of Sm-Fe-N ferromagnets produced by the mechanical alloying technique
[J].
Improved efficiency for preparing hard magnetic Sm2Fe17N x powders by plasma assisted ball milling followed by nitriding
[J].
Anisotropic Sm-Fe-N particles prepared by surfactant-assisted grinding method
[J].
Changing phase equilibria: A method for microstructure optimization and properties improvement in preparing anisotropic Sm2Fe17N3 powders
[J].
High-coercivity samarium-iron-nitrogen from nitriding melt-spun ribbons
[J].
. Sm2Fe17N3 magnet powder made by reduction and diffusion method
[J].
Sm-(Fe, Mn) Magnet Powder made by reduction and diffusion method
[J].
Modified process for high performance anisotropic Sm2Fe17N3 magnet powder
[J].
The preparation of Sm2Fe17N x
[J].
Sm2Fe17N x 的制备
[J].
The influence of mechanical milling on the structure and magnetic properties of Sm-Fe-N powder produced by the reduction-diffusion process
[J].
Investigation of optimal route to fabricate submicron-sized Sm2Fe17 particles with reduction-diffusion method
[J].
Improvement of magnetization of submicron-sized high coercivity Sm2Fe17N x powder by using hydrothermally synthesized sintering-tolerant cubic hematite
[J].
Nanoparticle approach to the formation of Sm2Fe17N3 hard magnetic particles
[J].
Chemical synthesis of magnetically hard and strong rare earth metal based nanomagnets
[J].We report a general chemical approach to synthesize strongly ferromagnetic rare-earth metal (REM) based SmCo and SmFeN nanoparticles (NPs) with ultra-large coercivity. The synthesis started with the preparation of hexagonal CoO+Sm O (denoted as SmCo-O) multipods via decomposition of Sm(acac) and Co(acac) in oleylamine. These multipods were further reduced with Ca at 850 °C to form SmCo NPs with sizes tunable from 50 to 200 nm. The 200 nm SmCo NPs were dispersed in ethanol, and magnetically aligned in polyethylene glycol (PEG) matrix, yielding a PEG-SmCo NP composite with the room temperature coercivity (H ) of 49.2 kOe, the largest H among all ferromagnetic NPs ever reported, and saturated magnetic moment (M ) of 88.7 emu g, the highest value reported for SmCo NPs. The method was extended to synthesize other ferromagnetic NPs of Sm Co, and, for the first time, of Sm Fe N NPs with H over 15 kOe and M reaching 127.9 emu g. These REM based NPs are important magnetic building blocks for fabrication of high-performance permanent magnets, flexible magnets, and printable magnetic inks for energy and sensing applications.© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Preparation of submicron-sized Sm2Fe17N3 fine powder by ultrasonic spray pyrolysis-hydrogen reduction (USP-HR) and subsequent reduction-diffusion process
[J].
Direct preparation of submicron-sized Sm2Fe17 ultra-fine powders by reduction-diffusion technique
[J].
Magnetic field-induced nitridation of Sm2Fe17
[J].
Preparation of Sm-Fe-N by high-pressure N2 nitridation and Sm2Fe17 by a diffusion process
[J].
Diffusion of nitrogen atoms during the preparation of Sm2Fe17N y per-manent magnet alloy by gas-solid reaction method
[J].
气-固相反应法制备Sm2Fe17N y 永磁合金过程中氮原子的扩散
[J].研究了氮原子在Sm_2Fe_(17)合金中的扩散行为,测定了扩散温度、时间与样品平均氮含量的关系,以及氮原子在样品中的分布;计算了氮原子在Sm_2Fe_(17)合金中的扩散频率因子D_0和扩散激活能Q,运用扩散Fick第二定律计算了氮原子在Sm_2Fe_(17)粉末颗粒内部的分布及其与扩散温度、扩散时间、颗粒尺寸之间的关系.理论计算与实验结果一致.
Nitrogenation process in Sm2Fe17 under various N2-gas pressures up to 6 MPa
[J].
Preparation of Mn-diffused Sm-Fe-N core-shell powder by reduction-diffusion process
[J].Although the addition of other elements such as Mn to Sm2Fe17Nx compounds can change the magnetic properties, it also decreases saturation magnetization. In order to exploit the advantages of additional elements in Sm2Fe17Nx powder while maintaining a high saturation magnetization, a structure of Sm2Fe17Nx-core/Sm-2(Fe,M)(17)N-x-shell is promising. This is the first report of such a core-shell powder obtained by a reduction-diffusion process. Mn3O4 was mixed with Sm-Fe and Sm2O3 powders followed by the reduction by Ca above 860 degrees C, and then samples were nitrided and washed after reduction-diffusion process. Core-shell Sm-Fe-N fine powder with a Mn-enriched Sm-2(Fe,Mn)(17)N-x shell was thus successfully obtained. The thickness of the Mn enriched region was about 0.8 mu m, and the crystal structure of the core-shell powder was Th2Zn17. The saturation magnetization and coercivity of the core-shell powder were 138.8 A.m(2).kg(-1) and 1001.7 kA.m(-1), respectively. The saturation magnetization was slightly smaller than that of Mn-free Sm-Fe-N powder, whereas the coercivity of the core-shell powder was higher than that of Mn-free Sm-Fe-N powder. In addition, the saturation magnetization and coercivity were higher than those reported for Sm-Fe-Mn-N powder. The thermal stability of the Mn-diffused Sm-Fe-N core-shell powder was improved compared with Mn-free Sm-Fe-N powder.
Effect of Mn addition to Sm-Fe-N magnets on thermal stability of coercivity
[J].
Metal-coated Sm2Fe17N3 magnet powders with an oxide-free direct metal-metal interface
[J].
Effects of nonmagnetic overlay metals on coercivity of Sm2Fe17N3 magnet powders
[J].