过渡金属层状双氢氧化物(LDH)的储量丰富,且具有独特的结构、物理化学性质和优异的电催化活性[13,14,16~18]。Yuan等[13]用多步溶液法在FeCo LDH微米管上合成了非晶态空心CoS x 纳米颗粒(FeCo-LDH@CoS x),独特的结构和CoS x 与FeCo LDH间的协同作用使其具有优异的析氧反应(oxygen evolution reaction,OER)性能。Lei等[17]合成的Pt量子点修饰的硫掺杂NiFe LDH(Pt@S-NiFe LDHs)提高了界面电子传输、降低了气泡吸附和提高了金属基底相互作用,使其电催化(Hydrogen evolution reaction,HER)性能提高。用水热法和电沉积法合成的CoFe LDH@NiSe 异质结构[18]具有很强的界面耦合效应和更多的活性位点,使其析氢反应HER催化性能和OER催化性能提高。Fan等[19]合成的Ir嵌入NiCo LDH (Ir-NiCo LDH)使Ir和NiCo LDH间的强电子耦合和晶格畸变产生的大量缺陷使其全解水性能提高。同时,催化剂表面的活性位点数量和缺陷的增多也使其电催化活性提高[18,19]。FeOOH易于与其它过渡金属相互作用和具有丰富的活性位点,得到了深入的研究[20~22]。Zhang等[21]制备的FeOOH修饰NiCo LDH纳米花异质结构其强界面相互作用调节了电子结构和多金属间的协同作用,提高了OER活性。Bao等[22]构筑的 NiV LDH@FeOOH异质结构通过界面工程提高导电性、调节电子结构和加速气体释放的质量输运过程,提高了OER催化活性。在泡沫镍上电沉积生长的双功能NiFe LDH/FeOOH异质结构纳米片催化剂[23],具有优异的全裂解海水电催化活性和稳定性。Luo等[24]构筑的FeOOH-NiCoMo LDH/NF电催化剂其丰富的缺陷结构和高价态Mo插入LDH调节电子结构和协同耦合效应,使OER催化活性大大提高。同时,非晶态FeOOH独特的结构缺陷和更多的电催化活性面积,更有利于提高电催化性能[25~29]。Deng等[30]基于FeOOH和CoAl LDH间的强电子相互作用制备的多孔FeOOH-CoAl LDH异质结构,具有优异的OER活性。化合物中多种金属元素的相互协同作用,也能提高其催化性能[21,24,31]。本文将用原位方法在CoFeAl LDH表面生长非晶态FeOOH制备的CoFeAl-FeOOH-x (x = 1、3、6、9、12,代表反应时间)催化剂用于碱性溶液全解水反应并研究其催化性能。
1 实验方法
1.1 实验用原材料
实验用化学试剂,包括氯化钴(CoCl2)、氯化铁(FeCl3·6H2O)、氯化铝(AlCl3)、氟化铵(NH4F)、尿素(CH4N2O)、碳酸氢铵(NH4HCO3)、无水乙醇(C2H6O)和1.0 M氢氧化钾(KOH)。将泡沫镍(NF)用无水乙醇和去离子水分别超声清洗5 min,然后在60 ℃真空干燥箱中干燥12 h。
1.2 CoFeAl LDH和非晶态FeOOH修饰CoFeAl LDH的制备
用水热法在泡沫镍上制备CoFeAl LDH。先将0.8 mmol的CoCl2·6H2O、0.4 mmol的FeCl3·6H2O、0.4 mmol的AlCl3·6H2O、4 mmol的NH4F、28 mmol 的尿素和40 mL去离子水混合搅拌成均匀溶液,然后将其放置到50 mL聚四氟乙烯内衬的不锈钢高压反应釜中并垂直放入几片泡沫镍(尺寸为2 cm × 3 cm),再将反应釜放入100 烘箱中,反应6 h后自然冷却。待烘箱冷却到室温后将泡沫镍取出,依次用去离子水和无水乙醇反复充分清洗。最后将清洗好的泡沫镍放入到60 ℃真空干燥箱中保持12 h,烘干后得到CoFeAl LDH样品。
用常温溶液法将非晶态FeOOH修饰到CoFeAl LDH样品表面。先将CoFeAl LDH前驱体置入含有1 mmol FeCl3·6H2O和3 mmol NH4HCO3的60 mL无水乙醇中,调控搅拌时间(即反应时间x = 1、3、6、9和12 h)。然后将样品取出用去离子水和无水乙醇充分清洗后在40 ℃的烘箱中烘干12 h得到最终的样品,命名为CoFeAl-FeOOH-x (x = 1、3、6、9、12)。同时,用相同的方法在纯泡沫镍上制备反应时间为6 h的FeOOH样品作为对比样品,标记为Ni foam-FeOOH-6。
1.3 催化剂样品的表征
用Bruker D8 Advance X射线衍射仪测试样品的XRD谱,扫描范围为5°~80°。用JEOL JSM-6700F扫描电子显微镜(SEM)和JEOL-2100F透射电子显微镜(TEM)观察样品的形貌和微观结构。用Thermo Scientific Escalab Xi+光谱仪(Al Kα X射线激发源,1486.68 eV)测试样品的X射线光电子能谱,功率为100 W。
在常温下用电化学工作站(CHI 760E)测试样品的电化学性能。使用1.0 M KOH溶液电解液进行电解水,用Ag/AgCl (参比电极)、碳棒(辅助电极)和制备的催化剂样品(工作电极)搭建标准的三电极体系电化学电池。进行三电极体系测试时,电位Evs. RHE = EAg/AgCl + 0.198 V + 0.059 × pH校准为可逆氢电极(RHE)[31]。采用两电极体系进行全解水,电极均为制备的催化剂。
用95%的电压降(iR)补偿线性伏安曲线(LSV),扫描速率为10 mV·s-1。在所有测试前,将催化剂经过20个循环伏安(CV)测试达到稳定状态,扫描速率为20 mV·s-1,析氧电位范围为1.1~1.8 V (vs. RHE),析氢电位范围为-0.5~0 V (vs. RHE)。电化学阻抗谱(EIS)的频率范围为10-2~105 Hz,微扰正弦电压为5 mV。采用计时电位法测量催化剂的稳定性,电流密度为150 mA·cm-2。根据循环伏安(CV)曲线的双层电容(Cdl)推算催化剂的电化学活性面积(ECSA)[32],在非Faraday电位范围0.948~1.1 V (vs. RHE)内采集数据,扫描速率分别为40、60、80、100、120、140、160、180和200 mV·s-1。依据在1.024 V (vs. RHE)下的充放电电流密度差值(Δj = janodic - jcathodic)线性拟合扫描速率曲线,得到ECSA。
2 结果和讨论
2.1 样品的相结构和形貌
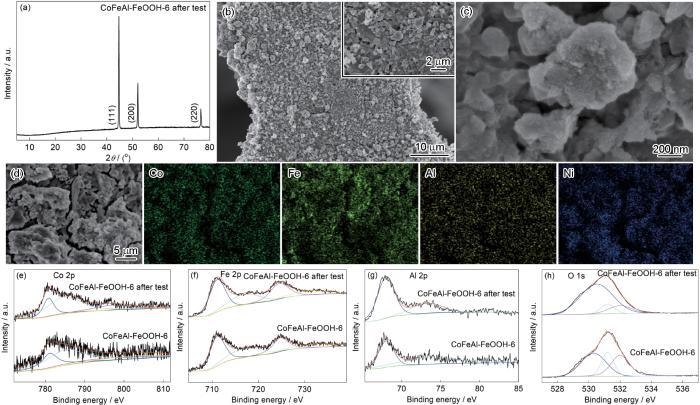
图1a给出了所制备样品的XRD谱。谱中44.5°、51.8°和76.4°处出现属于Ni(111)、(200)、(220)的强衍射峰,来自于泡沫镍基底(JCPDS No.70-1849)。在图1b中11.8°、23.4°和34.5°处出现属于LDH的(003)、(006)和(012)三个衍射峰(JCPDS No. 25-0521)[33]。在CoFeAl LDH的谱中(图1b)39.1°和46.8°两处出现属于LDH的 (015)和(018)特征衍射峰,表明在泡沫镍上成功地制备出CoFeAl LDH[34,35]。在FeOOH修饰的样品CoFeAl-FeOOH-x (x = 1、3、6、9、12)的谱中属于LDH的三个特征峰强度减弱,尤其是39.1°和46.8°两处的特征峰消失且没有出现FeOOH的衍射峰,表明生长在CoFeAl LDH表面的是非晶态FeOOH [30]。
图1
图1
CoFeAl-FeOOH-x (x = 1, 3, 6, 9, 12)和CoFeAl LDH的XRD谱以及CoFeAl-FeOOH-6和CoFeAl LDH XRD谱的放大
Fig.1
XRD patterns of the as-synthesized samples: CoFeAl-FeOOH-x (x = 1, 3, 6, 9, 12) and CoFeAl LDH (a) and the enlarged XRD patterns of CoFeAl-FeOOH-6 and CoFeAl LDH (b)
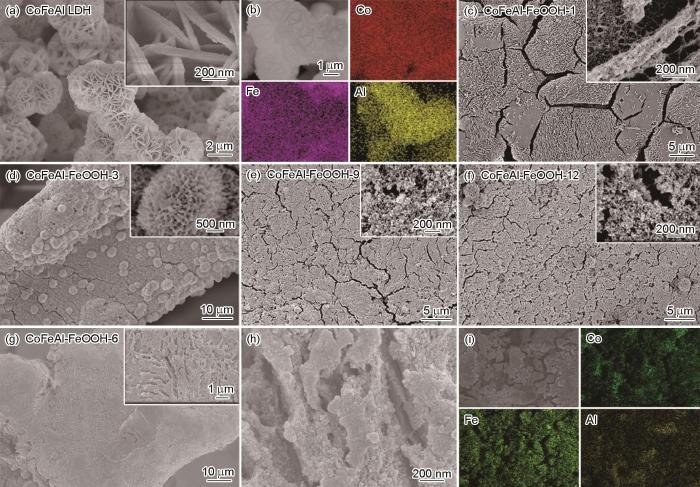
图2给出了样品的扫描电子显微镜照片。可以看出,CoFeAl LDH分布在泡沫镍的表面且由交叉互连的纳米片构成球形结构,Co、Fe和Al元素均匀分布(图2a,b)。修饰FeOOH后,样品的形貌发生了显著的变化。在图2c中可观察到CoFeAl-FeOOH-1样品表面粗糙的毛细管状分层结构。随着FeOOH修饰反应时间的延长,CoFeAl-FeOOH-3表现出由纳米颗粒组成且交叉互连的压舌板状形貌(图2d)。特别是反应时间延长到6 h后,CoFeAl-FeOOH-6样品由纳米颗粒组成的多孔层状板结构,和均匀分布的Co、Fe、Al和Ni元素(图2g~i)。CoFeAl-FeOOH-9和CoFeAl-FeOOH-12样品的形貌与CoFeAl-FeOOH-6相似(图2e~f),只是板状结构被严重破坏。
图2
图2
样品的SEM照片和对应的EDX元素分布
Fig.2
SEM and corresponding elemental mapping images of CoFeAl LDH (a, b), CoFeAl-FeOOH-1 (c), CoFeAl-FeOOH-3 (d), CoFeAl-FeOOH-9 (e), CoFeAl-FeOOH-12 (f) and CoFeAl-FeOOH-6 (g, i)
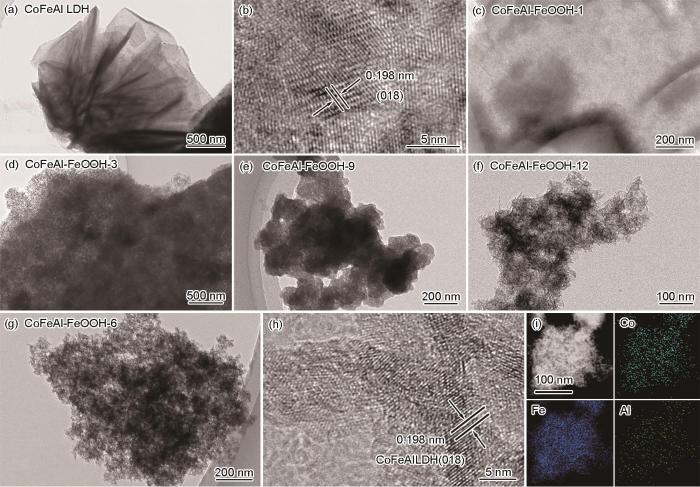
图3给出了透射电子显微镜对样品微观结构的表征。从图3a可见CoFeAl LDH具有薄片状结构,与SEM的观测结果相同。高分辨透射电子显微镜照片(HRTEM,图3b)表明CoFeAl LDH的晶格条纹间距为0.198 nm,对应于(018)晶面。CoFeAl-FeOOH-1也具有相似的纳米片状结构(图3c)。图3d表明,CoFeAl-FeOOH-3拥有多孔薄片状结构。在CoFeAl-FeOOH-6样品的照片中观测到多孔结构和小纳米颗粒(图3g),和0.198 nm的晶格间距(对应于CoFeAl LDH(018)晶面,图3h)以及均匀分布的Co、Fe、Al元素(图3i)。同时,CoFeAl-FeOOH-9 (图3e)和CoFeAl-FeOOH-12 (图3f)也具有与CoFeAl-FeOOH-6类似的多孔结构。从TEM照片可见,CoFeAl-FeOOH-6具有比CoFeAl-FeOOH-9和CoFeAl-FeOOH-12更大的孔尺寸和更薄的层状板,有利于电催化性能的提高。
图3
图3
样品的TEM、HRTEM和对应的EDX元素分布
Fig.3
TEM, HRTEM and corresponding elemental mapping images of CoFeAl LDH (a, b), CoFeAl-FeOOH-1 (c), CoFeAl-FeOOH-3 (d), CoFeAl-FeOOH-9 (e), CoFeAl-FeOOH-12 (f) and CoFeAl-FeOOH-6 (g, i)
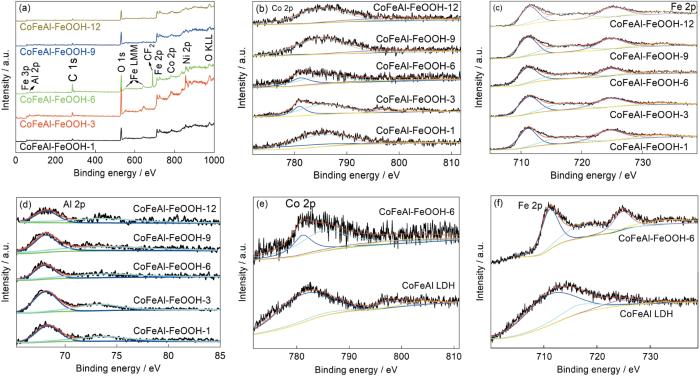
图4给出了样品的XPS光谱,用于分析催化剂的化学氧化态和组成。从图4a可见,所有的样品都含有Co、Fe、Al、Ni和O元素。CoFeAl-FeOOH-6的Co 2p高分辨XPS光谱(图4b)中出现了结合能分别为781.1 eV (Co 2p3/2)和797.1 eV (Co 2p1/2)的两个峰以及结合能分别为785.1 eV和802.3 eV处的卫星峰,表明存在Co2+ [30]。同时,CoFeAl-FeOOH-6中Fe 2p的XPS光谱中结合能分别为711.1 eV (Fe2p3/2)和724.5 eV (Fe2p1/2)处出现两个主峰(图4c)、在结合能分别为717.0 eV和732.5 eV处出现两个卫星峰,证实了Fe3+的存在和FeOOH的生成[30,36,37]。图4e,f表明,FeOOH修饰后Co 2p和Fe 2p的结合能向低能量方向轻微偏移,表明存在高价态的Co(Co3+)和低价态的Fe(Fe2+)[38,39]。另外,在CoFeAl-FeOOH-6样品中的Al 2p的高分辨XPS光谱中结合能分别为68.3 eV (Al 2p3/2)和73.6 eV (Al 2p1/2)处出现两个主峰,表明存在Al3+ [40]。
图4
图4
样品的XPS光谱分析
Fig.4
XPS analysis of the as-synthesized samples
(a) XPS survey spectra, (b) the high-resolution spectra of Co2p, (c) the high-resolution spectra of Fe 2p, (d) the high-resolution spectra of Al 2p and (e, f) high-resolution spectra comparison of Co 2p and Fe 2p in CoFeAl-FeOOH-6 and CoFeAl LDH
2.2 催化剂的OER催化性能
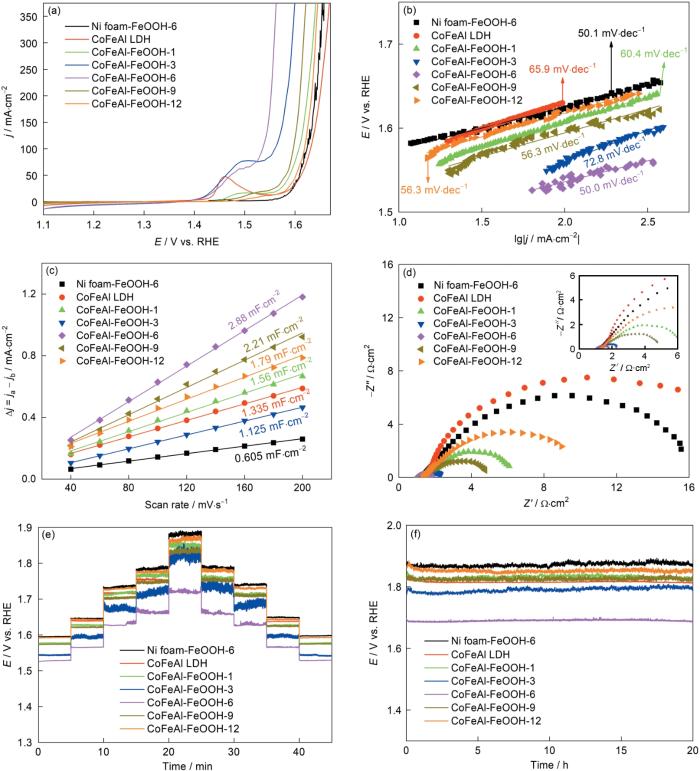
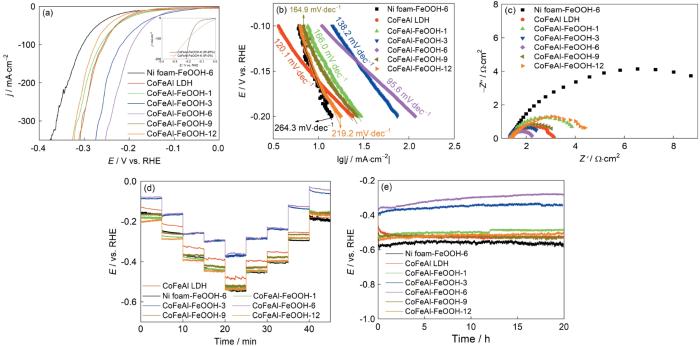
从图5a中的LSV曲线可以看出:过电位为298 mV时CoFeAl-FeOOH-6催化剂电流密度即可达到100 mA·cm-2,远低于其他催化剂需要的过电位(CoFeAl-FeOOH-1需要377 mV、CoFeAl-FeOOH-3 需要328 mV、CoFeAl-FeOOH-9 需要367 mV、CoFeAl-FeOOH-12需要391 mV、CoFeAl LDH需要401 mV和Ni foam-FeOOH-6需要393 mV)。同时,CoFeAl-FeOOH-6的电流密度达到300 mA cm-2时需要的过电位为331 mV,表明这种催化剂的大电流密度催化潜力很大。图5b给出了催化剂氧析出的Tafel斜率,可见CoFeAl-FeOOH-6的斜率(50.0 mV·dec-1)最小,表明这种催化剂的反应动力学更快[41]。根据CV曲线计算出的双层电容可用于评估催化剂的电催化活性面积(ECSA)。图5c表明,在制备的几种电催化剂中CoFeAl-FeOOH-6的电化学活性面积最大,即暴露的活性位点更多[37,42]。电化学阻抗谱(EIS)可用于评估催化剂的导电性。用R(Q(R))等效电路拟合了电化学阻抗谱(图5d),其中RS 、Rct 和CPE分别表示溶液电阻、电荷传输电阻和恒相角元[14,43]。催化剂CoFeAl-FeOOH-6、CoFeAl-FeOOH-3、CoFeAl-FeOOH-9、CoFeAl-FeOOH-1、CoFeAl-FeOOH-12、Ni foam-FeOOH-6 和CoFeAl LDH的Rct值分别为0.85、1.41、4.04、6.18、10.31、16.78和20.22 Ω·cm2。可以看出,CoFeAl-FeOOH-6催化剂的电荷传输电阻最小,表明其具有优异的电荷传输能力[44]。图5e给出了CoFeAl-FeOOH-6催化剂的多阶跃计时电势法(电流密度从0到150 mA·cm-2,间隔5 min)测试结果。可以看出,对于每一电流密度OER电位的突变和稳定的、无起伏的平台以及对称的逆过程证实:催化剂具有坚固性和优异的质量输运、电荷传输性能。在电流密度为150 mA·cm-2条件下20 h后CoFeAl-FeOOH-6的性能没有明显的衰减(图5f),表明这种催化剂的长期稳定性很高。测试稳定性后再测试催化剂的XRD、SEM和XPS能谱,以进一步证实其稳定性。稳定性测试前后XRD和SEM基本上没有变化(图6),表明CoFeAl-FeOOH-6的活性和结构的稳定性很高。可以看出,稳定性测试前后CoFeAl-FeOOH-6催化剂中Co2p、Fe2p和Al 2p的XPS高分辨能谱(图6e~g)没有明显的变化。在O1s高分辨能谱中三个特征峰位于530.5 eV,531.2 eV和532.0 eV处(图6h),表明存在Fe-O-Fe键,Fe-O-H键和H-O-H键[30,45,46]。这些结果表明,CoFeAl-FeOOH-6催化剂具有优异的OER活性和长时间稳定性。
图5
图5
样品的OER性能
Fig.5
OER performance of the as-synthesized samples
(a) LSV with 95% iR compensation, (b) corresponding Tafel plots, (c) capacitive current as a function of sweep rate for the as-synthesized samples, (d) Nyquist measured at 1.55 V (vs. RHE), (e) multistep chronopotentiometry response without iR compensation with current density of 10, 30, 75, 100 and 150 mA·cm-2, (f) chronopotentiometric curves of the as-synthesized catalysts at 150 mA·cm-2
图6
图6
CoFeAl-FeOOH-6稳定性测试前后的XRD谱(a),SEM照片(b~c),(d)对应的元素扫描和高分辨XPS能谱,Co 2p (e),Fe 2p (f),Al 2p (g)和O 1s (h)的XPS高分辨能谱
Fig.6
XRD pattern (a), SEM image (b, c), (d) corresponding elemental mapping image and the high-resolution spectra of Co 2p (e), Fe 2p (f), Al 2p (g) and O 1s (h) in CoFeAl-FeOOH-6 before and after long-term stability test
2.3 催化剂的HER性能
图7给出了催化剂样品的HER性能。如图7a所示,与其他催化剂相比,在电流密度为100 mA·cm-2的条件下CoFeAl-FeOOH-6催化剂的过电位(193 mV)最低。在相同的条件下,CoFeAl-FeOOH-3的过电位为217 mV、CoFeAl-FeOOH-9 的过电位为258 mV、CoFeAl-FeOOH-1的过电位为263 mV、CoFeAl-FeOOH-12的过电位为275 mV、Ni foam-FeOOH-6 的过电位为304 mV)和CoFeAl LDH的过电位为270 mV。即使电流密度提高到300 mA·cm-2,CoFeAl-FeOOH-6催化剂的过电位也只增大到242 mV。同时,CoFeAl-FeOOH-6催化剂的Tafel斜率为95.6 mV·dec-1,也是最小的。这表明,这种催化剂具有快速反应动力学(图7b)[41]。从电化学阻抗谱(图7c)可见,CoFeAl-FeOOH-6的电荷传输电阻最小,表明其具有快速的电荷传输能力。多阶跃计时电势法(图7d)给出了HER电位在电流密度变化时的突变以及在恒定电流密度下的稳定性和对称逆过程,证实这种催化剂的坚固性和快速的质量输运、电荷传输性能。在电流密度为150 mA·cm-2条件下CoFeAl-FeOOH-6催化剂20 h的计时电位测量(图7e)表明,这种催化剂具有长期稳定性。上述的结果证明,CoFeAl-FeOOH-6在碱性溶液中具有优异的HER活性。
图7
图7
样品的HER性能
Fig.7
HER performance of the as-synthesized samples
(a) LSV with 95% iR compensation (inset: the comparison of LSV curves with or without 95% iR compensation for CoFeAl-FeOOH-6), (b) The corresponding Tafel plots, (c) Nyquist measured at -0.226 V (vs. RHE), (d) Multistep chronopotentiometry response without iR compensation with current density of 10, 30, 75, 100 and 150 mA·cm-2, (e) Chronopotentiometric curves measured at the current density of 150 mA·cm-2
2.4 CoFeAl-FeOOH-6催化剂的全解水催化性能
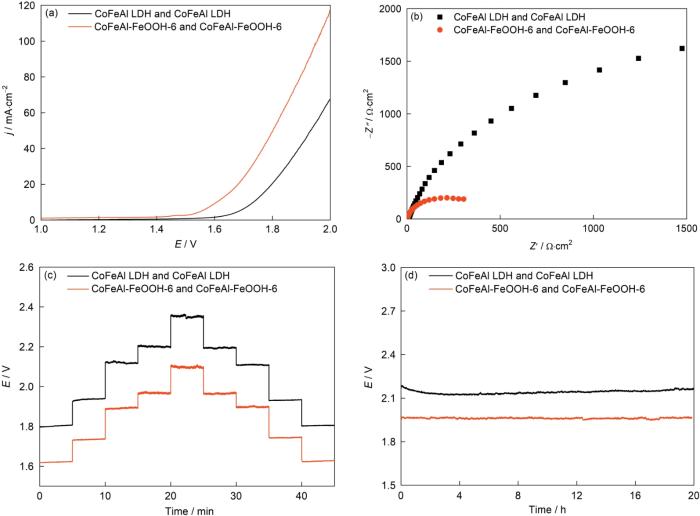
图8给出了在1.0 M KOH溶液中用两电极体系测试的CoFeAl-FeOOH-6和CoFeAl LDH催化剂的全解水性能[31],以CoFeAl LDH的结果作为对照。图8a表明,CoFeAl-FeOOH-6在1.60 V的电压下即可达到10 mA·cm-2的电流密度,远低于CoFeAl LDH需要的1.73 V。为了达到30 mA·cm-2的电流密度,CoFeAl-FeOOH-6也只需要1.73 V的电压。同时,与CoFeAl LDH相比,CoFeAl-FeOOH-6的电荷传输电阻更小(图8b),极利于电荷传输。同时,多阶跃计时电位测试(图8c)和稳定性测试(图8d)的结果表明,CoFeAl-FeOOH-6的电荷传输能力和坚固性很好。总之,CoFeAl-FeOOH-6具有优异的全解水性能。
图8
图8
CoFeAl-FeOOH-6和CoFeAl LDH的催化性能
Fig.8
Catalytic performance of the CoFeAl-FeOOH-6 and the CoFeAl LDH was measured by the two-electrode method, respectively
(a) LSV measurement at a scan rate of 2 mV·s-1 without iR compensation, (b) Nyquist plots measured at 1.352 V (vs. RHE), (c) Multistep chronopotentiometry response without iR compensation with the current density of 10, 30, 75, 100 and 150 mA·cm-2, (d) Chronopotentiometric curves measured at the current density of 100 mA·cm-2
3 结论
用两步法(水热和溶液法)可制备分层互联多孔的非晶态FeOOH修饰的CoFeAl LDH催化剂,并能用作双功能型电催化剂。CoFeAl-FeOOH-6催化剂在1.0 M KOH溶液中具有优异的OER和HER催化活性和很好的长期稳定性。作为全解水的阴极和阳极电极,CoFeAl-FeOOH-6电极在很低的电压(1.60 V)下即可实现10 mA·cm-2的电流密度且具有很好的大电流稳定性。FeOOH修饰的CoFeAl LDH催化剂催化的高性能,可归因于分层互连纳米片、多孔结构和协同效应有效地促进了电催化反应中电解液渗透、反应物和电荷传输以及质量输运。
参考文献
In situ growth of metallic Ag0 intercalated CoAl layered double hydroxides as efficient electrocatalysts for the oxygen reduction reaction in alkaline solutions
[J].
Perspective of hydrogen energy and recent progress in electrocatalytic water splitting
[J].
A highly efficient oxygen evolution catalyst consisting of interconnected nickel-iron‐layered double hydroxide and carbon nanodomains
[J].
Electrocatalytic oxygen evolution performance of high entropy FeCoNiMoCr alloy thin film electrode
[J].Thin film of high entropy FeCoNiMoCr alloy was deposited on Ti substrate by magnetron sputtering method to obtain high entropy film electrode. The surface morphology, composition, phase constituent, structure and performance of the electrode were characterized by means of surface profilometer, SEM-EDS, XRD and electrochemical workstation. The results show that the electrode surface is rough, the constituent elements are evenly distributed, the film thickness is about 2.40 μm, and the film is amorphous. The electrode showed good oxygen evolution performance and good stability in the alkaline solution. Under the condition of current density of 10.0 mA/cm2, the overpotential was 360 mV, the Tafel slope was 73.45 mV/dec. Under the condition of overpotential of 360 mV, the current density was not significantly attenuated after continuous use for 24 hours. The results of cyclic voltammetry and electrochemical impedance analysis show that due to the improved intrinsic catalytic activity, the film electrode have electrocatalytic oxygen evolution performance better than that of the noble metal oxide RuO2 (over potential 409 mV, Tafel slope 94.18 mV/dec).
FeCoNiMoCr高熵合金薄膜电极的电催化析氧性能
[J].用磁控溅射法在Ti基底上沉积了FeCoNiMoCr高熵合金薄膜并制成电极,用SEM和EDS观察和分析了电极表面和横截面的形貌和元素分布,用表面轮廓测量仪测量了电极的表面粗糙度,用XRD分析了电极的物相和结构,使用电化学工作站表征了电极的电化学性能。结果表明,电极的表面粗糙、元素分布均匀,电极上的膜厚约为2.40 μm,薄膜呈非晶态。电极在碱性溶液中表现出良好的析氧性能和稳定性。在电流密度为10.0 mA/cm<sup>2</sup>条件下,过电位为360 mV、Tafel斜率为73.45 mV/dec。在过电位为360 mV的条件下连续使用24 h,电流密度没有明显的衰减。循环伏安实验和电化学阻抗分析的结果表明,FeCoNiMoCr高熵合金薄膜本征催化活性的提高使电极的电催化析氧性能优于贵金属RuO<sub>2</sub>(过电位为409 mV,Tafel斜率为94.18 mV/dec)。
High-density cationic defects coupling with local alkaline-enriched environment for efficient and stable water oxidation
[J].
Hetero-interface manipulation in MoO x @Ru to evoke industrial hydrogen production performance with current density of 4000 mA·cm-2
[J].
A dual-site doping strategy for developing efficient perovskite oxide electrocatalysts towards oxygen evolution reaction
[J].
A highly active CoFe layered double hydroxide for water splitting
[J].Highly active, cost-effective, and durable catalysts for oxygen evolution reaction (OER) are required in energy conversion and storage processes. A facile synthesis of CoFe layered double hydroxide (CoFe LDH) is reported as a highly active and stable oxygen evolution catalyst. By varying the concentration of the metal ion precursor, the Co/Fe ratios of LDH products can be tuned from 0.5 to 7.4. The structure and electrocatalytic activity of the obtained catalysts were found to show a strong dependence on the Co/Fe ratios. The Co Fe LDH sample exhibited the best electrocatalytic performance for OER with an onset potential of 1.52 V (vs. the reversible hydrogen electrode, RHE) and a Tafel slope of 83 mV dec. The Co Fe LDH was further loaded onto a Ni foam (NF) substrate to form a 3D porous architecture electrode, offering a long-term current density of 100 mA cm at 1.65 V (vs. RHE) towards the OER.© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
NiSe nanowire film supported on nickel foam: an efficient and stable 3D bifunctional electrode for full water splitting
[J].
Preparation and electrocatalytic oxygen evolution performance of a novel porous MnNiCoCrFe high-entropy alloy as electrocatalytic electrode material
[J].A novel three-dimensional porous self-supporting electrode material for electrochemical catalytic oxygen evolution were prepared by chemical etching method from a bulk high-entropy alloy Mn50Fe12.5Co12.5Ni12.5Cr12.5. The electrochemical test results show that the overpotential of the prepared electrode material is only 281 mV at the current of 10 mA·cm-2 and the Tafel slope is 63 mV/dec in an alkaline solution of 1 mol/L KOH, which is better than that of commercial RuO2. At the same time, the working voltage does not increase significantly after continuous operation for 50 h at the current density of 50 mA·cm-2, which reflects the excellent stability during electrocatalytic oxygen evolution process of the Mn-rich high-entropy porous alloy as electrocatalytic electrode material. The Nyquist plots show that the free-standing structure of the bulk HEA catalyst has outstanding electron transfer ability compared with the ordinary supported catalyst.
MnNiCoCrFe多孔高熵合金的电催化析氧性能
[J].用化学腐蚀方法制备出3D多孔自支撑型Mn<sub>50</sub>Fe<sub>12.5</sub>Co<sub>12.5</sub>Ni<sub>12.5</sub>Cr<sub>12.5</sub>高熵合金。电化学测试结果表明,将这种高熵合金放入1 mol/L KOH的碱性溶液中,电流密度为10 mA·cm<sup>-2</sup>时过电位为281 mV,Tafel斜率为63 mV/dec,表明其电催化性能优于商业RuO<sub>2</sub>的性能。在电流密度为50 mA·cm<sup>-2</sup>的条件下连续工作50 h,工作电压没有明显的升高,表明这种富锰高熵电催化电极材料具有优异的析氧稳定性。电化学阻抗谱表明,这种自支撑型结构的块体高熵合金催化剂具有出色的导电性,与负载型催化剂相比其电子转移能力显著提高。
One-pot synthesis of reduced graphene oxide supported hollow Ag@ Pt core-shell nanospheres with enhanced electrocatalytic activity for ethylene glycol oxidation
[J].
High entropy-driven role of oxygen vacancies for water oxidation
[J].
Hollow CoS x nanoparticles grown on FeCo-LDH microtubes for enhanced electrocatalytic performances for the oxygen evolution reaction
[J].
Trinary layered double hydroxides as high‐performance bifunctional materials for oxygen electrocatalysis
[J].
Mn-doped Co-Al LDHs and its potential use for overall water splitting
[J].The layered double-metal Co-Al hydroxide (CoAl LDH) was first prepared via reflux precipitation method with CoCl2·6H2O and AlCl3·6H2O as raw material, and then the heteroelement Mn doped layered double-metal Co-Al hydroxide (Mn-CoAl LDH) was acquired by the same means. When the current density reaches 10 mA·cm-2, the fully hydrolyzable potential of Mn-CoAl LDH in 1 mol/L KOH alkaline electrolyte is 1.66 V, its performance is much better than that of undoped Co-Al layered bimetallic hydroxide (CoAl LDH), Ni2/3S1/3 /Nickel Foam (1.76 V) and commercial Pt/C (1.75 V). These results show that Mn-CoAl LDH catalyst has high activity of hydrogen evolution and oxygen evolution in alkaline environment, and is a kind of low cost and high performance bifunctional electric catalyst
Mn掺杂Co-Al 金属氢氧化物的制备及其全解水电化学性能
[J].以Co基氢氧化物为基础用异质元素掺杂方式引入Mn并与Co协同,制备出Mn掺杂Co-Al层状双金属氢氧化物 (Mn-CoAl LDH)。在1 mol/L的KOH碱性电解质中,电流密度达到10 mA·cm<sup>-2</sup>时Mn-CoAl LDH的全解水电势为1.66 V,其性能远优于Co-Al层状双金属氢氧化物(CoAl LDH)、Ni<sub>2/3</sub>S<sub>1/3 </sub>/Nickel Foam (1.76 V)和已经商业化的Pt/C (1.75 V)。这表明,Mn-CoAl LDH催化剂在碱性环境下具有较高的析氢和析氧活性,是一种低成本高性能的双功能电催化剂。
Partial sulfidation strategy to NiFe-LDH@FeNi2S4 heterostructure enable high-performance water/seawater oxidation
[J].
Pt-quantum-dot-modified sulfur-doped NiFe layered double hydroxide for high-current-density alkaline water splitting at industrial temperature
[J].
Designing a smart heterojunction coupling of cobalt-iron layered double hydroxide on nickel selenide nanosheets for highly efficient overall water splitting kinetics
[J].
Atomic Ir-doped NiCo layered double hydroxide as a bifunctional electrocatalyst for highly efficient and durable water splitting
[J].
Vertically aligned FeOOH/NiFe layered double hydroxides electrode for highly efficient oxygen evolution reaction
[J].
Simple construction of NiCo-LDH@FeOOH nanoflower heterostructure by chemical etching strategy for efficient oxygen evolution reaction
[J].
Interface engineering of NiV-LDH@FeOOH heterostructures as high-performance electrocatalysts for oxygen evolution reaction in alkaline conditions
[J].
NiFe layered double hydroxide/FeOOH heterostructure nanosheets as an efficient and durable bifunctional electrocatalyst for overall seawater splitting
[J].
Synergistic coupling of FeOOH with Mo-incorporated NiCo LDH towards enhancing the oxygen evolution reaction
[J].
Electrocatalytic activity of amorphous RuO2 electrode for oxygen evolution in an aqueous solution
[J].
Photochemical route for accessing amorphous metal oxide materials for water oxidation catalysis
[J].Large-scale electrolysis of water for hydrogen generation requires better catalysts to lower the kinetic barriers associated with the oxygen evolution reaction (OER). Although most OER catalysts are based on crystalline mixed-metal oxides, high activities can also be achieved with amorphous phases. Methods for producing amorphous materials, however, are not typically amenable to mixed-metal compositions. We demonstrate that a low-temperature process, photochemical metal-organic deposition, can produce amorphous (mixed) metal oxide films for OER catalysis. The films contain a homogeneous distribution of metals with compositions that can be accurately controlled. The catalytic properties of amorphous iron oxide prepared with this technique are superior to those of hematite, whereas the catalytic properties of a-Fe(100-y-z)Co(y)Ni(z)O(x) are comparable to those of noble metal oxide catalysts currently used in commercial electrolyzers.
Unification of catalytic water oxidation and oxygen reduction reactions: amorphous beat crystalline cobalt iron oxides
[J].Catalytic water splitting to hydrogen and oxygen is considered as one of the convenient routes for the sustainable energy conversion. Bifunctional catalysts for the electrocatalytic oxygen reduction reaction (ORR) and the oxygen evolution reaction (OER) are pivotal for the energy conversion and storage, and alternatively, the photochemical water oxidation in biomimetic fashion is also considered as the most useful way to convert solar energy into chemical energy. Here we present a facile solvothermal route to control the synthesis of amorphous and crystalline cobalt iron oxides by controlling the crystallinity of the materials with changing solvent and reaction time and further utilize these materials as multifunctional catalysts for the unification of photochemical and electrochemical water oxidation as well as for the oxygen reduction reaction. Notably, the amorphous cobalt iron oxide produces superior catalytic activity over the crystalline one under photochemical and electrochemical water oxidation and oxygen reduction conditions.
Water‐plasma‐enabled exfoliation of ultrathin layered double hydroxide nanosheets with multivacancies for water oxidation
[J].
Lateral-size-mediated efficient oxygen evolution reaction: insights into the atomically thin quantum dot structure of NiFe2O4
[J].
Amorphous FeOOH decorated hierarchy capillary-liked CoAl LDH catalysts for efficient oxygen evolution reaction
[J].
Co3S4/Fe3S4 heterostructured bifunctional catalyst evolved from CoFe LDH for effective overall water splitting in alkaline solution
[J].
A mini review on nickel-based electrocatalysts for alkaline hydrogen evolution reaction
[J].
CoFe-based layered double hydroxide for high removal capacity of hydrogen sulfide under high humid gas stream
[J].
Central-collapsed structure of CoFeAl layered double hydroxides and its photocatalytic performance
[J].
Laminated graphene oxide-supported high-efficiency microwave absorber fabricated by an in situ growth approach
[J].
Ultrathin CoFe-borate layer coated CoFe-layered double hydroxide nanosheets array: a non-noble-metal 3D catalyst electrode for efficient and durable water oxidation in potassium borate
[J].
Electrodeposition of hierarchically structured three-dimensional nickel–iron electrodes for efficient oxygen evolution at high current densities
[J].
Chemical and structural origin of lattice oxygen oxidation in Co-Zn oxyhydroxide oxygen evolution electrocatalysts
[J].
Integrating hydrogen production with anodic selective oxidation of sulfides over a CoFe layered double hydroxide electrode
[J].
Alumina-supported CoPS nanostructures derived from LDH as highly active bifunctional catalysts for overall water splitting
[J].
Amorphous MoSx developed on Co(OH)2 nanosheets generating efficient oxygen evolution catalysts
[J].
Self‐assembly of single‐layer CoAl‐layered double hydroxide nanosheets on 3D graphene network used as highly efficient electrocatalyst for oxygen evolution reaction
[J].
Facile one-pot synthesis of novel hierarchical Bi2O3/Bi2S3 nanoflower photocatalyst with intrinsic pn junction for efficient photocatalytic removals of RhB and Cr (VI)
[J].
Hierarchical CoFe oxyhydroxides nanosheets and Co2P nanoparticles grown on Ni foam for overall water splitting
[J].
Amorphous FeOOH quantum dots assembled mesoporous film anchored on graphene nanosheets with superior electrochemical performance for supercapacitors
[J].
Amorphous FeOOH oxygen evolution reaction catalyst for photoelectrochemical water splitting
[J].Reaching the goal of economical photoelectrochemical (PEC) water splitting will likely require the combination of efficient solar absorbers with high activity electrocatalysts for the hydrogen and oxygen evolution reactions (HER and OER). Toward this goal, we synthesized an amorphous FeOOH (a-FeOOH) phase that has not previously been studied as an OER catalyst. The a-FeOOH films show activity comparable to that of another OER cocatalyst, Co-borate (Co-Bi), in 1 M Na2CO3, reaching 10 mA/cm(2) at an overpotential of ∼550 mV for 10 nm thick films. Additionally, the a-FeOOH thin films absorb less than 3% of the solar photons (AM1.5G) with energy greater than 1.9 eV, are homogeneous over large areas, and act as a protective layer separating the solution from the solar absorber. The utility of a-FeOOH in a realistic system is tested by depositing on amorphous Si triple junction solar cells with a photovoltaic efficiency of 6.8%. The resulting a-FeOOH/a-Si devices achieve a total water splitting efficiency of 4.3% at 0 V vs RHE in a three-electrode configuration and show no decrease in efficiency over the course of 4 h.