传统的混凝、絮凝和过滤等方法不能有效去除持久性药物化合物[10],因此开发高效和经济的去除方法迫在眉睫。半导体光催化技术高效、绿色、成本低且反应条件温和,可用于降解药物化合物[11]。铋基半导体结构可控、环境友好、原料充足和光催化活性高,已用于光催化和可再生能源等领域[12,13]。Bi2O3是一种典型的铋基半导体光催化剂,其组成简单、带隙能相对较窄(2.2~2.8 eV)且较高的氧化能力,是一种较好的可见光催化材料[14]。Bi2O3有6种主要晶型,其晶体结构和物理性质不同。β-Bi2O3的带隙能约为2.4 eV,更容易被可见光激发,其晶体结构中沿着晶体c轴方向的孔道是防止载流子复合的通道[15],具有更高的可见光催化活性。但是在全光谱范围内,纯β-Bi2O3的电子-空穴对复合率较高。将纯β-Bi2O3改性与其他半导体、贵金属等构建异质结构,可提高其催化性能[16,17]。Bi具有窄带隙、各向异性费米表面高度、低载流子密度等特性[18],Bi纳米粒子还具有纳米粒子的表面等离子体共振(SPR)效应[19]。因此,Bi纳米粒子可替代贵金属与光催化材料复合提高其光催化性能。特别是Bi纳米粒子可在铋基半导体合成过程中原位还原,从而形成紧密接触的异质界面。Yu等[20]用混合溶液燃烧合成的Bi/BiOCl纳米片,对罗丹明B的120 min降解效率是BiOCl的2.6倍。在甲酰胺的辅助下可用一步水热法将Bi纳米粒子原位还原在Bi2O2CO3和BiOCOOH上,制备出具有优异光催化降解性能的Bi/Bi2O2CO3和Bi/BiOCOOH异质结材料[21,22]。
1 实验方法
1.1 实验用试剂
葡萄糖酸钠(C6H11NaO7,分析纯(AR))、五水硝酸铋(Bi(NO3)3·5H2O,AR)、甲酰胺(CH3NO,AR)、聚乙二醇4000((OC2H4)nOH2,AR)、浓硝酸(HNO3,AR)、叔丁醇(C4H10O,AR)、乙二胺四乙酸二钠(CHN2Na2O8,AR)、1,4-苯醌(C6H4O2,AR)、硫酸钠(Na2SO4,AR)、盐酸左氧氟沙星(C18H21ClFN3O4,AR)和无水乙醇(C2H6O,AR)。所有试剂未经进一步纯化。实验用水为蒸馏水。
1.2 前驱体和光催化剂的制备
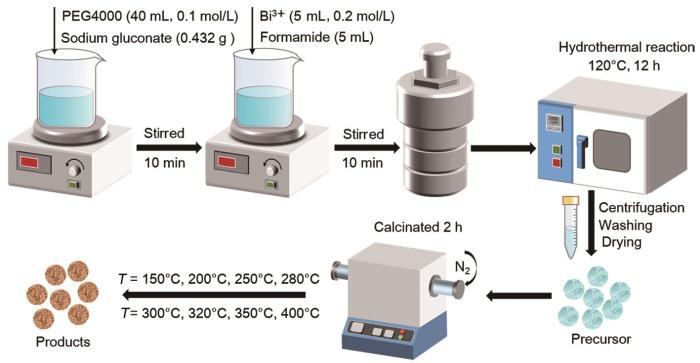
在葡萄糖酸钠的辅助下用水热法可控合成含铋前驱体:将0.432 g葡萄糖酸钠溶于30 mL的水中,然后加入40 mL的 PEG4000 (0.1 mol/L)水溶液,磁性搅拌10 min后加入0.2 mol/L的Bi3+溶液5 mL和甲酰胺5 mL,继续搅拌10 min得到均匀透明溶液。将透明溶液转移到不锈钢反应釜中,在120℃水热反应12 h后自然冷却至室温。离心收集产物并分别用水和乙醇充分洗涤后在60℃真空干燥24 h,得到含铋前驱体。只改变反应物的加入量,合成出其他样品。
光催化剂的制备:将前驱体置于瓷船中在氮气氛中在150~400℃的不同温度下煅烧2 h,得到光催化剂。
前驱体和光催化剂的合成流程,如图1所示。
图1
1.3 性能表征
用D8 Advance型X射线粉末衍射仪(XRD)分析样品的化学成分。用型号为Escalab250XI光谱仪测试样品的X射线光电子能谱(XPS)。用型号为Vario EL Cube元素分析仪进行样品的元素分析。用型号为Supra55的扫描电子显微镜(SEM,Carl Zeiss AG)、Tecnai12的透射电子显微镜(TEM,Phillips)和Tecnai G2 F30 S-Twin高分辨率透射电子显微镜(HRTEM,FEI)测试样品的形貌与尺寸。用型号为Cary5000的紫外-可见-近红外吸收光谱仪(Varian)测试样品的紫外-可见漫反射光谱(UV-Vis DRS)。用型号为Pyris 1热重分析仪(TGA,PerkinElmer)在氮气气氛中进行样品的热重(TG)分析。用型号为Autosorb IQ3表面积分析仪(Quantachrome)采集Brunauer-Emmett-Teller(BET)样品的比表面积和孔径等数据。用型号为 CHI660E的电化学工作站测试样品的光电流。
用型号为GHX-2的光化学反应仪进行光催化实验,配备250 W氙灯和紫外光截止滤光片(λ > 420 nm)作为可见光源。将0.0500 g光催化剂放入20 mg/L的LVFH水溶液(50 mL)中并将其置于一个100 mL石英容器中,用循环水浴控制在25℃下进行光催化反应。为了保证吸附-脱附达到平衡,在光照前先将溶液置于暗处搅拌约1.5 h,然后用可见光下照射进行光催化反应。在给定的时间间隔内取样4 mL,然后离心以去除催化剂。用紫外-可见分光光度计(TU-1901,Persee)测定LVFH的浓度,波长为292 nm。根据Ct/C0与可见光辐照时间的关系评价光催化剂对LVFH的降解效率,其中Ct为一定时间的LVFH的浓度,C0为吸附-脱附平衡后的LVFH的浓度。
2 结果和讨论
2.1 前驱体的形貌和物相组成
图2
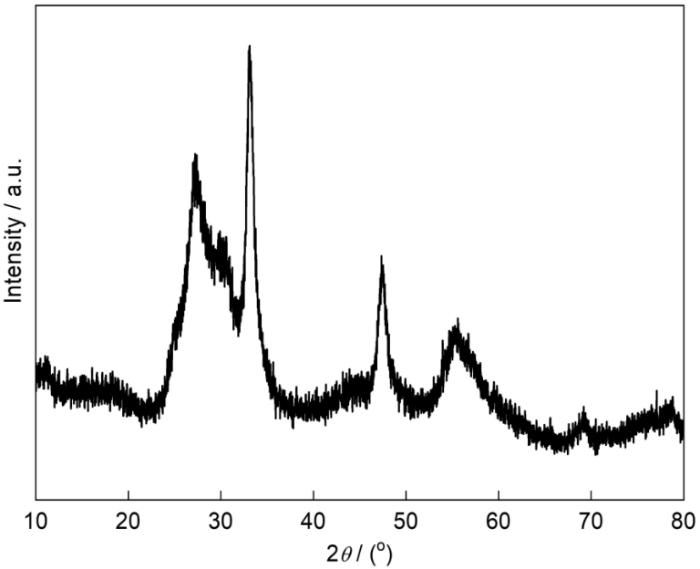
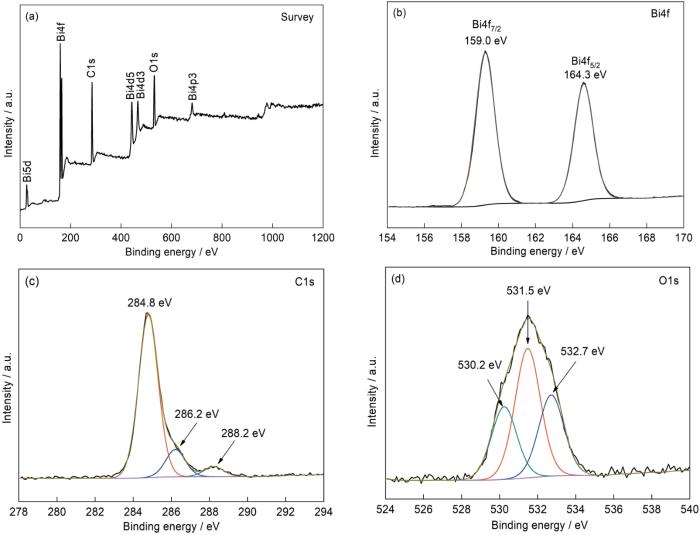
图3给出了前驱体的XRD谱。可以看出,前驱体的特征衍射峰较宽,强度较弱,表明组成前驱体的晶体颗粒较小、结晶化程度较低。图4给出了前驱体样品的XPS谱。从图4a可见,前驱体中有Bi、O和C元素。C元素的峰强度较高,表明其含量较高。图4b、c和d给出了前驱体的Bi4f、O1s和C1s的高分辨率XPS光谱。谱中位于159.0和164.3 eV处的两个强峰,分别为Bi3+的Bi4f7/2和Bi4f5/2的峰[26]。C1s的高分辨率光谱可拟合出三个较强的峰,位于284.8和286.2 eV处的峰归属于无定型碳的峰,而288.2 eV处的峰是前驱体中的O=C-O键引起的[27]。同时,O1s的高分辨谱中位于530.2 eV处的峰是Bi-O键的特征峰[28],位于531.5和532.7 eV处的两个峰分别归因于羧酸盐的O原子和O-H的特征峰[29,30]。根据XRD和XPS的结果推测,前驱体可能是铋与葡萄糖酸的配合物。从表1可以看出,前驱体中含有非金属元素C、O、H,以及微量N元素。由此可以推测,其可能的组成是(BiO)4(C6H11O7)(OH)3。
图3
图4
图4
前驱体的XPS全谱和Bi4f、C1s和O1s的高分辨谱
Fig.4
XPS spectra of the precursor (a) survey spectrum, (b) Bi4f spectrum, (c) C1s spectrum and (d) O1s spectrum
表1 前驱体的元素分析结果
Table 1
| Chemical formula | Element | EA results | Theoretical calculation |
|---|---|---|---|
| (BiO)4(C6H11O7)(OH)3 | C | 6.28 | 6.29 |
| H | 1.37 | 1.22 | |
| O | 17.97 | 19.54 | |
| N | 0.07 | 0 |
2.2 中间产物的生长机制
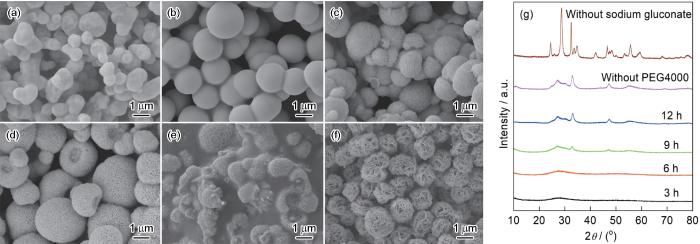
图5给出了实验过程中不同阶段中间产物的形貌和XRD谱以研究鸟巢状前驱体的生长机理。可以看出,反应时间为3 h的中间产物是不规则类球状聚集体(图5a)。反应时间为6 h的中间产物,是单分散性良好直径约为1.5 μm表面光滑的球体结构(图5b)。随着反应时间延长到9 h,球体的表面变得不光滑,某些球体结构的表面生长出纳米片(图5c)。在反应12 h的周期内,Oswald熟化过程后生成了鸟巢状中间产物(图5d)。图5g给出了这种中间产物的XRD谱。可以看出,水热反应时间为3,6 h的中间产物只有一个峰型较宽的特征峰,表明其结晶性较差。反应时间为9 h的中间产物其XRD的特征峰与前驱体的类似。图5e给出了不加PEG4000的中间产物,可见其是形貌不规则的鸟巢状,分散性较差,其XRD谱与前驱体的相似。不加葡萄糖酸钠的中间产物是直径约为1.5 μm的花状结构(图5f),其XRD谱表明是四方相BiOCOOH (依据JCPDS No.35-0939) [22]。上述实验结果表明,在前驱体的生成过程中,葡萄糖酸与Bi3+键合生成配合物,作为结构导向剂控制了鸟巢状分等级微纳结构的生成。而PEG4000则是一种分散剂和保护剂,以生成规则的、均一性和单分散性较好的薄纳米片。
图5
图5
水热时间不同的样品的SEM照片
Fig.5
SEM images of samples after hydrothermal treatment for 3 h (a), 6 h (b), 9 h (c) and 12 h (d) and SEM images of samples prepared without PEG4000 (e) and sodium gluconate (f) and XRD patterns (g) of the corresponding products
2.3 Bi/β-Bi2O3 复合材料的制备
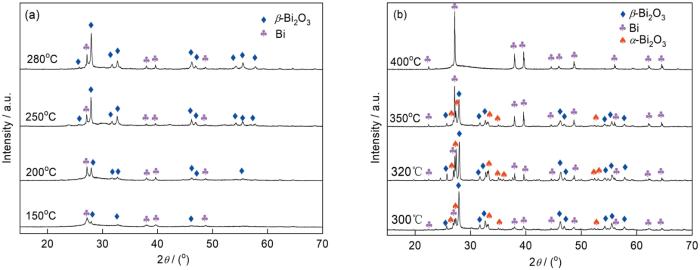
将含铋的前驱体在一定的温度下热处理后,分解生成Bi2O3[24,31]。图6a给出了前驱体样品在150~400℃的不同温度煅烧后的XRD谱。在150~280℃煅烧的样品其谱中出现了可归属于斜方六面体相Bi (依据JCPDS No.44-1246)和归属于四方相的β-Bi2O3 (依据JCPDS No.27-0050)的特征衍射峰。其原因是,在煅烧过程中前驱体分解生成了Bi2O3,而Bi2O3成为参与骨架构建的葡萄糖酸高温碳化成碳的还原剂。碳将样品中的部分Bi3+还原为单质Bi。同时,随着煅烧温度的提高β-Bi2O3的衍射峰强度逐渐提高,表明生成了Bi/β-Bi2O3复合材料。在不同温度煅烧的样品,其成分含量不同。在300~350℃煅烧的样品(图6b),其XRD谱中出现了明显的α-Bi2O3 (依据JCPDS No. 41-1449)的特征衍射峰,其强度先增强后减弱。β-Bi2O3的衍射峰强度均逐渐减弱,但是Bi的特征衍射峰却逐渐增强。其可能的原因是,部分β-Bi2O3先相变为α-Bi2O3再被碳还原为Bi单质。在400℃煅烧的样品,在其XRD谱中只观察到纯Bi的特征衍射峰,表明样品中的Bi3+已经完全还原为单质Bi。煅烧过程中发生的相变为前驱体→Bi/β-Bi2O3(150~280℃)→Bi/β-Bi2O3/α-β-Bi2O3(300~350℃)→Bi(400℃)。
图6
图6
煅烧温度不同的样品的XRD谱
Fig.6
XRD patterns of samples calcinated at different temperatures (a) 150~280oC, (b) 300~400oC
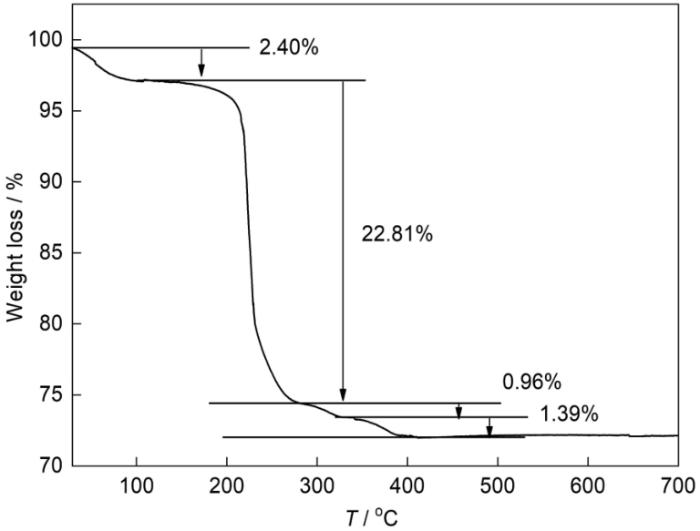
根据TG分析结果,研究了含铋前驱体在N2气氛中煅烧过程中的化学变化和相变。从图7可见,TG曲线出现四个质量损失阶段。从室温到100℃,样品的水蒸发产生的质量损失率为2.40%。在100~280℃约为22.81%的质量损失率,可归因于大量前驱体分解为β-Bi2O3和部分Bi3+还原为Bi纳米粒子。在280~340℃质量损失率约为0.96%,可归因于部分Bi3+继续还原为单质Bi。在340~400℃约为1.39%的质量损失率,可归因于Bi2O3全部还原为单质Bi。前驱体的总质量损失率为27.56%,与根据前文推导的分子式理论计算出的损失率(27.07%)基本一致。TG结果也印证了XRD给出的相变结果,表明样品的化学成分和相结构与煅烧温度有密切的关系。
图7
图8
图8
280℃煅烧的Bi/β-Bi2O3异质结光催化剂的XPS全谱和Bi4f的高分辨谱
Fig.8
XPS spectra of Bi/β-Bi2O3 heterojunction photocatalyst calcinated at 280oC (a) survey spectrum; (b) Bi4f spectrum
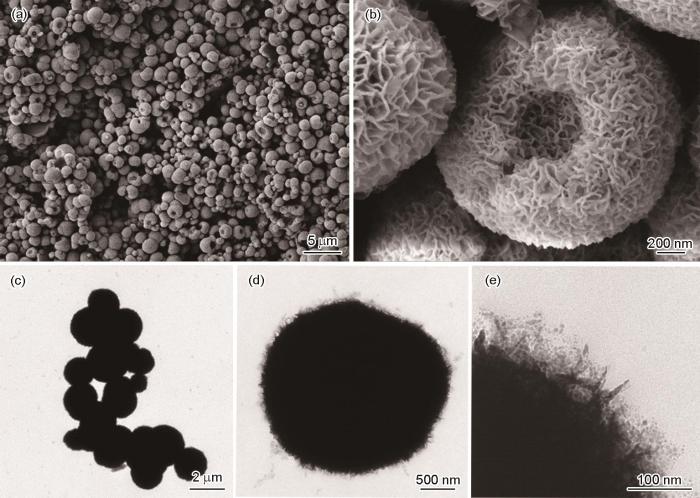
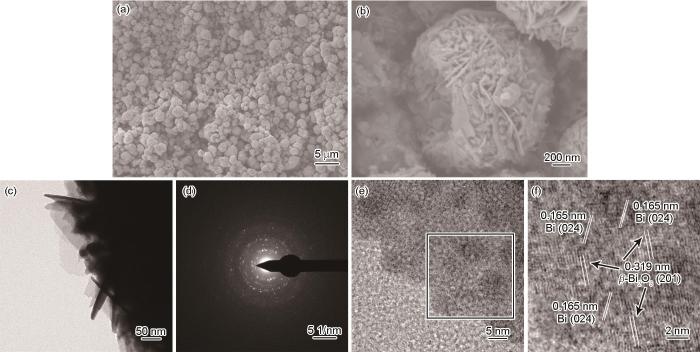
图9a给出了在280℃煅烧生成的Bi/β-Bi2O3的SEM照片。可以看出,Bi/β-Bi2O3仍呈现出平均直径约为2 μm的鸟巢状结构。从放大的照片(图9b)可见,与前驱体相比Bi/β-Bi2O3组成的纳米片发生萎缩,但是轮廓更清晰。其原因是,低结晶性的含Bi前驱体热分解成结晶性良好的Bi/β-Bi2O3,但是基本上保持了鸟巢状结构,表明含铋前驱体在煅烧过程中成了自牺牲模板。从图9c也可见,组成鸟巢结构的纳米片轮廓分明,相应的选区电子衍射图(图9d)出现清晰的衍射斑点,表明其具有良好的结晶性。这也证明了上述SEM的结果。进一步用HRTEM表征了Bi/β-Bi2O3的微观结构。图9f给出了图9e中用红方框标记区域的HRTEM图像,可观察到两种不同晶面的条纹,其中间距为0.319 nm的条纹对应四方相β-Bi2O3的(201)晶面,间距为0.165 nm的条纹归属于金属Bi的(024)晶面。这些结果表明,这种原位还原生成的Bi纳米粒子能与β-Bi2O3紧密接触构建Bi/β-Bi2O3异质结构。这有利于光生载流子的迁移,使半导体的光催化活性提高。
图9
图9
在280℃煅烧的Bi/β-Bi2O3质结光催化剂的SEM、TEM图像、选区电子衍射以及HRTEM图像
Fig.9
SEM (a, b), TEM (c), selected-area electron diffraction pattern (d) and HRTEM images (e, f) of Bi/β-Bi2O3 heterojunction photocatalyst calcinated at 280oC
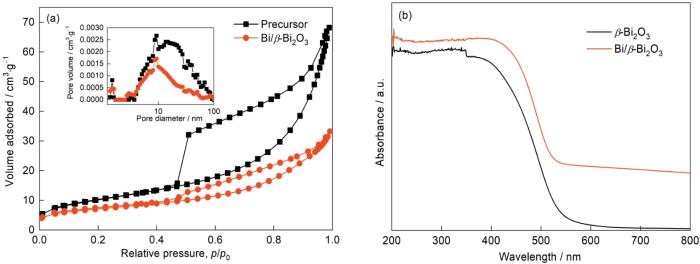
根据N2吸附-脱附等温线测定了前驱体和在280℃煅烧生成的Bi/β-Bi2O3的比表面积和孔径分布。如图10a所示,两条等温线均属于带有H3迟滞环的IV型等温线,表明煅烧前后的样品均有介孔结构[33]。前驱体的BET表面积为37.26 m2/g,在280℃煅烧后为24.81 m2/g。用BJH法确定了对应的孔径分布(见插图),表明样品的大多数孔径分布在2~50 nm,分别在9.1和9.2 nm处出现尖锐的峰,进一步证实有介孔。样品中还有少量的大孔,可能是Bi/β-Bi2O3纳米片的堆积所致。煅烧后比表面的减小源于热处理过程中粒子尺寸的增大,样品骨架的崩塌也使一些小孔消失。较大的比表面积和介孔结构,有利于材料对污染物的吸附和传质。图10b给出了在280℃煅烧生成的Bi/β-Bi2O3和按照文献[31]制备的纯β-Bi2O3样品的UV-Vis DRS谱。可以看出,Bi/β-Bi2O3不仅在紫外光区有较强的吸收,在530 nm前的可见光区也有较好的吸收,与纯β-Bi2O3对光的吸收性能一致。同时,Bi/β-Bi2O3在530~800 nm处有一个连续的吸收带。这表明,原位生成的Bi明显拓宽了β-Bi2O3对可见光的吸收。用可见光照射可产生更多的电荷载流子,使材料的可见光催化性能提高。
图10
图10
前驱体和Bi/β-Bi2O3样品的N2等温线(插图为其对应的孔径分布图)、纯β-Bi2O3和在280℃煅烧的Bi/β-Bi2O3样品的UV-Vis DRS谱
Fig.10
Typical N2 adsorption-desorption isotherm of precursor and Bi/β-Bi2O3 heterojunction photocatalyst (The inset shows the corresponding pore size distribution) (a); DRS UV-vis spectrum of pure β-Bi2O3 and as-prepared Bi/β-Bi2O3 samples calcinated at 280oC (b)
2.4 Bi/β-Bi2O3 的光催化性能
以盐酸左氧氟沙星为代表性抗生素类污染物,评价了这种光催化剂的可见光催化活性。图11a给出了在280℃煅烧生成的Bi/β-Bi2O3光催化降解LVFH的UV-Vis光谱时间演化曲线。可以看出,随着光照射时间的增加LVFH所有的吸收峰均降低,特别是292 nm处的吸光峰经过140 min可见光照射后基本消失,表明LVFH基本上全被降解。图11b给出了在不同温度煅烧生成的样品对LVFH的降解效率曲线。可以看出,在没有催化剂的情况下LVFH几乎没有降解。在200、250和280℃煅烧制备的Bi/β-Bi2O3的光催化活性,随着煅烧温度的提高而提高。在280℃煅烧制备的Bi/β-Bi2O3材料,用可见光照射140 min对LVFH降解效率高达97.75%。在更高温度煅烧制备的Bi/β-Bi2O3/α-Bi2O3和Bi,其光催化活性都降低了。在350℃煅烧生成的Bi/β-Bi2O3/α-Bi2O3用可见光照射140 min只能降解39.99%的LVFH;而在400℃煅烧生成的Bi单质对LVFH的降解率更低,只有18.38%。纯β-Bi2O3的降解率,只有54.19%。这些结果表明,在280℃煅烧生成的Bi/β-Bi2O3,其可见光催化活性最好。XRD测试结果表明,所制备的催化剂结晶程度、化学成分和含量随着煅烧温度的变化而变化。在低于280℃的温度煅烧,样品中的β-Bi2O3的含量较低且结晶度较差,使样品的光催化性能较差。β-Bi2O3具有较小的带隙能,与α-Bi2O3相比其可见光催化活性更好[34]。另一方面,原位还原生成的Bi纳米粒子的SPR效应增强了材料对可见光的吸收,且其与β-Bi2O3形成的异质结促进了光生载流子的分离。但是,过高的Bi含量反而降低材料的光催化活性且其本身的光催化性能并不高[21]。总之,在可见光照射下负载适量的Bi纳米粒子能提高β-Bi2O3的光催化性能。对上述的光催化降解过程进行准一级反应动力学方程拟合,可得到图11c所示的曲线。在140 min内拟合的线性相关性较好,表明这几个样品对LVFH的光催化降解过程符合准一级反应动力学。根据拟合计算出无催化剂和200、250、280、300、320、350、400℃煅烧生成的催化剂以及纯β-Bi2O3的表观速率常数(kapp),分别为0.0009、0.0018、0.0076、0.0257、0.0201、0.0131、0.0037、0.0012和0.0054 min-1。这表明,在不同温度煅烧生成样品其光催化活性高低的排序为:Bi/β-Bi2O3(280℃) > Bi/β-Bi2O3/α-Bi2O3 (300℃) > Bi/β-Bi2O3 (320℃) > Bi/β-Bi2O3 (250℃) > Bi/β-Bi2O3/α-Bi2O3 (350℃) > Bi/β-Bi2O3 (200℃) > Bi (400℃)。β-Bi2O3的kapp值最高,分别为纯Bi和β-Bi2O3样品的21.4倍和4.8倍。在可见光照射下光催化的循环实验结果,如图11d所示。可以看出,Bi/β-Bi2O3异质结光催化剂经5个光催化循环后对LVFH的光催化降解率仍为83.46%,表明其可重复利用性较好。同时,比较了在280℃制备的Bi/β-Bi2O3与文献报道的相关光催化剂降解LVFH的性能。从表2可以看出,Bi/β-Bi2O3异质结光催化剂具有优异的降解LVFH性能。
图11
图11
以在280℃煅烧的Bi/β-Bi2O3为光催化剂降解LVFH溶液UV-Vis光谱时间演变曲线、不同光催化剂对LFVH降解效率曲线、其对应的准一级反应动力学拟合曲线以及以Bi/β-Bi2O3为光催化剂降解LFVH的循环实验
Fig.11
Temporal evolution of the UV-vis absorption spectra of LFVH solution photodegradation by Bi/β-Bi2O3 photocatalyst under visible-light irradiation (a); degradation efficiency curves of LFVH by different photocatalysts (b); the corresponding fitting plots of pseudo-first-order rate reaction dynamics (c) and cyclic experiments for LFVH degradation using Bi/β-Bi2O3 as a photocatalyst (d)
表2 不同光催化剂降解LVFH性能的比较
Table 2
| Photocatalyst and dosage / g·L-1 | Concentration of LVFH / mg·L-1 | Light source | Degradation efficiency / % | Ref. |
|---|---|---|---|---|
| BiOBr/BiOI / 1 | 20 | 500 W Xe lamp (λ > 420 nm) | 97 (120 min) | [35] |
| Bi2SiO5/BiVO4 / 0.5 | 10 | 300 W Xe lamp (λ > 420 nm) | 80.3 (120 min) | [36] |
| Ag3PO4/BiVO4 / 1 | 10 | 500 W Xe lamp (λ > 400 nm) | 92.44 (180 min) | [37] |
Ag/AgCl/Bi2O3/BiFeO3@zeolite / 1~1.2 | 18 | 350 W Xe lamp | 72.09~73.55 (150 min) | [38] |
| α-Fe2O3/Cu2O / 1 | 31 | 500 W halogen tungsten lamp (400~1100 nm) | 79.4 (240 min) | [39] |
| AuNPs/h-BN / 1 | 10 | 300 W Xe lamp (λ > 400 nm) | 84.4 (210 min) | [40] |
| Ag/ZnO / 1 | 25 | 500 W halogen tungsten lamp | 56 (180 min) | [41] |
| Bi/β-Bi2O3 / 1 | 20 | 250 W Xe lamp (λ > 420 nm) | 97.75 (140 min) | This work |
2.5 Bi/β-Bi2O3 的光催化机理
为了确定Bi/β-Bi2O3在光催化降解LVFH过程中的活性物种,将1,4-苯醌(BQ)、乙二胺四乙酸二钠盐(EDTA)和叔丁醇(TBA)分别加到反应液中,作为超氧自由基(•O
图12
图12
以Bi/β-Bi2O3为光催化剂加入捕获剂TBA、BQ和EDTA前后降解LFVH的对比曲线以及在可见光照射下Bi、β-Bi2O3和Bi/β-Bi2O3的瞬时光电流密度
Fig.12
Effect of different scavengers on the photodegradation of LFVH with Bi/β-Bi2O3 as the photocatalyst (a) and photo-current density of Bi、β-Bi2O3 and Bi/β-Bi2O3 samples under visible-light irradiation (b)
基于以上结果提出了Bi/β-Bi2O3异质结具有优异光催化活性的可能机理,如图13所示。β-Bi2O3具有较小的带隙能(2.45 eV)[31],被可见光照射时激发出电子-空穴对。在β-Bi2O3原位生成的Bi纳米粒子具有类似于贵金属的SPR效应,极大地拓展了β-Bi2O3的可见光吸收范围,使β-Bi2O3吸收更多的可见光产生更多的电子-空穴对。另外一方面,Bi纳米粒子也能被可见光激发生电子-空穴对。因为Bi的费米能级(Ef = -0.17 eV)[42]高于β-Bi2O3 (ECB = 0.39 eV)[31]的导带,Bi纳米粒子上的光生电子可迁移到β-Bi2O3的导带上,Bi纳米粒子中残留空穴使其具有更正的电势,接受来自β-Bi2O3价带上的电子使Bi恢复到初始状态。在此过程中,Bi纳米粒子起助催化剂的作用[21]。β-Bi2O3导带上的光生电子将吸附的溶解O2还原为H2O2,再进一步捕获电子生成•OH。同时,β-Bi2O3价带电势(EVB = 2.84 eV)比E0(•OH/OH-)(1.99 eV vs. NHE)[43]更正,所以β-Bi2O3价带中的h+也将OH-氧化为•OH。•OH和h+都是强氧化性物种,皆能将吸附在Bi/β-Bi2O3材料表面的LVFH分子光降解。光催化降解盐酸左氧氟沙星的过程,历经脱氯化氢、哌嗪环开环、脱羧、脱甲基、脱氨基和脱氟等反应生成一系列中间产物。这些中间产物继续降解生成乙酸、乙二酸和甲酸等小分子,都在足够的光照时间下降解成CO2和H2O,从而完成矿化[39,40]。综上所述,Bi/β-Bi2O3的异质结材料优异光催化降解LVFH性能归因于β-Bi2O3较好的可见光催化活性,以及原位生成的Bi纳米粒子的SPR效应拓宽了β-Bi2O3对可见光的吸收范围和使光生电子-空穴对有效分离。
图13
图13
在可见光照射下Bi/β-Bi2O3异质结对LVFH降解过程中光生载流子的迁移机理示意图
Fig.13
Scheme of the photocatalytic mechanism of Bi/β-Bi2O3 heterojunction photocatalyst under visible-light irradiation
3 结论
(1) 胺为碱源在葡萄糖酸钠和PEG4000辅助下可合成具有鸟巢状微纳结构含Bi前驱体,在280℃下将其煅烧可制备出Bi/β-Bi2O3异质结可见光催化剂。
(2) 280℃煅烧生成的Bi/β-Bi2O3具有最高的可见光催化活性,用可见光照射140 min对LFVH降解率达到97.75%;Bi/β-Bi2O3优异的可见光催化性能,可归因于β-Bi2O3优异的可见光催化活性、SPR效应拓宽的β-Bi2O3对可见光的吸收范围以及较高的电子-空穴对的分离和转移效率以及鸟巢状分等级结构的较大比表面积和介孔结构。
(3) 这种Bi/β-Bi2O3异质结光催化剂可重复使用,5次光催化循环后降解率为83.46%。
参考文献
Construction of flower-like Zn2+/BiOBr with enhanced visible photocatalytic activity for the degradation of levofloxacin
[J].
Occurrence and toxicity of antibiotics in the aquatic environment: A review
[J].
Occurrence, distribution, and potential ecological risks of antibiotics in a seasonal freeze-thaw basin
[J].
Antibiotic concentrations and antibiotic resistance in aquatic environments of the WHO Western Pacific and South-East Asia regions: a systematic review and probabilistic environmental hazard assessment
[J].Antibiotic resistance poses human health risks, and there are concerns about the effect of environmental antibiotic residues in the selection and spread of antibiotic resistance. The aim of this study was to identify antibiotic residue levels that are likely to select for resistance and relative contributions from different aquatic sources, of various aquatic environmental compartments of the WHO Western Pacific region (WPR) and the WHO South-East Asia region (SEAR), including in China and India.A systematic review of empirical studies that measured antibiotic concentrations in aquatic environments, published between 2006 and 2019, and a probabilistic environmental hazard assessments approach, were used to identify antibiotic concentrations that are likely to select for resistance in various aquatic environmental compartments of the WPR and SEAR, including in China and India. The assessment involved the use of measured environmental concentrations and predicted no-effect concentrations (PNECs).The systematic review found 218 relevant studies of 5230 screened from the WPR and 22 relevant studies of 2625 screened from the SEAR; some of these relevant studies were largely from China (n=168) and India (n=15). 92 antibiotics in the WPR and 45 in the SEAR were detected in various aquatic compartments. Antibiotic concentrations that most likely exceeded PNECs (0-100%) were observed in wastewater, and influents and effluents of wastewater treatment plants. Antibiotic concentrations that most likely exceeded PNECs were also observed in aquatic environmental compartments. The highest risk for the development of resistance was in tap or drinking water of the WPR and China for ciprofloxacin (62·5%). The relative contributions of potential sources of antibiotic contamination in waterways, such as hospitals, municipals, livestock, and pharmaceutical manufacturing, was determined for each antibiotic.The concentrations of antibiotic residues found in wastewater and wastewater treatment plants of the WPR and SEAR make them potential hotspots for the development of antibiotic resistance, which creates human health risks from environmental exposure via drinking water. These findings can help decision makers to target risk reduction measures against environmental residues of priority antibiotics in high-risk sites, and help to focus research efforts in these world regions.Swedish Research Council.Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Analysis of quinolone-resistance in commensal and diarrheagenic Escherichia coli isolates from infants in Lima, Peru
[J].
Controllable synthesis of Ce-doped ZnO: TiO2 nanospheres for photocatalytic degradation of MB dye and levofloxacin under sunlight light irradiation
[J].
Remarkably enhanced sulfate radical-based photo-Fenton-like degradation of levofloxacin using the reduced mesoporous MnO@MnO x microspheres
[J].
Occurrence, distribution and their correlation with different parameters of antibiotics and antibiotic resistance genes in lakes of China: A review
[J].
Quinolone-induced painful peripheral neuropathy: a case report and literature review
[J].
Novel 3-D flower like Bi3O4Cl/BiOCl p-n heterojunction nanocomposite for the degradation of levofloxacin drug in aqueous phase
[J].
Progress on simultaneous photocatalytic degradation of pollutants and production of clean energy: A review
[J].
Photocatalytic degradation of tetracycline hydrochloride by g-C3N4 modified Bi2O3
[J].
g-C3N4改性Bi2O3对盐酸四环素的光催化降解
[J].使用液相沉淀法和热聚合法制备Bi<sub>2</sub>O<sub>3</sub>/g-C<sub>3</sub>N<sub>4</sub>复合催化剂,用SEM、XRD、XPS、FT-IR和紫外可见漫反射等手段对其微观形貌、晶体结构和光催化性能进行了表征。结果表明,这种Bi<sub>2</sub>O<sub>3</sub>/g-C<sub>3</sub>N<sub>4</sub>复合光催化剂的形貌较好、分布均匀,具有较高的光催化性能;复合催化剂Bi<sub>2</sub>O<sub>3</sub>/g-C<sub>3</sub>N<sub>4</sub>-30%的光催化性能最好,用300 W模拟可见光氙灯照射2 h后对盐酸四环素(TCH)的去除率为70%;捕获实验的结果表明,光催化降解盐酸四环素(TCH)的主要活性物种为超氧自由基(·O2-)。
Bi-based visible light-driven nano-photocatalyst: the design, synthesis, and its application in pollutant governance and energy development
[J].
A review on the preparation, microstructure, and photocatalytic performance of Bi2O3 in polymorphs
[J].
Synergistic effect of crystal and electronic structures on the visible-light-driven photocatalytic performances of Bi2O3 polymorphs
[J].
Construction of a Z-scheme heterojunction bifunctional photocatalyst with Ag-modified AgBr embedded in β-Bi2O3 flowers
[J].β-BiO demonstrates excellent photocatalytic activity under visible light, but it has a very high photogenerated e-h recombination rate and quite low quantum efficiency. AgBr also shows excellent catalytic activity but Ag is easily reduced to Ag under light radiation, which limits its application in the photocatalysis field, and there are few reports about the application of AgBr in photocatalysis. In this study, the spherical flower-like porous β-BiO matrix was first obtained, and then the spherical-like AgBr was embedded between the petals of the flower-like structure to avoid direct light radiation. The only light through the pores on the β-BiO petals could be transmitted onto the surfaces of AgBr particles to form a nanometer point light source, which photo-reduced Ag on the surface of the AgBr nanospheres to construct the Ag-modified AgBr/β-BiO embedded composite and a typical Z-scheme heterojunction was constructed. Under this bifunctional photocatalyst and visible light, the RhB degradation rate reached 99.85% in 30 min, and the photolysis water hydrogen production rate reached 6.288 mmol g h. This work is as an effective method for not only the preparation of the embedded structure, quantum dot modification and flower-like morphology but also for the construction of Z-scheme heterostructures.
Preparation and photocatalytic properties of composite photocatalyst β-Bi2O3/BiOCOOH with hierarchical structure
[J].
多级结构形貌β-Bi2O3/BiOCOOH复合光催化剂的制备及其光催化性能
[J].用水热法合成具有多级结构形貌的甲酸氧铋,将其作为自牺牲前驱体调节热处理温度制备出不同的产物,其中一种是具有可见光响应的新型β-Bi<sub>2</sub>O<sub>3</sub>/BiOCOOH复合光催化材料。使用X射线衍射(XRD)、紫外-可见漫反射光谱(UV-Vis DRS)、扫描电子显微镜(SEM)和光电化学响应等手段表征不同温度热处理后产物的晶型结构、光吸收性能、形貌、光电流等物理化学性质。以罗丹明B作为光催化活性评价的探针分子,研究了在不同温度热处理产物的可见光光催化性能。结果表明,热处理温度从250℃提高到330℃、400℃、450℃,甲酸氧铋发生以下转变:BiOCOOH→β-Bi<sub>2</sub>O<sub>3</sub>/BiOCOOH→β-Bi<sub>2</sub>O<sub>3</sub>→α-Bi<sub>2</sub>O<sub>3</sub>。在330℃和350℃热处理后的β-Bi<sub>2</sub>O<sub>3</sub>/BiOCOOH复合光催化剂可见光催化性能最优,其降解速率比β-Bi<sub>2</sub>O<sub>3</sub>、α-Bi<sub>2</sub>O<sub>3</sub>分别高6.7倍和100倍。对罗丹明B表现出最佳脱色率的样品,光照90 min的矿化率可达88%。与纯相物质(β-Bi<sub>2</sub>O<sub>3</sub>、BiOCOOH)相比β-Bi<sub>2</sub>O<sub>3</sub>/BiOCOOH复合材料具有更大的光电流响应和更小的阻抗。结合UV-vis DRS和莫特-肖特基曲线可推测,β-Bi<sub>2</sub>O<sub>3</sub>与BiOCOOH可紧密结合形成Z-型光催化结构,具有更高的光生载流子分离效率并实现光生电荷的分离。
A semimetal bismuth element as a direct plasmonic photocatalyst
[J].
Exploring the optical potential of nano-bismuth: Tunable surface plasmon resonances in the near ultraviolet-to-near infrared range
[J].
One-step solution combustion synthesis of Bi/BiOCl nanosheets: Reaction mechanism and photocatalytic RhB degradation
[J].
Formamide-assisted one-pot synthesis of a Bi/Bi2O2CO3 heterojunction photocatalyst with enhanced photocatalytic activity
[J].
Formamide-Assisted one-step synthesis of BiOCOOH and Bi/BiOCOOH micro-/nanostructures with tunable morphologies and composition and their photocatalytic activities
[J].
Self-assembled Co-doped β-Bi2O3 flower-like structure for enhanced photocatalytic antibacterial effect under visible light
[J].
Hierarchical self-assembly of 0D/2D β-Bi2O3 crossandra flower morphology exhibits excellent photocatalytic activity against bromophenol dyes
[J].
Hierarchical photocatalysts
[J].As a green and sustainable technology, semiconductor-based heterogeneous photocatalysis has received much attention in the last few decades because it has potential to solve both energy and environmental problems. To achieve efficient photocatalysts, various hierarchical semiconductors have been designed and fabricated at the micro/nanometer scale in recent years. This review presents a critical appraisal of fabrication methods, growth mechanisms and applications of advanced hierarchical photocatalysts. Especially, the different synthesis strategies such as two-step templating, in situ template-sacrificial dissolution, self-templating method, in situ template-free assembly, chemically induced self-transformation and post-synthesis treatment are highlighted. Finally, some important applications including photocatalytic degradation of pollutants, photocatalytic H2 production and photocatalytic CO2 reduction are reviewed. A thorough assessment of the progress made in photocatalysis may open new opportunities in designing highly effective hierarchical photocatalysts for advanced applications ranging from thermal catalysis, separation and purification processes to solar cells.
Photocatalytic degradation and reduction properties of AuAg/Bi2O3 composite
[J].
AuAg/Bi2O3复合材料的光催化降解和还原性能
[J].先用聚丙烯酰胺凝胶法制备出Bi<sub>2</sub>O<sub>3</sub>颗粒,然后用光还原法将粒径为6~18 nm的AuAg合金纳米颗粒修饰在Bi<sub>2</sub>O<sub>3</sub>颗粒表面,制备出AuAg/Bi<sub>2</sub>O<sub>3</sub>复合光催化剂。AuAg合金纳米颗粒的等离子体共振吸收效应(SPR)使AuAg/Bi<sub>2</sub>O<sub>3</sub>复合物能吸收波长为~577 nm的可见光,拓展了Bi<sub>2</sub>O<sub>3</sub>的光响应范围,还促进了Bi<sub>2</sub>O<sub>3</sub>中光生电荷的分离。以甲基橙(MO)、罗丹明(RhB)和铬离子(Cr(VI))作为目标反应物,在模拟太阳光和可见光照射下考察了AuAg/Bi<sub>2</sub>O<sub>3</sub>的光催化降解和还原活性,发现AuAg合金纳米颗粒修饰提高了Bi<sub>2</sub>O<sub>3</sub>的光催化性能。用模拟太阳光照射2 h后,RhB和MO的降解率以及Cr(VI)的还原效率分别提高了~34.2%,~38.0%和~56.7%。同时,AuAg/Bi<sub>2</sub>O<sub>3</sub>还具有良好的光催化和结构稳定性。基于以上结果,提出了AuAg合金纳米颗粒对Bi<sub>2</sub>O<sub>3</sub>光催化性能的改性机理。
Transformation of amorphous Bi2O3 to crystal Bi2O2CO3 on Bi nanospheres surface for photocatalytic NO x oxidation: Intensified hot-electron transfer and reactive oxygen species generation
[J].
Study on highly visible light active Bi2O3 loaded ordered mesoporous titania
[J].
Unravelling surface changes on Cu-Ni alloy upon immersion in aqueous media simulating catalytic activity of aerobic biofilms
[J].
Morphology normalization of peony flower-like Bi2O2CO3 boosts photocatalytic seawater purification
[J].
Composite soft template-assisted construction of a flower-like β-Bi2O3/Bi2O2CO3 heterojunction photocatalyst for the enhanced simulated sunlight photocatalytic degradation of tetracycline
[J].A three-dimensional (3D) flower-like beta-Bi2O3/Bi2O2CO3 heterojunction photocatalyst was synthesized via decomposition of the precursor fabricated using a composite soft template, which was constructed from DL-aspartic acid and nonionic amphiphilic triblock copolymer (Pluronic F127). The morphology, phase structure, composition, effect of reactant concentration, and possible formation mechanism were systematically studied. The results showed that DL-aspartic acid was chosen as the coordination- and structure-directing agent, while F127 was used as the capping agent during preparation of the precursor. The as-prepared flower-like beta-Bi2O3/Bi2O2CO3 heterojunction obtained after calcination of the self-sacrificing precursor at 290 degrees C showed excellent photocatalytic performance in the degradation of the refractory colorless antibiotic agent tetracycline (TC) under simulated sunlight irradiation, with 98.79% TC degradation being achieved within 60 min of irradiation. This excellent photocatalytic performance was attributed to the narrow band gap, heterojunction structure, and 3D hierarchical structure. The results further revealed that photogenerated holes (h(+)) and hydroxyl radicals ((OH)-O-center dot) dominated the photocatalytic process. Furthermore, the beta-Bi2O3/Bi2O2CO3 heterojunction catalyst was not photocorroded after six consecutive cycles, suggesting an excellent photostability.
In-situ synthesis of metal Bi to improve the stability of oxygen vacancies and enhance the photocatalytic activity of Bi4O5Br2 in H2 evolution
[J].
Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984)
[J].
Stabilized β-Bi2O3 nanoparticles from (BiO)4CO3(OH)2 precursor and their photocatalytic properties under blue light
[J].
Novel approach for preparation of three-dimensional BiOBr/BiOI hybrid nanocomposites and their removal performance of antibiotics in water
[J].
Preparation and characterization of Bi2SiO5/BiVO4 n-n isotype heterojunction composites as a visible-light-induced photocatalyst for tetracycline and levofloxacin degradation
[J].
An effective strategy for boosting photoinduced charge separation of Ag3PO4 by BiVO4 with enhanced visible light photodegradation efficiency for levofloxacin and methylene blue
[J].
Ag/AgCl/Bi2O3/BiFeO3@zeolite for photocatalytic degradation of levofloxacin hydrochloride
[J].
Interfacial charge transfer effects of α-Fe2O3/Cu2O heterojunction and enhancement mechanism of its photocatalytic oxidation
[J].
Construction of AuNPs/h-BN nanocomposites by using gold as interfacial electron transfer mediator with highly efficient degradation for levofloxacin hydrochloride and hydrogen generation
[J].
Comparison of the performance of Ag-deposited ZnO and TiO2 nanoparticles in levofloxacin degradation under UV/visible radiation
[J].
Plasmonic Bi metal as cocatalyst and photocatalyst: The case of Bi/(BiO)2CO3 and Bi particles
[J].
Bi plasmon-enhanced mesoporous Bi2MoO6/Ti3+ self-doped TiO2 microsphere heterojunctions as efficient visible-light-driven photocatalysts
[J].