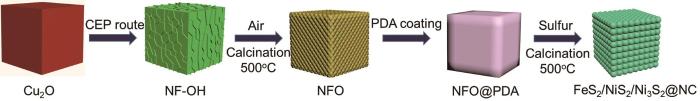
过渡金属硫化物的理论比容量较高,例如NiS2和FeS2的理论容量分别为870 mAh·g-1和890 mAh·g-1。但是,过渡金属硫化物的导电性较差,且在Li+嵌入脱出时体积变化较大,使材料粉碎而导致容量下降[5,6]。为了解决这些问题,Zheng等[7]用溶剂热法制备具有不同核壳结构的CuCo2S4纳米微球。核壳结构能降低机械应力和体积膨胀,提高Li+存储的可逆性能,在1.0 A·g-1循环1000次后比容量仍为773.7 mAh·g-1。Gu等[8]使用ZnSn(OH)6立方体为模板,进行水热反应和碳包覆制备空心立方SnO2/ZnS@C复合材料。这种中空复合材料能降低体积变化并提供更多的活性位点,使离子迁移速率和循环性能提高。在电流密度为0.2 A·g-1的条件下循环200次后这种复合材料的容量仍为818 mAh·g-1。鉴于此,本文以Cu2O为自牺牲模板,通过简单安全的协同-刻蚀沉淀路线(CEP)设计中空结构并引入两种金属元素,制备中空FeS2/NiS2/Ni3S2@NC立方体复合材料,将其作为锂离子电池负极进行电化学性能测试,研究中空立方体结构对体积膨胀和NC层对材料导电性和结构稳定性的影响。
1 实验方法
1.1 Cu2O立方前驱体的制备
将300 mL的0.01 mol/L CuCl2浅蓝色溶液置于55℃恒温水浴中,再缓慢滴加30 mL的2 mol/L Na(OH)2溶液制得棕色的CuO溶液。将其搅拌30 min后再缓慢滴加30 mL的0.6 mol/L抗坏血酸溶液,将CuO还原为Cu2O,反应3 h后得到砖红色沉淀物。将制得的产物分别用去离子水(DI)和无水乙醇充分离心洗涤(3次)后在60℃干燥12 h,得到的产物即为Cu2O立方前驱体,将其标记为Cu2O。
1.2 中空NiO/Fe2O3 纳米立方体材料的制备
在0.2 g的Cu2O粉末中加入200 mL体积比为1∶1含有DI水和无水乙醇的混合溶液,超声搅拌使Cu2O分散均匀后再依次加入40 mg的NiCl2·6H2O、46.7 mg的FeSO4·7H2O和10 mg的聚乙烯吡咯烷酮(PVP),超声搅拌30 min后用恒压漏斗缓慢滴加80 mL的0.8 mol/L Na2S2O3溶液,反应3 h后得到淡黄色产物。将产物分别用DI水和无水乙醇离心洗涤3次后在60℃干燥12 h,将得到Ni(OH)2/Fe(OH)3产物标记为NF-OH。
将NF-OH在坩埚中研磨后置于管式炉中,在空气中以2℃·min-1的速率升温到500℃,煅烧3 h后冷却至室温,得到的砖红色产物即为NiO/Fe2O3,标记为NFO。
1.3 中空FeS2/NiS2/Ni3S2@NC纳米立方体材料的制备
将0.05 g的NFO倒入100 mL的10 mmol/L Tris-缓冲液(三烃甲基氨基甲烷)中,超声搅拌使其分散均匀后加入0.1 g盐酸多巴胺,在室温下连续搅拌10 h。将产物分别用DI水和无水乙醇洗涤后离心分离3次,然后将其在60℃干燥12 h,将得到的产物标记为NFO@PDA。
最后将NFO@PDA进行高温硫化和碳化处理:将NFO@PDA与升华硫按1:5的质量比混合后在研钵中研磨,混合均匀后将其放入管式炉中。在N2气氛下以2℃·min-1的速率升温到500℃,煅烧3 h后冷却至室温。将得到的产物浸泡在二硫化碳中以去除多余的硫,将其标记为FeS2/NiS2/Ni3S2@NC。制备的过程如图1所示。
图1
图1
制备中空FeS2/NiS2/Ni3S2@NC立方体的示意图
Fig.1
Schematic diagram of preparation of hollow FeS2/NiS2/Ni3S2@NC cube
为了比较金属硫化物材料与包覆NC层复合材料的性能,在相同条件下制备中空FeS2/NiS2/Ni3S2立方体。
1.4 性能表征
用SU8220型扫描电镜和Titan G2 F30型透射电镜表征材料的形貌、微观结构及元素分布。用Smartlab 9kW型X射线衍射仪测定样品的相结构,扫描速率为8 (°)/min,扫描范围为10°~80°。用Thermo Electron型拉曼光谱仪表征碳材料的石墨化程度。用MPA型在红外光谱仪测试材料的红外吸收光谱,波长范围为4000~500 cm-1。用3Flex 5.02型分析仪表征N2的吸附和解吸性能,测定样品的比表面积和孔径分布。用Thermo ESCALAB 250XI型X射线光电子能谱仪(XPS)分析样品的化学成分。
依次将活性物质(FeS2/NiS2/Ni3S2@NC和FeS2/NiS2/Ni3S2)、导电剂(乙炔黑)和粘结剂(PVDF/NMP,0.025 g·mL-1)按照7∶2∶1的质量比,磁力搅拌10 h混合均匀。在适当大小的铜箔表面均匀涂覆搅拌好的浆料,放在真空烘箱中120℃干燥10 h。计算得到电极上活性物质的质量负荷约为1.0 mg。在充满氩气的手套箱中组装扣式电池(CR2032),以用其测试这种材料的电化学性能。在室温静置适当时间后,使用CHI660E电化学工作站的LAND CT3001A电池测试系统用循环伏安法(CV)和电化学阻抗谱法(EIS)测试不同电流密度下电池的恒流充放电性能。
2 结果和讨论
2.1 中空FeS2/NiS2/Ni3S2@NC立方体复合材料的物相结构和组成
比对图2a中衍射峰的位置,表明制备过程的第一步得到的立方体成分为Cu2O(JCPDS#05-0667)。图2b给出了NF-OH和NFO的XRD谱。比对结果表明,高温煅烧后物质的组分发生了变化。在NF-OH的XRD谱中19.26°、33.06°和38.54°处的衍射峰分别与Ni(OH)2 (JCPDS#14-0117)的(001)、(100)和(101)晶面有关,在18.47°、43.04°和62.73°处的峰分别对应Fe(OH)3(JCPDS#22-0346)的(111)、(400)和(440)晶面。这表明,经CEP路线得到的产物其组分为Ni(OH)2和Fe(OH)3。在NFO的XRD谱中出现了分别位于37.25°、43.27°和62.88°处的3个主衍射峰,对应NiO(JCPDS#47-1049)的(111)、(200)和(220)晶面。位于30.27°、35.68°和63.01°处的衍射峰,分别对应Fe2O3(JCPDS#25-1402)的(206)、(119)和(4012)晶面。这些结果表明,Ni和Fe的氧化物成分为NiO和Fe2O3。从XRD谱可见,两种材料衍射峰的峰位均较宽,说明这两种材料的结晶度较低,Ni(OH)2/Fe(OH)3可能与其在室温下合成有关,NiO/Fe2O3可能与其表面的颗粒粒径较小有关[9]。
图2
图2
Cu2O、NF-OH和NFO的XRD谱、FeS2/NiS2/Ni3S2@NC与FeS2/NiS2/Ni3S2的XRD谱的对比以及 FeS2/NiS2/Ni3S2@NC的拉曼谱
Fig.2
XRD patterns of Cu2O (a), XRD patterns of NF-OH and NFO (b), XRD patterns of FeS2/NiS2/Ni3S2@NC and FeS2/NiS2/Ni3S2 (c) and Raman spectra of FeS2/NiS2/Ni3S2@NC (d)
图2c给出了FeS2/NiS2/Ni3S2@NC与FeS2/NiS2/Ni3S2的XRD谱的对比,先确定金属硫化物的成分。位于28.51°、33.08°、37.11°、40.78°、47.41°和56.28°处的衍射峰分别与FeS2 (JCPDS#42-1340)的(111)、(200)、(210)、(211)、(220)和(311)晶面有关。位于27.69°、32.08°、35.99°、39.56°、46.01°和54.55°处的衍射峰分别与NiS2(JCPDS#11-0099)的(200)、(210)、(211)、(220)、(311)和(230)晶面有关。位于29.60°、49.35°、58.64°和72.48°处的衍射峰与Ni3S2 (JCPDS#27-0341)的(111)、(220)、(311)和(400)晶面有关。对比衍射峰的位置可以确认,金属元素Ni和Fe是以FeS2、NiS2和Ni3S2的形式存在。同时,FeS2/NiS2/Ni3S2@NC与FeS2/NiS2/Ni3S2的XRD谱的比对结果表明,FeS2/NiS2/Ni3S2@NC中C的峰并不明显,表明材料中的碳主要以无定形的形式存在。
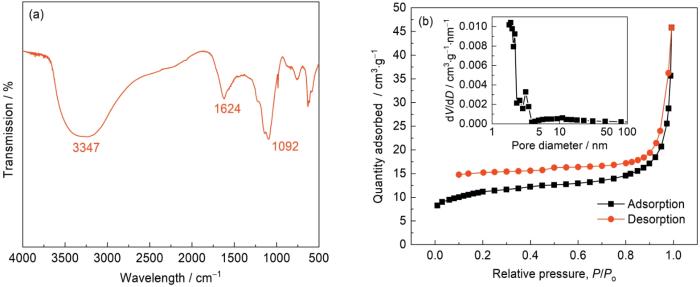
图3
图3
FeS2/NiS2/Ni3S2@NC的红外光谱、N2吸附/脱附等温线和孔径分布
Fig.3
IR spectra (a) and N2 absorption/desorption isotherm and pore size distribution curve (b) of FeS2/NiS2/Ni3S2@NC
图4a给出了FeS2/NiS2/Ni3S2@NC的X射线光电子能谱(XPS)的全谱,表明样品由Ni、Fe、S、C、N五种元素组成。由图4b给出的Ni 2p XPS谱拟合出了两个自旋轨道双星和两个振动卫星(记为“Sat.”)。Ni2+的2p3/2和2p1/2处的峰位于854.2和871.8 eV,而位于857.5和875.2 eV的拟合峰属于Ni3+的2p3/2和2p1/2。另外,Ni 2p3/2和Ni 2p1/2的卫星峰分别位于862.6和880.4 eV[13,14]。图4c给出了Fe 2p的XPS谱,位于707.3和719.6 eV的拟合峰对应Fe2+,711.4和724.2 eV处的峰,表明存在Fe3+[15,16]。在图4d给出的S 2p谱中,163.7和164.9 eV处的拟合峰分别对应S 2p3/2和S 2p1/2,表明产物含有S-S键[17]。同时,从图4e可以看出,C 1s的XPS谱中284.7、285.9和288.4 eV分别对应C-C/C=C、C-N和C=O[18,19]。在图4f给出的拟合后的N 1s XPS谱中,三个主要峰分别为398.3、399.5和401 eV,对应吡啶氮、吡咯氮和石墨氮[10,20]。XPS表征结果和IR结果,进一步证明已经成功地将N元素掺杂进碳材料中。
图4
图4
FeS2/NiS2/Ni3S2@NC的全谱以及Ni 2p、 Fe 2p、 S 2p、 C 1s和N 1s的XPS谱
Fig.4
Survey scan of FeS2/NiS2/Ni3S2@NC (a) and XPS high-resolution spectrum of Ni 2p (b), Fe 2p (c), S 2p (d), C 1s (e), N 1s (f)
2.2 微观形貌
图5
图5
Cu2O、NF-OH、FeS2/NiS2/Ni3S2以及FeS2/NiS2/Ni3S2@NC的SEM像
Fig.5
SEM images of Cu2O (a), NF-OH (b), FeS2/NiS2/Ni3S2 (c), FeS2/NiS2/Ni3S2@NC (d)
和
生成[Cu2(S2O
图5d给出了NF-OH立方体高温煅烧、包覆PDA和硫化碳化处理后的形貌。可以看出,高温煅烧后FeS2/NiS2/Ni3S2@NC立方体的表面出现较大的颗粒结构,因为一部分金属硫化物嵌入进碳层。形貌比对结果表明,FeS2/NiS2/Ni3S2@NC与FeS2/NiS2/Ni3S2 (图5c)的表面差异较大,进一步表明已经在FeS2/NiS2/Ni3S2表面包覆上NC层。从图6a可以看出,FeS2/NiS2/Ni3S2@NC内部是中空结构。FeS2/NiS2/Ni3S2立方体的边长约为750 nm,表面塌陷。FeS2/NiS2/Ni3S2的结构不稳定,引入NC层可提高中空结构的稳定性。FeS2/NiS2/Ni3S2@NC立方体的边长约为800 nm,据此推测NC层的厚度约为50 nm。根据图6b给出的高倍率下FeS2/NiS2/Ni3S2@NC的TEM照片,NC层的厚度约为40.1 nm。
图6
图6
FeS2/NiS2/Ni3S2@NC不同放大倍率下的TEM图像、FeS2/NiS2/Ni3S2@NC的HRTEM图像以及FeS2/NiS2/Ni3S2@NC对应的EDS元素分布
Fig.6
TEM images of FeS2/NiS2/Ni3S2@NC with different magnifications (a, b), HRTEM image of FeS2/NiS2/Ni3S2@NC (c) and and corresponding EDS elemental mapping images (d) of FeS2/NiS2/Ni3S2@NC
2.3 电化学性能
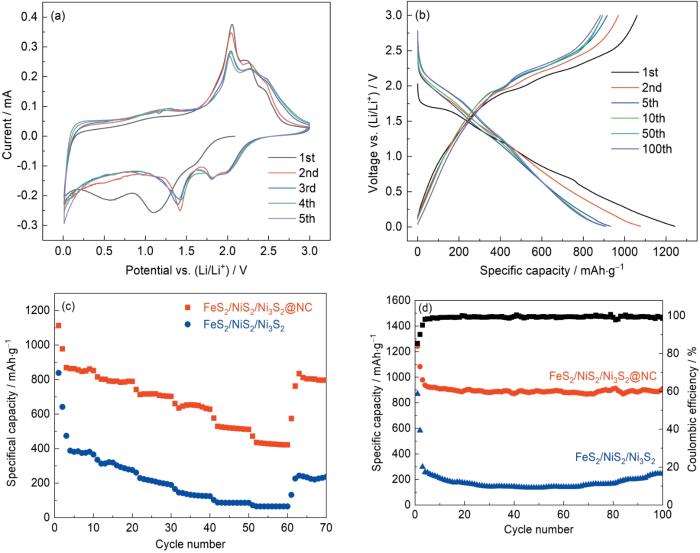
图7a给出了用FeS2/NiS2/Ni3S2@NC复合材料组装的CR2032扣式半电池的电化学性能。可以看出,在电压为0.01~3.0 V (vs. Li/Li+),扫描速率为0.1 mV·s-1条件下FeS2/NiS2/Ni3S2@NC有五个初始循环的CV曲线,涉及到的反应式有
图7
图7
扫描速率为0.1 mV·s-1时FeS2/NiS2/Ni3S2@NC的CV曲线、电流密度为0.2 A·g-1时FeS2/NiS2/Ni3S2@NC第1、2、5、10、50和100次循环的GCD分布、FeS2/NiS2/Ni3S2@NC和FeS2/NiS2/Ni3S2阳极的倍率性能以及电流密度为0.2 A·g-1时FeS2/NiS2/Ni3S2@NC和FeS2/NiS2/Ni3S2的循环性能
Fig.7
CV curves of FeS2/NiS2/Ni3S2@NC at the scan rate of 0.1 mV·s-1 (a), GCD profiles of FeS2/NiS2/Ni3S2@NC for the 1st, 2nd, 5th, 10th, 50th and 100th cycles at the current density of 0.2 A·g-1 (b), the rate capability (c) and the cycling performance (d) at the current density of 0.2 A·g-1 of FeS2/NiS2/Ni3S2@NC and FeS2/NiS2/Ni3S2
第一次阴极扫描时在0.58 V处出现一个还原峰,但是在后续的几圈循环中消失,其原因是在电极表面生成了固体电解质界面层(SEI)。位于1.11和1.62 V处的峰,与NiS2、Ni3S2和FeS2部分锂化进而转化成Ni、Fe和Li2S有关[23~25]。在阳极扫描过程中,位于2.06和2.21 V的氧化峰与Ni0氧化成NiS2和Ni3S2有关,位于2.44 V的氧化峰与Fe0的氧化有关[25~27]。位于1.11和1.62 V的还原峰分别移动到1.41和1.80 V,是结构重排以及Li+与电极材料发生复杂的化学反应造成的[5]。位于2.06、2.21和2.44 V的氧化峰略微移动到2.05、2.26和2.46 V,重合度较好。从第三个循环开始峰位不再随着循环次数的增加而变化,表明FeS2/NiS2/Ni3S2@NC电极具有良好的循环可逆性和稳定性。
图7b给出了电流密度为0.2 A·g-1时FeS2/NiS2/Ni3S2@NC电极在0.01~3.0 V (vs. Li/Li+)电压窗口下第1、2、5、10、50和100次循环的放电/充电电压分布。在第一次循环中初始库仑效率(CE)为85.36%,放电比容量和充电比容量分别为1242.2和1070.3 mAh·g-1。电池的初始库仑效率较低,主要原因是充放电过程中电解液的还原分解和SEI膜的生成[28]。在第二个循环中,库仑效率提高到90.08%,放电容量降低到1076.6 mAh·g-1,对应的充电容量为969.8 mAh·g-1。第5个循环的库仑效率为98.35%,第10、50和100圈循环的库伦效率接近100%,表明充放电过程具有良好的可逆性和循环稳定性。
图7c给出了FeS2/NiS2/Ni3S2@NC和FeS2/NiS2/Ni3S2在不同电流密度下的倍率性能。可以看出,在不同电流密度下FeS2/NiS2/Ni3S2@NC材料具有比单纯的金属硫化物材料更高的比容量和循环稳定性。随着电流密度从0.1 A·g-1增大到0.2、0.5、1.0、2.0和3.0 A·g-1,平均比容量分别为895.5、791.8、711.4、643.7、518.9和427.5 mAh·g-1。随着电流密度恢复到0.1 A·g-1,比容量随之迅速恢复到806.0 mAh·g-1,表明FeS2/NiS2/Ni3S2@NC电极具有优异的锂存储可逆性和循环稳定性。相比之下,FeS2/NiS2/Ni3S2电极的倍率性能很低,在对应的电流密度下平均比容量分别为460.7、300.6、208.8、132.4、86.4和65.2 mAh·g-1,而电流密度返回到0.1 A·g-1后比容量只能恢复到231.5 mAh·g-1。其原因可能是中空FeS2/NiS2/Ni3S2立方体没有NC层的支撑,材料由低电流密度向高电流密度充放电时体积膨胀使结构粉碎,降低了电极材料的倍率性能[29]。这表明,中空FeS2/NiS2/Ni3S2@NC纳米立方体复合材料能极大地缓解体积膨胀,从而提高中空结构的稳定性和降低容量衰减,使其具有优异的倍率性能。
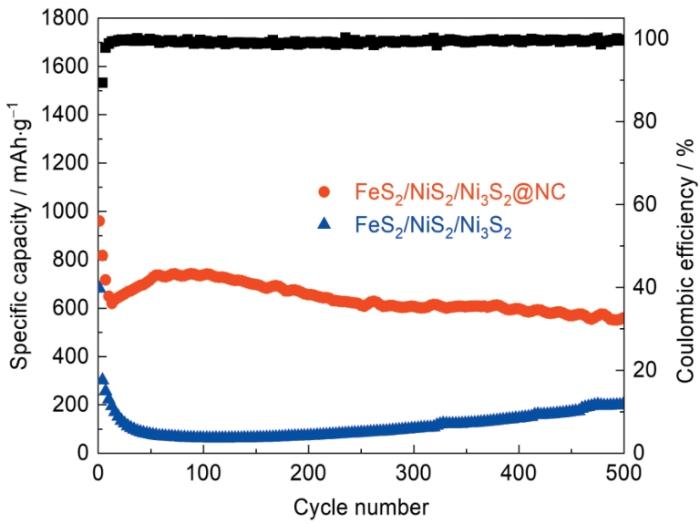
图7d给出了FeS2/NiS2/Ni3S2@NC和FeS2/NiS2/Ni3S2在0.2 A·g-1的循环性能。可以看出,FeS2/NiS2/Ni3S2@NC电极的可逆容量为899.4 mAh·g-1,约为FeS2/NiS2/Ni3S2电极(245.7 mAh·g-1)的3倍。FeS2/NiS2/Ni3S2@NC电极的库伦效率达到了99.52%,表明Li+嵌入/脱出过程的可逆性极好。在前5次循环中FeS2/NiS2/Ni3S2@NC电极的比容量逐渐降低,是SEI膜的生成所致。此后的95圈循环几乎是一条直线,表明这种材料的循环稳定性极为优异。图8给出了FeS2/NiS2/Ni3S2@NC和FeS2/NiS2/Ni3S2电极材料在1.0 A·g-1电流密度下循环500次的对比图,可见FeS2/NiS2/Ni3S2@NC电极材料在高电流密度下仍具有较高的容量(561.8 mAh·g-1),而无碳层的FeS2/NiS2/Ni3S2电极材料在前4圈循环的比容量就从681.9 mAh·g-1骤降到302.1 mAh·g-1。这个结果表明,NC层的引入有助于维持电极材料的比容量和降低容量的损失。从图中还可见,FeS2/NiS2/Ni3S2电极材料在200圈后比容量有上升的趋势且在大约450圈平稳,其原因可能是电极逐渐活化[30]。
图8
图8
电流密度为1.0 A·g-1时FeS2/NiS2/Ni3S2@NC和FeS2/NiS2/Ni3S2循环500圈后性能的对比
Fig.8
Composion at the current density of 1.0 A·g-1 after 500 cycles of FeS2/NiS2/Ni3S2@NC and FeS2/NiS2/Ni3S2
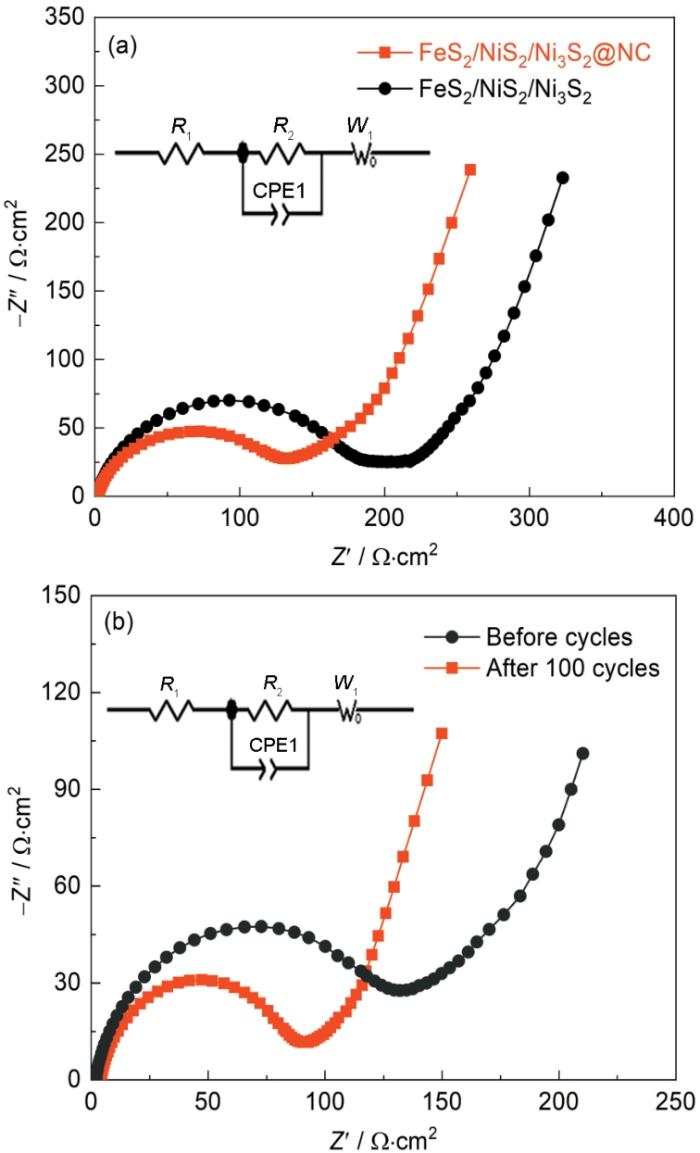
图9给出了FeS2/NiS2/Ni3S2和FeS2/NiS2/Ni3S2@NC电极材料循环前后的电化学阻抗谱(EIS)对比,图中的EIS曲线均由半圆和斜线组成,高频到中频范围内的半圆与界面电荷转移过程有关,低频区域的斜率线(也称为Warburg阻抗)与Li+的固态扩散有关[31,32]。图9a给出的拟合结果表明,FeS2/NiS2/Ni3S2@NC的电荷转移电阻(Rct)远小于FeS2/NiS2/Ni3S2的电荷转移电阻,表明FeS2/NiS2/Ni3S2@NC材料具有更好的电化学反应动力学和更高的电荷转移速度。从图9b可以明显地看出,100圈循环后的阻抗比循环前的小。这表明,循环后中空FeS2/NiS2/Ni3S2@NC立方体材料的电极反应动力学得到了优化,中空结构和碳材料的引入使其电化学性能更加稳定。
图9
图9
FeS2/NiS2/Ni3S2@NC和FeS2/NiS2/Ni3S2未循环前的阻抗对比和FeS2/NiS2/Ni3S2@NC循环100圈后阻抗对比图
Fig.9
EIS spectra of FeS2/NiS2/Ni3S2@NC and FeS2/NiS2/Ni3S2 (a) before the cycle and FeS2/NiS2/Ni3S2@NC after 100 cycles (b)
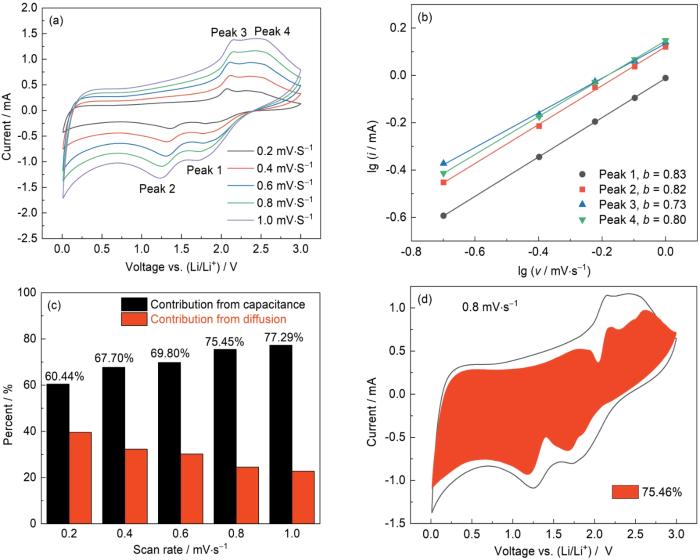
图10a给出了FeS2/NiS2/Ni3S2@NC电极的存储行为和锂离子电池中的反应动力学。扫描速率为0.2~1.0 mV·s-1时的CV曲线表明,随着扫描速率的提高阳极峰的峰值电流逐渐增大,阴极峰的峰值电流逐渐减小,CV曲线围成的面积不断增大。在一般情况下,CV曲线峰电流i与扫描速率υ之间的关系跟
图10
图10
FeS2/NiS2/Ni3S2@NC在不同扫速下的CV曲线、不同氧化还原峰处的lg(i)与lg(ʋ)的比值、不同扫速下的赝电容和扩散控制电容贡献率以及0.8 mV·s-1下的赝电容贡献
Fig.10
CV curves of FeS2/NiS2/Ni3S2@NC at various scan rates (a), the ratios of lg(i) to lg(ʋ) at different oxidation/reduction peaks (b), the contribution of capacitance and diffusion controlled capacitance at different scan rates (c), the proportions of capacitance in the total charge contribution (d) at a scan rate of 0.8 mV·s-1
和
3 结论
以Cu2O为自牺牲模板,采用简单安全的CEP路线可制备倍率性能和循环可逆性优异的中空FeS2/NiS2/Ni3S2@NC复合材料。引入NC层,能提高FeS2/NiS2/Ni3S2@NC的导电性和加快反应动力学。部分金属硫化物纳米颗粒嵌入NC层能抑制金属纳米颗粒的积累和溶解,提高锂离子电池的循环稳定性。中空立方体结构能提供充足的空间,减少Li+嵌入和脱离引起的机械应力,降低体积膨胀和容量的损耗,显著提高FeS2/NiS2/Ni3S2@NC材料的比容量和循环性能。
参考文献
Preparation and electrochemical behavior of MoP nanoparticles as anode material for lithium-ion batteries
[J].Molybdenum phosphide (MoP) is successfully prepared by a facile 2-step process. First as precursor, nano metal Mo-powders were prepared via DC arc plasma method, which then react with red phosphorus through solid-phase reaction to yield MoP nanoparticles. The prepared MoP nanoparticles were further characterized by means of XRD and TEM. Results show that the MoP nanoparticles are spherical with particle diameter of 20-50 nm. As the anode material for lithium-ion batteries, MoP nanoparticles deliver the initial discharge capacity of 746 mAh/g at the current density of 100 mA/g and the capacity maintains at 241.9 mAh/g after 50 charge-discharge cycles. As the current density increased to 2000 mA/g the discharge capacity decreases to 99.90 mAh/g. The constant capacity of 247.60 mAh/g can be restored when the current density is back to 100 mA/g.
MoP纳米粒子锂离子电池负极材料的制备及其电化学性能
[J].用直流电弧等离子体法制备金属钼纳米粉体再使其与赤磷发生固相反应,用两步法制备出磷化钼纳米粒子。使用X射线衍射(XRD)和透射电镜(TEM)等手段表征磷化钼纳米粒子的结构并进行了电化学性能测试。结果表明,MoP纳米粒子呈球状,粒径为20~50 nm;在电流密度为100 mA/g的条件下MoP纳米粒子负极材料的首次放电比容量达到746 mAh/g,50次循环后放电比容量为241.9 mAh/g;电流密度为2000 mA/g时放电比容量为99.90 mAh/g,电流密度恢复到100 mA/g其放电比容量仍然保持247.60 mAh/g。用作锂离子电池的负极材料,MoP纳米粒子具有优异的稳定性和可逆性。
Preparation and electrochemical properties of B-doped MnO2
[J].
B掺杂MnO2的制备及其电化学性能
[J].
High‐energy lithium‐ion batteries: recent progress and a promising future in applications
[J].
Lithium-ion battery modeling for aerospace applications
[J].
NiS2 nanoparticles anchored on open carbon nanohelmets as an advanced anode for lithium-ion batteries
[J].Low intrinsic conductivity and large volume expansion seriously restrict the efficient lithium storage performance of metal sulfides. Here, we fabricate a hybrid material of NiS nanoparticles/carbon nanohelmets (NiS/CNHs) to address the above issues. As an anode material in lithium-ion batteries, NiS/CNHs exhibit excellent cycling stability (490 mA h g after 3000 cycles at 5 A g) and rate properties (412 mA h g at 10 A g), outperforming other NiS -based anode materials. These remarkable performances originate from the three-dimensional helmet-like integrated architecture of NiS/CNHs, which reduces the electrode resistance due to the tight combination between NiS and CNHs, provides efficient diffusion paths for the electrolyte and Li owing to the amorphous nanoporous carbon structure, and significantly mitigates the aggregation and buffers the large volumetric expansion of NiS nanoparticles upon long-term cycling thanks to the open three-dimensional architecture and well-dispersed NiS nanoparticles on it.This journal is © The Royal Society of Chemistry.
FeS2 encapsulated with mesoporous carbon for high-performance lithium-ion batteries
[J].
Porous core-shell CuCo2S4 nanospheres as anode material for enhanced lithium-ion batteries
[J].
Fabrication of hollow SnO2/ZnS@C nanocubes as anode materials for advanced lithium-ion battery
[J].
Electrochemical performance of spinel LiMn2O4 in Water-in-salt aqueous electrolyte
[J].
尖晶石锰酸锂正极在Water-in-salt电解液中的电化学性能
[J].尖晶石型锰酸锂正极作为一种主流的水系锂电池正极材料被广泛用于水系锂离子电池,研究表明其电化学性能高度依赖于锰酸锂材料自身化学组分、颗粒尺寸、形貌和晶体结构等材料属性。本文针对性选取了LiMn<sub>2</sub>O<sub>4</sub>、铝掺杂LiAl <sub>x</sub> Mn<sub>2-</sub> <sub>x</sub> O<sub>4</sub>、富锂Li<sub>1+</sub> <sub>x</sub> Mn<sub>2-</sub> <sub>x</sub> O<sub>4</sub>三种典型的尖晶石型锰酸锂,通过一系列分析、表征手段研究循环前后其晶体结构、材料形貌以及化学组分的变化,探究在高盐浓度Water-in-salt电解液中3种材料电化学性能不同的原因。研究发现充放电时未经处理的尖晶石LiMn<sub>2</sub>O<sub>4</sub>因为严重的Mn溶解和Jahn-Teller效应产生了不可逆的相变和形貌变化,容量衰减严重,循环性能差;铝掺杂一定程度上抑制了尖晶石锰酸锂的Jahn-Teller畸变,但不能完全解决Mn溶解和晶格变形问题,也存在较严重的容量衰减;富锂Li<sub>1+</sub> <sub>x</sub> Mn<sub>2-</sub> <sub>x</sub> O<sub>4</sub>可以有效抑制尖晶石锰酸锂在水系电解液中的Mn溶解和Jahn-Teller畸变,晶体结构稳定,综合电化学性能好,适合用于水系锂离子电池,提高其整体电化学性能。
Yolk-shell-structured zinc-cobalt binary metal sulfide@N-doped carbon for enhanced lithium-ion storage
[J].
Nanoporous carbon microspheres as anode material for enhanced capacity of lithium ion batteries
[J].
Preparation of nitrogen-doped carbon dots for highly sensitive detection of amoxicillin
[J].In this paper, the nitrogen-doped carbon dots (NCDs) were synthesized using the natural material dendrobe as the raw material by one-step hydrothermal method. The synthesized carbon dots were characterized by transmission electron microscopy(TEM), X-ray photoelectron spectroscopy(XPS), Fourier transform infrared (FTIR) spectroscopy, ultraviolet-visible(UV-Vis) absorption spectroscopy and photoluminescence spectroscopy (PL). The experiment results show that NCDs are spherical or quasi-spherical, uniformly dispersed with the size ranges from 1 to 5 nm, which can emit strong blue fluorescence. The surface of the synthesized NCDs is rich in water-soluble groups such as COOH, OH and NH2. The optimal excitation and emission wavelengths of NCDs are 350 and 435 nm, respectively. Meanwhile, the fluorescence emission has good luminescence stability. The fluorescence quantum yield of the carbon dots is as high as 29.19%. The effects of different substances on the fluorescence of NCDs were measured in a buffer solution with pH=7.4. Under the same conditions, only amoxicillin is able to significantly quench the fluorescence of NCDs, indicating the synthesized NCDs can selectively interact with amoxicillin. A sensitive sensor was constructed for detecting amoxicillin by changing the fluorescence intensity of carbon dots. The linear detection range for amoxicillin is from 2.6 to 30 μmol/L and the detection limit is 0.15 μmol/L.
氮掺杂碳点的制备及其对阿莫西林高灵敏检测
[J].
Hierarchical NiCo2S4@NiFe LDH heterostructures supported on nickel foam for enhanced overall-water-splitting activity
[J].
Hierarchical micro-nano structure based NiCoAl-LDH nanosheets reinforced by NiCo2S4 on carbon cloth for asymmetric supercapacitor
[J].
Facile synthesis of Fe3S4 microspheres as advanced anode materials for alkaline iron-based rechargeable batteries
[J].
Fe3S4@reduced graphene oxide composites as novel anode materials for high performance alkaline secondary batteries
[J].
Fe3S4 nanoparticles wrapped in an rGO matrix for promising energy storage: outstanding cyclic and rate performance
[J].
Carbon-coated NiCo2S4 multi-shelled hollow microspheres with porous structures for high rate lithium ion battery applications
[J].
Double-layer N, S-codoped carbon protection of MnS nanoparticles enabling ultralong-life and high-rate lithium ion storage
[J].
Self-templated formation of hierarchically yolk-shell-structured ZnS/NC dodecahedra with superior lithium storage properties
[J].Hierarchical ZnS/NC dodecahedra are successfully constructed via a two-step synthetic method combining a sulfidation process and subsequent carbonization treatment, benefiting from the inherent merits of zeolitic imidazolate frameworks as ideal precursors/self-sacrificing templates. Studies reveal that the sulfidation time plays a vital role in the morphological evolution and lithium storage performances of final products. To our knowledge, this is the first example of carbon-based ZnS hierarchical materials with yolk-shell structures. When used as anode materials for lithium-ion batteries (LIBs), the resultant ZnS(x h)/NC (x is the sulfidation time) electrodes showed high lithium storage abilities, excellent cycling stabilities, and good rate capabilities. The optimal ZnS(72 h)/NC sample shows a well-defined multi-yolk-shell structure and delivers a high reversible specific capacity (757 mA h g-1 after 100 cycles at 200 mA g-1), extraordinary rate capability, and intriguing long-term cycling stability (∼500 mA h g-1 at 2 A g-1 after 1000 cycles). Such a type of architecture simultaneously integrates several attractive design principles for high-performance LIB anodes including the yolk-shell structure, nitrogen-doped carbon coupling, and ultrafine ZnS nanoparticles.
Design of Ni(OH)2 nanocages@MnO2 nanosheets core-shell architecture to jointly facilitate electrocatalytic dynamic for highly sensitive detection of dopamine
[J].
Preparation of NiCo2O4 nanocages and its applications in supercapacitors
[J].
NiCo2O4纳米笼的制备及其在超级电容器中的应用
[J].
Engineering nano-NiS2 embedded in graphitized carbon skeleton in hollow spherical structure as stable anode material for reversible Li+ storage
[J].
Cation-adsorption-assisted Ni3S2/carbon nanowalls composites with three-dimensional interconnected porous structures for high-performance lithium-ion battery anodes
[J].
Raspberry-like hierarchical structure FeS2 decorated by dual-carbon framework as high-performance cathode for rechargeable lithium batteries
[J].
Building a Ni3S2 nanotube array and investigating its application as an electrode for lithium ion batteries
[J].
Molybdenum-doped nickel disulfide (NiS2:Mo) microspheres as an active anode material for high-performance durable lithium-ion batteries
[J].
Effective combination of FeS2 microspheres and Fe3S4 microcubes with rGO as anode material for high-capacity and long-cycle lithium-ion batteries
[J].
Construction of FeS2@C coated with reduced graphene oxide as high-performance anode for lithium-ion batteries
[J].
Hierarchical mulberry-like Fe3S4/Co9S8 nanoparticles as highly reversible anode for lithium-ion batteries
[J].
Preparation and performance of TiS3 nanoflakes as anode material for lithium-ion batteries
[J].
锂离子电池负极材料TiS3纳米片的制备和性能
[J].
Construction of a poly(anthraquinone sulfide)/carbon nanotube composite with enhanced Li‐ion storage capacity
[J].
A novel binary metal sulfide hybrid Li-ion battery anode: three-dimensional ZnCo2S4/NiCo2S4 derived from metal-organic foams enables an improved electron transfer and ion diffusion performance
[J].
NiS2 nanodots on N, S-doped graphene synthesized via interlayer confinement for enhanced lithium-/sodium-ion storage
[J].