高级氧化法(AOPs)的降解速率高、实用性强、运行成本低、能处理毒性强和难降解的有机污染物[1,2]。目前,典型的AOPs工艺H2O2/Fe2+芬顿氧化法已得到了较多的应用[3]。但是,传统的H2O2/Fe2+芬顿氧化法有活性物种
金属-有机骨架(MOFs)是一类以金属离子为节点、有机配体为组件桥连的多孔网状结构的新材料[12]。这种材料在气体储存[13,14]、分离[15,16]和光催化[17]等领域有良好的应用前景。MOFs中由富马酸和Fe(Ⅲ)构筑的MIL-88A(Fe)是一种有代表性的Fe-MOF材料[18~20],每个Fe(Ⅲ)与6个富马酸配体配位形成Fe―O簇,再由富马酸连接成三聚体而构成具有孔和笼的三维骨架结构。丰富的Fe―O簇使MIL-88A(Fe)被光激发产生e--h+,有机配体也能被光辐射激发而产生配体-金属电荷转移(LMCT),使其光催化活性提高[21, 22]。因此,MIL-88A(Fe)能在有PMS的条件下形成光-芬顿反应体系。同时,MIL-88A(Fe)表面的丰富配位不饱和位点还能活化PMS,与光生电子反应抑制光生e--h+复合并生成SO
研究表明,控制合成条件和添加活性剂可改变MIL-88A(Fe)的物理性质而影响其结晶度和结构缺陷,使其催化性能提高[26]。Bagherzadeh等[27]改变添加表面活性剂的量制备出不同尺寸的纳米棒状晶体,发现晶体粒径与带隙宽度呈反比。Wang等[28]研究了反应温度对MIL-88A(Fe)性能的影响,发现在95 ℃制备的材料其光催化活化性能更高。Zhao等[29]调节反应体系中的DMF-H2O混合溶剂比例使MIL-88A(Fe)的形态改变,制备出棒状、纺锤状和类金刚石晶体。DMF与H2O体积比为1∶1的纺锤状MIL-88A(Fe),其结构和吸附性能最佳。调整溶剂热时长能改变MIL-88A(Fe)晶体的尺寸制备出3D-MOF类似物,较大的晶体横纵比可降低材料内部对电子转移的限制,暴露出更多的表面原子和活性位点[30,31]。改变MIL-88A(Fe)晶体的形貌,能显著影响其在PMS光芬顿体系中的催化活性。鉴于此,本文用溶剂热法制备六种不同的MIL-88A(Fe)样品,将其在模拟太阳光下活化PMS降解RhB,并重复使用MIL-88A(Fe)/PMS/Light体系,研究溶剂热时长对MIL-88A(Fe)结构和催化性能的影响、可重复性和稳定性,并提出活化PMS和降解RhB的机制。
1 实验方法
1.1 实验用材料
反丁烯二酸、三氯化铁(Ⅲ)六水合物、过硫酸氢钾(2KHSO5·KHSO4·K2SO4)、罗丹明B C28H31CN2O3/RhB、甲醇(MeOH)、NaNO3、叔丁醇(TBA)、对苯醌(p-BQ)、组氨酸(C6H9N3O2/L-His)、碘化钾、NaOH、NaCl、Na2SO4、NH4Cl、HCl、NaH2PO4·2H2O、NaHCO3、N-N-二甲基甲酰胺(DMF)以及无水乙醇,均为分析纯。
1.2 催化剂的制备
用溶剂热法制备催化剂MIL-88A(Fe),制备过程在图1中给出。
图1
图1
不同溶剂热时长MIL-88A(Fe)的制备过程示意
Fig.1
Preparation process of MIL-88A(Fe) with different hydrothermal duration
将10 mmol的FeCl3
1.3 催化剂性能的表征
用扫描电镜(SEM, JSM-6710F)观察样品的形貌。测试催化剂的X射线衍射谱(XRD, Bruker D8),用Cu-Kα1辐射(λ = 0.15406 nm)。根据X射线光电子能谱(XPS, EscaLab 250Xi)分析样品表面的元素组成。用N2吸附/解吸测定仪(ASAP 2020,USA)测定样品的比表面积、孔容和孔径分布,分别用Brunauer-Emmet-Teller (BET)方程和Barrett-Joyner-Halenda (BJH)法计算。用傅里叶红外(FT-IR)谱分析样品的表面官能团性质。用配备积分球的紫外-可见漫反射光谱仪(UV-Vis-DRS)分析催化剂对光的吸收性能,参比为BaSO4。测试样品的瞬态光电流响应和电阻抗谱(EIS)。
将0.175 g催化剂加入浓度为20 mg/L的有机染料RhB溶液中,催化剂的浓度为0.35 g/L。进行20 min的暗反应,达到吸附平衡后加入PMS,然后用(已预热10 min的)500 W氙灯照射使催化剂活化PMS降解污染物。实验反应时长为1 h,每隔10 min取样4 mL。将取出的样品加入0.2 mL的饱和硫代硫酸钠溶液以终止反应。随后离心取出上清液,采用直接比色法测定RhB,在554 nm处测定吸光值。用抽滤法收集反应后的催化剂,用DMF和无水乙醇交替充分洗涤,在真空条件下烘干后进行下一次循环实验,循环实验共进行5次。污染物的光催化降解率为
式中,
2 结果和讨论
2.1 MIL-88A(Fe)的形貌
不同溶剂热时长的MIL-88A(Fe)形貌,在图2中给出。不同形貌的MIL-88A(Fe)对光的吸收能力不同,也影响其催化性能[32]。可以看出,溶剂热时长为4 h的MIL-88A(Fe)具有良好的钻石状形貌,与Liao等[33]的结果相同;随着溶剂热时长增加到8 h,产物中出现孔洞,有破碎的迹象(图2b);溶剂热时长12 h的产物为四棱锥状,形似钻石状,在晶体底面的中心出现亮斑(图2c);溶剂热时长为16 h的产物大部分为无规则状,部分为四棱柱状;溶剂热时长为20 h的产物,已成为完全的生长态纺锤状[34],晶体的尺寸变大,但是结晶度不高,表面也不光滑;溶剂热时长为24 h的产物呈光滑纺锤体状,结晶度高,达到了完全生长阶段。溶剂热时长小于24 h的MIL-88A(Fe),其晶体生长经过了两次循环。随着溶剂热时长的增加晶体的尺寸变大,由钻石状变为纺锤状[35]。同时,从图2可见,MIL-88A(Fe)晶体破损后单个晶体块生长为独立的MIL-88A(Fe)晶体。图 2c中的亮斑是新生MIL-88A(Fe)晶体开始生长的衔接面,这一晶体生长规律与文献[35]报道的“成核-聚集-溶解-再结晶”机制是一致的。重要的是,M-4、M-8、M-12、M-16、M-20和M-24六种样品的晶体横纵比分别为0.67、0.57、3.33、2.86、0.53、0.37,M-12的晶体纵横比最大(3.33),为3D-MOF类似物。与其他五种MIL-88A(Fe)晶体相比,其表面的铁离子和有机配体连接处有更多的缺陷和不饱和配位位点[31]。这决定了M-12的催化活性最好,在催化剂的光芬顿性能实验中得到了印证。
图2
图2
不同溶剂热时长MIL-88A(Fe)的SEM照片
(a) M-4; (b) M-8; (c) M-12; (d) M-16; (e) M-20; (f) M-24
Fig.2
SEM images of MIL-88A(Fe) with different hydrothermal duration
2.2 MIL-88A(Fe)的晶相结构
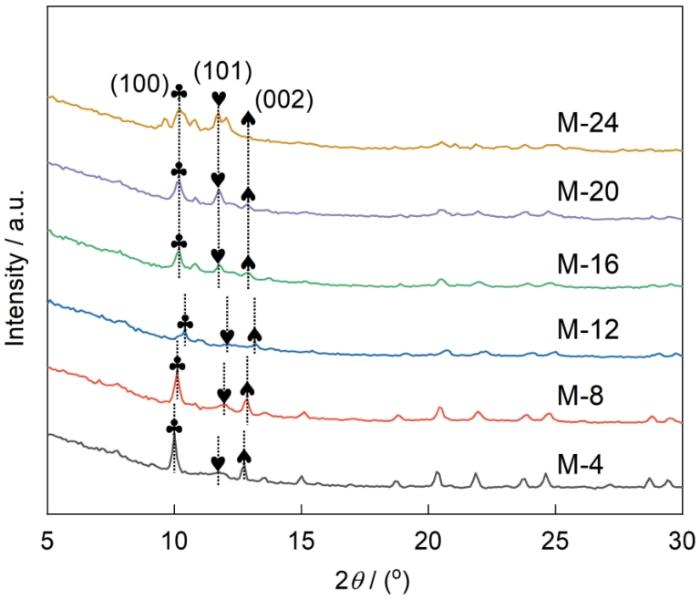
图3给出了M-4、M-8、M-12、M-16、M-20和M-24的XRD谱。可以看出,MIL-88A(Fe)样品XRD谱中的特征峰与文献报道的MIL-88A(Fe)的特征峰一致[37,38],表明成功地合成出MIL-88A(Fe)。还可以看出,M-4、M-8、M-12与(100)、(101)、(002)晶面对应的峰均向高角度偏移,是晶体结构坍塌后表面粗糙和表面缺陷所致[39]。同时,峰强的降低是结晶度降低引起的,与SEM表征结果一致。随着溶剂热时长的增加,M-16、M-20、M-24样品的(100)面和(101)面的衍射峰强度逐渐提高,而(002)面的衍射峰的强度逐渐降低,可归因于MIL-88A(Fe)的晶体取向从(002)转变到(100)和(101)。这表明,反应时长的增加促进了MIL-88A(Fe)(100)和(101)晶面的生长。
图3
图3
不同溶剂热时长MIL-88A(Fe)的XRD谱
Fig.3
XRD patterns of MIL-88A(Fe) with different hydrothermal duration
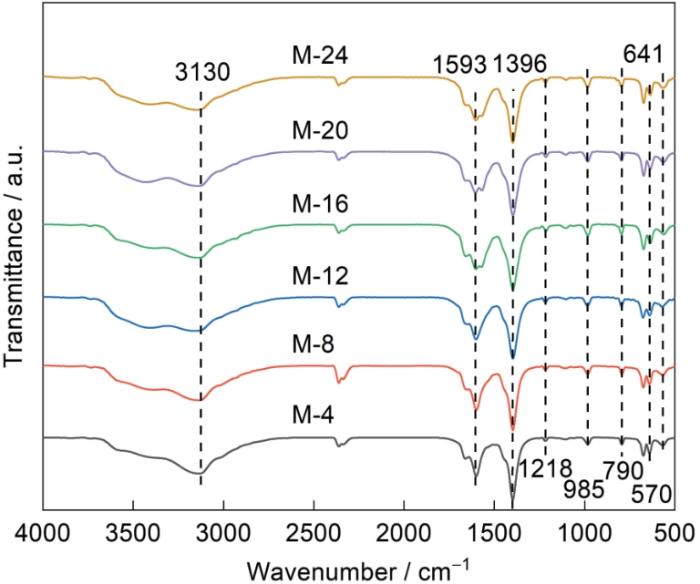
图4给出了MIL-88A(Fe)样品的FT-IR光谱。可以看出,M-4、M-8、M-12、M-16、M-20和M-24具有相似的FT-IR振动,表明溶剂热时长不影响MIL-88A(Fe)的基本结构。位于3000~3500 cm-1处的宽峰对应物理吸附在MIL-88A(Fe)上的水产生的O―H伸缩振动[40];790 cm-1对应三嗪单元特征峰[41],985和641 cm-1分别对应MIL-88A(Fe)的Fe―O振动和Fe―O―H弯曲振动[42];1396和1593 cm-1分别归因于MIL-88A(Fe)羧基(―COOH)的对称和不对称振动[28];570 cm-1处的峰归因于Fe―O键的伸缩振动,表明无机金属与富马酸的羧基之间生成了Fe―O簇[43,44]。
图4
图4
不同溶剂热时长MIL-88A(Fe)的FT-IR谱
Fig.4
FT-IR spectra of MIL-88A(Fe) with different hydrothermal duration
2.3 比表面积和孔径对催化剂的光催化性能的影响
溶剂热时长的不同影响MIL-88A(Fe)的比表面积和孔径等结构参数,而比表面积和孔径对催化剂的光催化性能有较大的影响。图5a、b给出了六种MIL-88A(Fe)样品的N2吸附-脱附等温线。可以看出,所有的等温线均为典型的Ⅰ型,表明样品中有微孔。孔径分布曲线(图5c,d)也表明其具有微孔结构。样品的比表面积(SBET)、孔体积(Vp)、孔径(Dp)和单位面积反应速率常数的计算结果,列于表1。可以看出,M-4、M-8和M-12的SBET和Vp依次递减,M-16、M-20和M-24的SBET和Vp依次递减,而Dp的变化与SBET、Vp的变化相反。这一结果,与不同溶剂热时长导致的“成核-聚集-溶解-再结晶”的晶体结构变化趋势一致。
图5
图5
不同溶剂热时长MIL-88A(Fe)的N2吸脱附等温线和孔径分布
Fig.5
N2 adsorption/desorption isotherms (a, b) and pore size distribution curves (c, d) of MIL-88A(Fe) with different hydrothermal duration
表1 不同溶剂热时长MIL-88A(Fe)的比表面积、孔体积、孔径及单位反应速率常数
Table 1
| Catalyst | SBET / m2·g-1 | Vp / cm3·g-1 | Dp / nm | Unit reaction rate constant / min-1·m-2 |
|---|---|---|---|---|
| M-4 | 153.67 | 0.114 | 1.36 | 0.364 × 10-3 |
| M-8 | 140.13 | 0.096 | 1.49 | 0.441 × 10-3 |
| M-12 | 92.80 | 0.072 | 1.56 | 0.938 × 10-3 |
| M-16 | 126.28 | 0.079 | 1.25 | 0.657 × 10-3 |
| M-20 | 152.02 | 0.086 | 1.13 | 0.387 × 10-3 |
| M-24 | 226.32 | 0.162 | 1.44 | 0.164 × 10-3 |
2.4 催化剂对光的吸收范围和带隙结构的影响
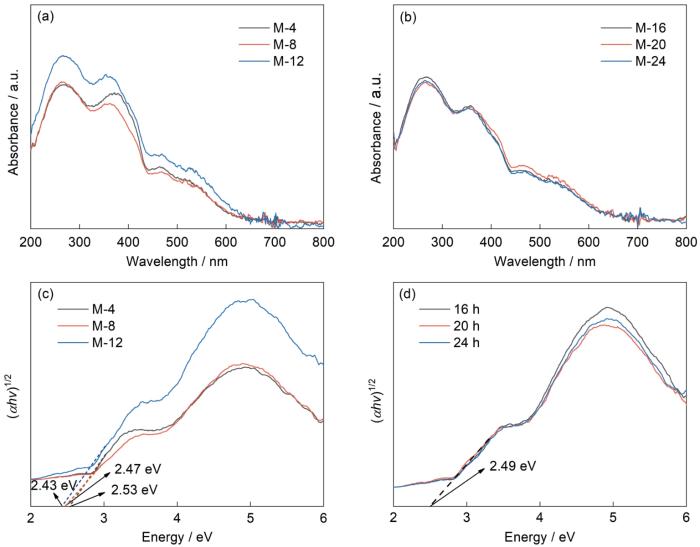
图6给出了UV-vis DRS的室温测试结果,据此可分析催化剂对光的吸收范围和带隙结构。可以看出,六种样品在400~650 nm可见光区有明显的吸收,吸收边在637 nm附近重合,其中M-12在可见光区的吸收值最高,其他样品对可见光的吸收值基本相同,都远比M-12的小。
图6
图6
不同溶剂热时长MIL-88A(Fe)的UV-Vis DRS谱
Fig.6
UV-Vis DRS spectra (a, b) and band gap width representation (c, d) of MIL-88A(Fe) with different solvent thermal durations
使用Kubelka-Munk函数
可计算催化剂的禁带宽度。
2.5 M-12中元素的化学状态
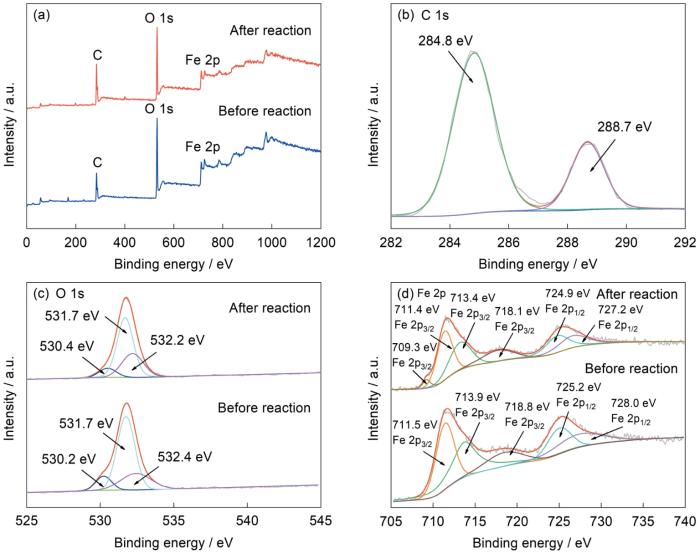
图7给出了根据XPS测定的反应前后M-12中元素的化学状态。图7a给出了样品的XPS全谱,可见样品中有C、O、Fe元素。图7b给出了C 1s的XPS高分辨谱,谱中284.8和288.7 eV处的特征峰分别对应M-12的苯甲酸环(C4H4O4)中的羧酸酯键和C―C键[45]。图7c给出了O 1s的XPS高分辨谱,反应前M-12的谱中530.3、531.6和532.2 eV处的峰分别属于苯甲酸环羧酸基团中的O、Fe―O键和催化剂表面吸附的水分子[33,45,46];反应后三个峰均向较高的BE值偏移(530.4、531.7、532.3 eV),是催化剂表面吸附了羟基基团(―OH)所致[47]。图7d给出了Fe 2p的XPS高分辨谱,反应前M-12的Fe 2p3/2轨道谱中711.5和713.9 eV处的特征峰,分别属于Fe2+及Fe3+,Fe 2p1/2轨道中725.2和728.0 eV处的特征峰也表明存在Fe2+及Fe3+[45,46]。同时,718.8 eV处的峰属于Fe 2p3/2的卫星峰。反应后的M-12中Fe 2p3/2和Fe 2p1/2轨道的峰,均向较低的BE偏移。同时,Fe3+因与PMS和金属中心的界面接触而被还原,在709.3 eV处出现了Fe 2p3/2的新峰。这表明,在反应过程中Fe3+与Fe2+之间的氧化还原转换很活跃[47],有利于PMS分子降解RhB。
图7
图7
反应前后M-12的XPS谱
Fig.7
XPS spectra of M-12 before and after reaction (a) full spectrum; (b) XPS spectra of C 1s; (c) XPS spectra of O 1s; (d) XPS spectra of Fe 2p
2.6 MIL-88A(Fe)的光电流瞬时响应和电化学阻抗谱
图8
图8
不同溶剂热时长MIL-88A(Fe)的光电流瞬态响应图及EIS谱
Fig.8
Photocurrent transient response diagram (a, b) and EIS spectrum diagram (c, d) of MIL-88A(Fe) with different solvent thermal duration
2.7 催化剂的光芬顿性能
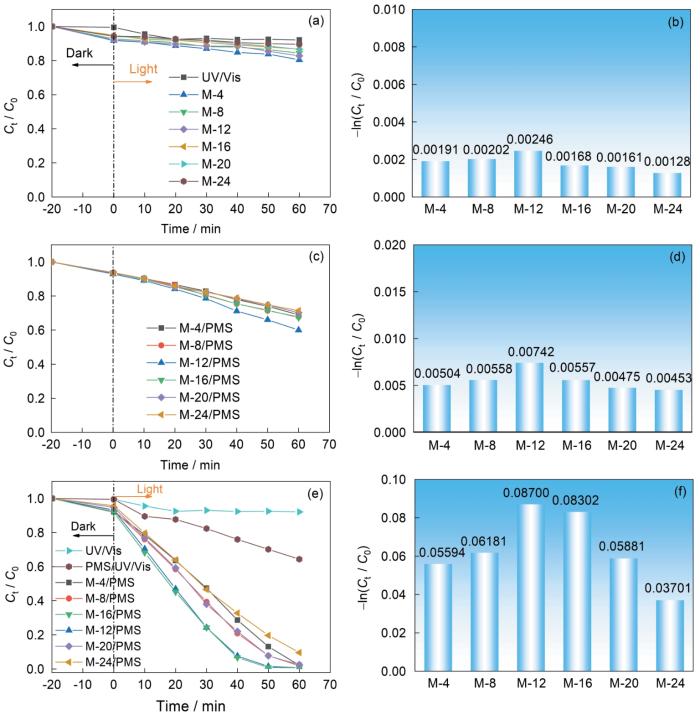
图9a,b给出了没有PMS时溶剂热时长对MIL-88A(Fe)降解RhB的影响。在模拟太阳光下不添加催化剂和氧化剂降解RhB,反应60 min后RhB浓度的变化极小。在光辐射条件下,反应体系中只有M-4、M-8、M-12、M-16、M-20以及M-24,反应60 min后对RhB的降解率分别为19.56%、15.65%、17.26%、13.26%、13.38%和10.45%。其原因是,催化剂产生的e--h+氧化了RhB,但是e--h+的高速复合抑制了RhB的降解。这表明,M系列催化剂具有一定的光催化活性。在没有光辐射条件下将M-4、M-8、M-12、M-16、M-20、M-24分别与PMS复合添加形成M/PMS类Fenton体系,对RhB的60 min降解率分别为31.01%、32.20%、40.02%、32.70%、30.05%和28.60%(图9c,d)。其原因是,M系列催化剂可直接活化PMS,表明本文制备的系列催化剂具有一定的类芬顿活性。而在光辐射条件下,M-4、M-8、M-12、M-16、M-20、M-24分别与PMS复合添加形成的M/PMS/Light体系对RhB的降解率分别为97.86%、98.13%、99.33%、99.31%、97.48%和90.39%,降解动力学常数分别为0.05594、0.06181、0.8700、0.08302、0.05881和0.03701 min-1(图9e,f)。其原因是:其一、PMS能捕获催化剂CB上的e-产生SO
图9
图9
在有光照、无光照和有光照并添加PMS条件下不同溶剂热时长的MIL-88A(Fe)对RhB的降解
Fig.9
Effect of addition of PMS on RhB degradation of MIL-88A(Fe) prepared with different solvent thermal duration under light (a, b), no light (c, d) and light (e, f) conditions
2.8 催化剂投加量的影响
选择PMS的初始浓度为0.5 mmol/L、RhB的浓度为20 mg/L,研究了M-12的投加量(0.05、0.20、0.35、0.50、0.65 g/L)对RhB降解效率的影响。从图10可以看出,RhB的降解率和动力学常数与催化剂的投加量呈正相关。M-12投加量从0.05 g/L增加到0.50 g/L,在60 min内RhB的降解率从64.8%提高到97.5%,动力学常数从0.01565 min-1增大到0.06064 min-1。这表明,增大催化剂的投加量在反应体系中产生更多的活性位点,促进了RhB的催化降解。而随着M-12投加量进一步增多到0.65 g/L,在60 min内RhB降解率为97.4%,但是降解效率没有变化。其原因是:其一,在PMS的活化过程中反应体系中的活性位点已经饱和;其二,溶液中大量的催化剂提高了悬浮液的浊度并引起光散射,从而降低了光的利用率[48]。因此,在后续实验中选择催化剂的投加量为0.35 g/L。
图10
图10
M-12投加量对RhB降解的影响
Fig.10
Effects of M-12 dosage on the degradation efficiency (a) and reaction rate (b) of RhB
2.9 PMS投加量的影响
在M-12/PMS体系中,PMS是活性自由基的主要来源。因此,PMS的投加量通过影响反应体系中的活性自由基的数量而影响RhB的降解。选择M-12的投加量为0.35 g/L,RhB的浓度为20 mg/L,在不改变其他条件的条件下研究了PMS浓度(0.25、0.50、0.75、1、1.25、1.5 mmol/L)对RhB降解的影响。图11表明,随着PMS的投加量从0.25 mmol/L提高到1 mmol/L,光照反应60 min后RhB的降解率从84.5%提高到99.4%,K值从0.02997 min-1增大到0.08435 min-1。其原因是,随着PMS投加量的增加,体系中产生了更多的SO
图11
图11
PMS投加量对RhB降解的影响
Fig.11
Effects of PMS dosage on the degradation efficiency (a) and reaction rate (b) of RhB
2.10 水体pH值的影响
水体pH值的变化使氧化剂的存在形式、污染物和催化剂的表面电荷发生变化而影响催化反应的进行。因此,本文在PMS的初始浓度为1 mmol/L、催化剂投加量为0.35 g/L、RhB的初始浓度为20 mg/L的条件下在3~11范围内调节溶液的初始pH值(pH = 3、5、7、9、11),研究了pH值对RhB降解的影响。图12给出了在pH值不同的条件下RhB浓度随时间的变化。在RhB结构中同时存在氨基和羧基,在纯水溶液中RhB以两性离子的形式存在。无论使用何种催化剂,脱质子状态的羧酸基团使RhB溶液的pH值接近5.3。RhB的pKa (酸解离常数) = 3.22,当溶液的pH值低于pKa时RhB分子的羧基发生质子化,而氨基只有在弱碱性条件下(pKb (碱解离常数) = 13.75)才发生质子化。但是,质子化的羧基使发色基团失去一个电子对,使其比质子化的氨基更有利于RhB的降解[49]。可以看出,反应体系中的pH值为3、5、7、9、11时,光照60 min后M-12/PMS对RhB的降解率分别为99.2%、99.1%、98.9%、98.6%、72.8%。反应前后溶液pH值的变化如图12(内嵌图)所示,可见反应液的pH值分别由3、5、7、9、11降到2.75、2.95、3.13、3.28、6.56。结合上文的结果,初始pH = 3~9时处于酸性环境的反应过程发生羧基质子化。这有利于破坏RhB分子结构,对RhB的降解几乎没有影响;而pH = 11时催化体系受到显著的抑制,因为在强碱环境中SO
图12
图12
pH值对RhB降解的影响
Fig.12
Influence of pH value on the degradation efficiency (a) and reaction rate (b) of RhB
2.11 无机阴离子的影响
废水中的无机阴离子会改变溶液的pH值和PMS结构,还会与反应体系中的模拟污染物竞争活性氧物质,因此影响对污染物的降解。如图13所示,在反应体系中分别加入浓度为5 mmol/L的Cl-、HCO
图13
2.12 催化剂的循环稳定性
为了评估MIL-88A(Fe)样品的循环稳定性,将反应结束后的M-12静置回收并用去DMF和无水乙醇充分清洗,在60 ℃烘干后进行循环实验。共进行5次循环实验。如图14所示,5次循环实验后M-12的催化活性依旧良好,60 min对RhB的降解率分别为99.41%、99.04%、98.01%、97.82%、91.82%,对RhB的去除率都高于90%。这表明,这种催化剂可重复使用。
图14
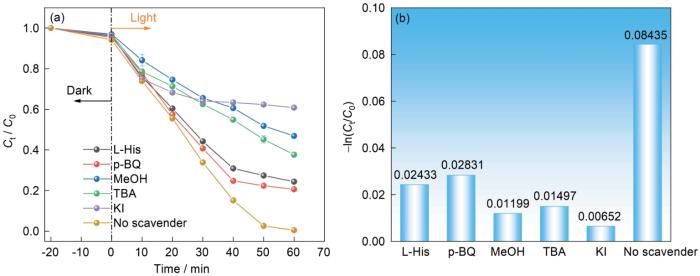
2.13 活性自由基的催化机理
为了研究M-12/PMS/Light体系对RhB的光催化活化降解机理,先向反应体系投加各种与活性物质对应的淬灭剂,确定降解过程中的主要活性物种。然后结合催化剂的能带位置,分析光催化活化过程中电子的转移机制。PMS在水中发生自分解反应产生强氧化性的1O2,PMS活化后产生氧化性更强的SO
图15
图15
捕获活性物种的实验
Fig.15
Effects of free radical quenching on the degradation efficiency (a) and reaction rate (b) of RhB
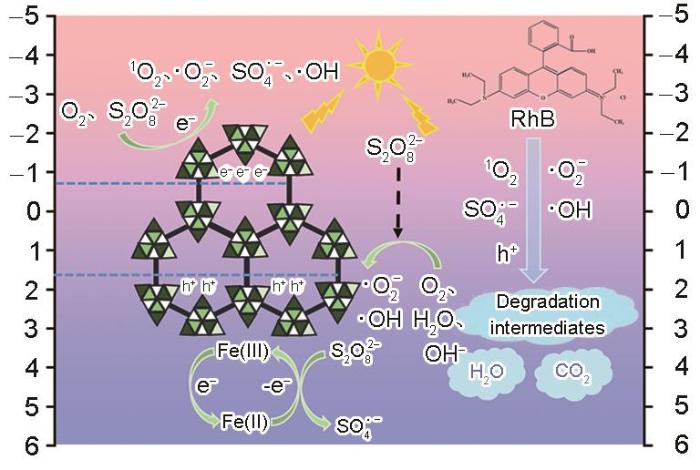
结合催化剂的能带位置和结构,进一步分析M-12光催化降解RhB的机理。从UV-Vis-DRS可知,M-12的禁带宽度为2.43 eV,由
和
可计算催化剂的价带(VB)和导带(CB)位置。
图16
图16
M-12/PMS/light体系降解RhB机理的示意图
Fig.16
RhB degradation mechanism in M-12/PMS/light system
综上所述,M-12光催化活化PMS降解RhB的过程,可表示为
3 结论
(1) 溶剂热时长对MIL-88A(Fe)的形貌有较大的影响,但其基本结构骨架不变。在无机金属与富马酸的羧基之间生成了Fe―O簇,Fe3+和Fe2+之间的氧化还原转换很活跃。随着溶剂热时长的增加晶体的形貌历经了“成核-聚集-溶解-再结晶”的过程。
(2) 溶剂热时长为12 h的催化剂为3D-MOF类似物,其M-12晶体横纵比最大(3.33),表面铁离子与有机配体连接处有更多的缺陷和不饱和配位位点,使其光电流响应最强,具有最大的光生电子-空穴分离效率、最低的电子转移电阻和最高的电导率和最佳的光催化活性。
(3) 在M-12/PMS/Light体系中,反应60 min后RhB的去除率达到99.33%。M-12/PMS/Light体系pH值的适应范围较广、无机阴离子的影响较低、样品的结构和催化性能稳定。
(4)·OH、SO
参考文献
Application of advanced oxidation processes and toxicity assessment of transformation products
[J].Advanced Oxidation Processes (AOPs) are the techniques employed for oxidation of various organic contaminants in polluted water with the objective of making it suitable for human consumption like household and drinking purpose. AOPs use potent chemical oxidants to bring down the contaminant level in the water. In addition to this function, these processes are also capable to kills microbes (as disinfectant) and remove odor as well as improve taste of the drinking water. The non-photochemical AOPs methods include generation of hydroxyl radical in absence of light either by ozonation or through Fenton reaction. The photochemical AOPs methods use UV light along with HO, O and/or Fe to generate reactive hydroxyl radical. Non-photochemical method is the commonly used whereas, photochemical method is used when conventional O and HO cannot completely oxidize organic pollutants. However, the choice of AOPs methods is depended upon the type of contaminant to be removed. AOPs cause loss of biological activity of the pollutant present in drinking water without generation of any toxicity. Conventional ozonation and AOPs can inactivate estrogenic compounds, antiviral compounds, antibiotics, and herbicides. However, the study of different AOPs methods for the treatment of drinking water has shown that oxidation of parent compound can also lead to the generation of a degradation/transformation product having biological activity/chemical toxicity similar to or different from the parent compound. Furthermore, an increased toxicity can also occur in AOPs treated drinking water. This review discusses various methods of AOPs, their merits, its application in drinking water treatment, the related issue of the evolution of toxicity in AOPs treated drinking water, biocatalyst, and analytical methods for identification of pollutants /transformed products and provides future directions to address such an issue.Copyright © 2018 Elsevier Inc. All rights reserved.
Scalability of advanced oxidation processes (AOPs) in industrial applications: a review
[J].
Recent advances in advanced oxidation processes (AOPs) for the treatment of nitro- and alkyl-phenolic compounds
[J].
Recent trends in applications of advanced oxidation processes (AOPs) in bioenergy production: review
[J].
Assessment of Fenton systems based on metabisulphite as a low-cost alternative to hydrogen peroxide
[J].
Sulfadiazine degradation using hybrid AOP of heterogeneous Fenton/persulfate system coupled with hydrodynamic cavitation
[J].
A comprehensive review on reactive oxygen species (ROS) in advanced oxidation processes (AOPs)
[J].
Critical review of natural iron-based minerals used as heterogeneous catalysts in peroxide activation processes: characteristics, applications and mechanisms
[J].
Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: a review
[J].
Carbon nanotube filter functionalized with iron oxychloride for flow-through electro-Fenton
[J].
A review on Fenton-like processes for organic wastewater treatment
[J].
Photocatalytic Cr(VI) elimination over BUC-21/N-K2Ti4O9 composites: big differences in performance resulting from small differences in composition
[J].A series of BUC-21/N-K2Ti4O9 composites (B1NX) were facilely fabricated from BUC-21 and N-K2Ti4O9 via ball-milling, and they were fully characterized using various techniques. The photocatalytic reduction of Cr(VI) over the B1NX composites was investigated systematically under various conditions, including different light sources, pH values, hole scavengers, and inorganic ions, in both real lake water and tap water. The BUC-21/N-K2Ti4O9 composites demonstrated remarkable photocatalytic Cr(VI) reduction performance, good reusability, and stability under both UV and white light irradiation. The introduction of N-K2Ti4O9 into BUC-21 not only broadened its light absorption region to white light, but also strongly inhibited the recombination of the photo-generated electrons and holes. Mechanisms of photocatalytic Cr(VI) reduction under both UV light and white light were proposed and verified by electrochemical measurements, active species capture experiments, and ESR measurements.
Advances on CO2 storage. Synthetic porous solids, mineralization and alternative solutions
[J].
Hydrogen storage in metal-organic frameworks: a review
[J].
The PLA/ZIF-8 nanocomposite membranes: the diameter and surface roughness adjustment by ZIF-8 nanoparticles, high wettability, improved mechanical property, and efficient oil/water separation
[J].
A novel granular MOF composite with dense and ordered MIL-100(Fe) nanoparticles grown on porous alumina: green synthesis and enhanced adsorption of tetracycline hydrochloride
[J].
Influence of preparation process parameters on relative amount of two-phase 1T/2H and performance of WS2
[J].
制备条件对WS2中1T/2H相的影响
[J].用简单的溶剂热法制备1T/2H相WS<sub>2</sub>纳米材料,改变前驱体中WCl<sub>6</sub>/TAA的摩尔比和反应温度调控WS<sub>2</sub>中1T相的含量。用X射线衍射(XRD)分析、X射线光电子能谱分析(XPS)和扫描电镜(SEM)观察探讨反应条件对产物中1T相含量的影响,并用共催化降解实验证实了W-200 (W-12)有最佳的助催化性能。用透射电镜(TEM)观察和拉曼光谱分析证实了W-200具有最佳的1T相含量。对比反应前后材料的状态发现,WS<sub>2</sub>在使用过程中会产生硫空位和具有优异的可循环使用性。
Role of solvent-host interactions that lead to very large swelling of hybrid frameworks
[J].An unusually large expansion upon solvent adsorption occurs without apparent bond breaking in the network of a series of isoreticular chromium(III) or iron(III) diarboxylates labeled MIL-88A to D [dicarbox = fumarate (88A); terephthalate (1,4-BDC) (88B); 2,6-naphthalenedicarboxylate (2,6-NDC) (88C); and 4-4'-biphenyldicarboxylate (4-4'-BPDC) (88D)]. This reversible "breathing" motion was analyzed in terms of cell dimensions (extent of breathing), movements within the framework (mechanism of transformation), and the interactions between the guests and the skeleton. In situ techniques show that these flexible solids are highly selective absorbents and that this selectivity is strongly dependent on the nature of the organic linker.
Fabrication approaches and organic pollutants degradation performances via advanced oxidation processes of MIL-88A(Fe) and its composites
[J].
金属-有机骨架MIL-88A(Fe)及其复合物的合成与高级氧化降解水体有机污染物的研究进展
[J].
Fabrication of metal organic framework Zn-BTC/rGO nanocomposites with different morphologies and their supercapacitor performance
[J].
金属有机骨架Zn-BTC/rGO复合材料的制备和性能
[J].用超声震荡法合成不同形貌的石墨烯负载Zn-BTC金属有机骨架材料,并将其用做电极组装超级电容器。用TG曲线、SEM观察、XRD谱、Brunauer-Emmett-Teller模型和Raman谱等手段表征材料的结构、形貌和电化学性能,使用电化学工作站和三电极体系测试了超级电容样品的电化学性能。结果表明,合成的一维棒状Zn-BTC均匀锚定在褶皱的石墨烯纳米片层上,其比电容为182.4 C·g<sup>-1</sup> (1 A·g<sup>-1</sup>),优于石墨烯负载的二维片状Zn-BTC (139.3 C·g<sup>-1</sup>)、石墨烯(97.9 C·g<sup>-1</sup>)和一维棒状Zn-BTC (62.8 C·g<sup>-1</sup>)。使用石墨烯负载的一维棒状Zn-BTC组装的对称超级电容器,在电流密度为1 A·g<sup>-1</sup>、比容量为57.7 F·g<sup>-1</sup>、功率密度为1390 W·kg<sup>-1</sup>条件下的最大能量密度为1.99 Wh·kg<sup>-1</sup>,经过2000次充放电循环后其比容量保持率为90.3%。
Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants
[J].
Elimination of emerging organic contaminants in wastewater by advanced oxidation process over iron-based MOFs and their composites
[J].<p id="p00005">The emergence of emerging organic contaminants(EOCs) has attracted increasing attention due to their wide distribution and persistence in aquatic ecosystems, as well as the potential threat to the health and safety of aquatic organisms. Traditional water treatment processes represented by activated sludge processes are generally insufficient to eliminate these persistent pollutants. For efficient removal of EOCs, advanced oxidation technology based on new materials is one of the most important advanced treatment technologies. Fe-MOFs and their composites have been widely used in many fields, especially in the catalytic oxidation of pollutants in wastewater. With the aid of the improvement of synthesis methods, post-synthetic modification and being composited with specific functional materials, Fe-MOFs can be used to effectively improve the adsorption performance, enhance the light absorption characteristics, and promote the effective separation of charge carriers. The review focuses on the progress of advanced oxidation processes(photocatalysis, Fenton-like reaction and sulfate radical($SO_{4}^{-·}$) mediated oxidation) of Fe-MOFs and their composites to remove emerging organic contaminants in wastewater. As well, the opportunities and challenges of Fe-MOFs in the field of EOCs removal are proposed. </p> <div class="mag_zhaiyao_sec"><strong class="mag_zhaiyao_title">Contents </strong><p class="mag_zhaiyao_p">1 Introduction</p><p class="mag_zhaiyao_p">2 Preparation of Fe?MOFs and their composites for oxidative degradation of EOCs</p><p class="mag_zhaiyao_p">2.1 MIL?100(Fe) and its composites</p><p class="mag_zhaiyao_p">2.2 MIL?101(Fe) and its composites</p><p class="mag_zhaiyao_p">2.3 MIL?53(Fe) and its composites</p><p class="mag_zhaiyao_p">2.4 MIL?88(Fe) and its composites</p><p class="mag_zhaiyao_p">3 The application of Fe?MOFs and their composites in advanced oxidation degradation of EOCs</p><p class="mag_zhaiyao_p">3.1 Advanced oxidative degradation of drugs in wastewater by Fe?MOFs and their composites</p><p class="mag_zhaiyao_p">3.2 Advanced oxidative degradation of environmental hormone in wastewater by Fe?MOFs and their composites</p><p class="mag_zhaiyao_p">3.3 Advanced oxidative degradation of pesticide in wastewater by Fe?MOFs and their composites</p><p class="mag_zhaiyao_p">3.4 Advanced oxidative degradation of multiple EOCs in wastewater by Fe?MOFs and their composites</p><p class="mag_zhaiyao_p">4 The influencing factors of advanced oxidation degradation of EOCs by Fe?MOFs and their composites</p><p class="mag_zhaiyao_p">4.1 The influence of physical properties of materials</p><p class="mag_zhaiyao_p">4.2 The influence of operation conditions</p><p class="mag_zhaiyao_p">4.3 The influence of active substances</p><p class="mag_zhaiyao_p">5 Conclusions and prospects</p></div>
铁基金属-有机骨架及其复合物高级氧化降解水中新兴有机污染物
[J].新兴有机污染物(Emerging organic contaminants,EOCs)是对人体健康及生态环境具有潜在或实质威胁的新型化学污染物。由于其被频繁使用且能在水生生态系统中持久性存在而对水生生物健康和安全造成严重威胁,故引起大众越来越多的关注。以活性污泥法为代表的传统水处理工艺通常不足以消除这些新兴有机污染物。为高效去除新兴有机污染物,基于新材料的高级氧化技术是最主要的深度处理技术之一。铁基金属-有机骨架(Fe-MOFs)及其复合物在诸多领域得到了广泛的应用,特别是在催化氧化去除水中有机污染物方面展现出良好的应用前景。通过合成方法改进、合成后改性以及与特定功能材料复合等方法可有效提升Fe-MOFs及其相关材料的吸附性能、增强其光吸收特性和促进载流子有效分离等。本文重点综述了Fe-MOFs及其复合物高级氧化(光催化、类芬顿和硫酸根自由基($SO_{4}^{-·}$)介导的氧化)去除水中新兴有机污染物的研究进展,并探讨了未来研究所面临的机遇和挑战。
Enhanced Fenton-like catalysis by iron-based metal organic frameworks for degradation of organic pollutants
[J].
A Cu/CuFe2O4-OVs two-electron centre-based synergistic photocatalysis-Fenton system for efficient degradation of organic pollutants
[J].
Remediation of antibiotic wastewater by coupled photocatalytic and persulfate oxidation system: a critical review
[J].
Design of MOFs and intellectual content in reticular chemistry: a personal view
[J].This article gives a personal perspective on the ideas leading to the development of reticular chemistry. The feasibility of achieving targeted materials with predetermined metrics and functionality by designed synthesis is defended.
Interplay between morphology and band gap energy in Fe-MIL-88A prepared via a high temperature surfactant-assisted solvothermal method
[J].
Activation of peroxydisulfate by MIL-88A(Fe) under visible light toward tetracycline degradation: effect of synthesis temperature on catalytic performance
[J].
Preparation of MIL-88A micro/nanocrystals with different morphologies in different solvents for efficient removal of Congo red from water: synthesis, characterization, and adsorption mechanisms
[J].
Metal-organic framework nanosheets: an emerging family of multifunctional 2D materials
[J].
2D metal-organic frameworks as multifunctional materials in heterogeneous catalysis and electro/photocatalysis
[J].
Exploring the effect of morphologies of Fe(III) metal-organic framework MIL-88A(Fe) on the photocatalytic degradation of rhodamine B
[J].Metal-organic frameworks built from [Fe-3(mu(3)-O)(COO)(6)] clusters and fumaric acid ligand, the so-called MIL-88 A(Fe) is a well-known environment-friendly promising material for many applications. In this paper, three different morphologies of MIL-88 A(Fe) such as rod, diamond and spindle have been synthesized separately by reacting FeCl3 center dot 6H(2)O and fumaric acid in 1 : 1 metal-ligand stoichiometric ratio using two different solvents such as water and DMF via hydrothermal method. The morphology of the products and their particle sizes were obtained using SEM and three distinct morphologies viz., rod, diamond and spindle were clearly distinguished by TEM. All the three samples were characterized by FT-IR, PXRD, UVDRS, PL, XPS and BET, and the effect of the morphologies of MIL-88 A(Fe) on the photocatalytic degradation of Rhodamine B (RhB) was studied under sunlight. The addition of an H(2)O(2)electron acceptor can markedly enhance the photocatalytic Rhodamine B degradation of MIL-88 A(Fe). Among these three, rod-shaped morphology of MIL-88 A(Fe) shows the higher photocatalytic effect for the degradation of Rhodamine B under sunlight due to its lower band gap, high surface area, and lower electron-hole recombination rate which enable them the transfer of electrons for the photocatalytic degradation. We found that 98% degradation of RhB in 50 min has taken place by using r-MIL-88 A(Fe) as the catalyst under sunlight.
Synthesis of (100) surface oriented MIL-88A-Fe with rod-like structure and its enhanced fenton-like performance for phenol removal
[J].
Photocatalytic Cr(VI) sequestration and photo-Fenton bisphenol A decomposition over white light responsive PANI/MIL-88A(Fe)
[J].
Synergetic molecular oxygen activation and catalytic oxidation of formaldehyde over defective MIL-88B(Fe) nanorods at room temperature
[J].
Rational construction of visible-light-driven MIL-88A(Fe)@PMo12 heterojunction with S-scheme electron transfer pathway to activate peroxymonosulfate for degradation of organic pollutants
[J].
A novel Bi3.64Mo0.36O6.55/MIL-88A(Fe) nanorod composite material for enhancing photocatalytic activity in photo-Fenton system
[J].
Photo-Fenton properties of MIL-88A(Fe)/Ti3C2 MXene with tunable active crystal facets: universal for degradation of common pollutants in wastewater
[J].
Coupling of heterogeneous advanced oxidation processes and photocatalysis in efficient degradation of tetracycline hydrochloride by Fe-based MOFs: synergistic effect and degradation pathway
[J].In this work, a Fe-based metal organic frameworks (MIL-88A) has been synthesized through a hydrothermal method and adopted as a high-efficiency catalyst for photocatalysis (PC) coupled with sulfate radical-based advanced oxidation processes (SR-AOPs) to degrade tetracycline hydrochloride (TC-HC1) under visible light irradiation. The effects of MIL-88A/persulfate (PS) molar ratio, initial solution pH and catalyst dosage were studied. The results indicated that the combining of the photocatalysis and SR-AOPs could remarkably enhance TC degradation and 200mg/L 100mL TC could be degraded completely at 0.25 g/L MIL-88A, unadjusted pH value and 4 mM PS in 80 mins. The results of scavenging experiments and ESR analysis demonstrated that SO4-center dot and O-center dot(2-) radicals were the predominant radicals for the TC degradation. The significant improvement of degradation rate in MIL-88A/PS/Vis process is attributed to the following two factors: (1) as a photocatalyst, MIL88A could be excited by visible light, therefore photogenerated electrons (e(-)) on the conduction band (CB) of MIL-88A could be trapped by PS to generate SO(4)(-center dot)radicals. In this process, PS acted as an electron acceptor and the electron/hole recombination was suppressed leading to the enhanced photocatalyst efficiency; (2) The PS can be activated not only by photoelectrons but also Fe (III) in MIL-88A leads to the generation of SO4-center dot radicals more rapidly and easily. A possible mechanism and degradation pathway for TC was proposed based on the trapping/ESR experiments and liquid chromatography-mass spectrometry (LC-MS) analysis. This work proposed a new idea and method in combining two oxidation processes in degradation of organic pollutants thus could be potentially used in environmental purification.
Constructing the novel ultrafine amorphous iron oxyhydroxide/g-C3N4 nanosheets heterojunctions for highly improved photocatalytic performance
[J].
Iron-based metal organic framework, MIL-88A, as a heterogeneous persulfate catalyst for decolorization of Rhodamine B in water
[J].
Accelerated photocatalytic degradation of organic pollutant over metal-organic framework MIL-53(Fe) under visible LED light mediated by persulfate
[J].
Ultrathin graphene oxide encapsulated in uniform MIL-88A(Fe) for enhanced visible light-driven photodegradation of RhB
[J].
Integration of plasmonic effect into spindle-shaped MIL-88A(Fe): steering charge flow for enhanced visible-light photocatalytic degradation of ibuprofen
[J].
Mechanistic investigation of photocatalytic degradation of Bisphenol-A using MIL-88A(Fe)/MoS2 Z-scheme heterojunction composite assisted peroxymonosulfate activation
[J].
Efficient degradation of Phenol by 1T/2H-MoS2/CuFe2O4 activated peroxymonosulfate and mechanism research
[J].
Activation of peroxydisulfate by V-Fe concentrate ore for enhanced degradation of carbamazepine: surface ≡V(III) and ≡V(IV) as electron donors promoted the regeneration of ≡Fe(II)
[J].
The mechanism of degradation of bisphenol A using the magnetically separable CuFe2O4/peroxymonosulfate heterogeneous oxidation process
[J].The removal of bisphenol A (BPA) in aqueous solution by an oxidation process involving peroxymonosulfate (PMS) activated by CuFe2O4 magnetic nanoparticles (MNPs) is reported herein. The effects of PMS concentration, CuFe2O4 dosage, initial pH, initial BPA concentration, catalyst addition mode, and anions (Cl(-), F(-), ClO4(-) and H2PO4(-)) on BPA degradation were investigated. Results indicate that nearly complete removal of BPA (50 mg/L) within 60 min and 84.0% TOC removal in 120 min could be achieved at neutral pH by using 0.6 g/L CuFe2O4 MNPs and 0.3 g/L PMS. The generation of reactive radicals (mainly hydroxyl radicals) was confirmed using electron paramagnetic resonance (EPR). Possible mechanisms on the radical generation from CuFe2O4/PMS system are proposed based on the results of radical identification tests and XPS analysis. The lack of inhibition of the reaction by free radical scavengers such as methanol and tert-butyl alcohol suggests that these species may not be generated in the bulk solution, and methylene blue probe experiments confirm that this process does not involve free radical generation. Surface-bound, rather than free radicals generated by a surface catalyzed-redox cycle involving both Fe(III) and Cu(II), are postulated to be responsible for the mineralization of bisphenol A.Copyright © 2016 Elsevier B.V. All rights reserved.
Structure-dependent catalysis of cuprous oxides in peroxymonosulfate activation via nonradical pathway with a high oxidation capacity
[J].
Enhanced artificial nitrogen fixation efficiency induced by construction of ternary TiO2/MIL-88A(Fe)/g-C3N4 Z-scheme heterojunction
[J].