316L不锈钢是常用的增材制造材料。对增材制造的316L不锈钢(SLM 316L)的腐蚀行为,已有较多的研究[6~12]。Lodhi等[6]研究了SLM 316L不锈钢在人工海水中的电化学腐蚀行为,发现起比锻造316L不锈钢的耐腐蚀性更好,因为其表面的钝化膜中富集了Cr2O3。Zhang等[7]研究了SLM 316L不锈钢和锻造316L不锈钢在3.5%NaCl溶液中的点蚀行为,发现SLM 316L不锈钢的点蚀电位明显高于锻造316L不锈钢。Zhao等[8]研究了SLM 316L不锈钢在3.5%NaCl溶液中的腐蚀行为,发现起点蚀先发生在熔池的边界。Zhao等[9]研究了SLM 316L不锈钢在深海环境下的耐蚀性能,发现其晶粒减小、晶界密度较高和耐蚀性能更好。Laleh等[10]研究了SLM 316L不锈钢在0.6 mol/L NaCl溶液中的耐蚀性能,发现其比商用316L不锈钢的耐蚀性能更高。
关于在酸性环境中的腐蚀,Trelewicz等[11]研究了粉末床融合增材制造316L不锈钢在0.1 mol/L HCl溶液中的腐蚀行为,并与经淬火处理的铸造316L不锈钢进行了对比。结果表明,粉末床融合增材制造316L不锈钢在成形过程中生成的不均匀固溶体和非平衡组织,使其耐蚀性降低。Geenen等[12]研究了SLM 316L不锈钢在0.5 mol/L H2SO4溶液中的腐蚀行为,发现其中出现了大量的孔洞,使其耐蚀性能低于经固溶退火处理的铸造316L不锈钢。本文研究SLM 316L不锈钢的微观组织及其在0.05 mol/L H2SO4 + 0.2 mol/L NaCl溶液中的钝化行为,并深入探讨微观组织和钝化膜对其耐蚀性能的影响。
1 实验方法
1.1 样品的制备
在高纯度Ar气保护下制备选区激光熔化316L不锈钢(SLM 316L),成形参数为:功率220 W,扫描速度850 mm/s,层厚0.05 mm。对比材料为商用冷轧316L(R 316L)不锈钢。两种材料的主要化学成分见表1。
表1 SLM 316L和R 316L的化学成分
Table 1
| Si | Mn | P | S | Ni | Cr | C | Mo | Fe | |
|---|---|---|---|---|---|---|---|---|---|
| SLM 316L | 0.25 | 0.71 | 0.007 | 0.006 | 10.58 | 16.41 | 0.013 | 2.67 | Bal. |
| R 316L | 0.447 | 1.167 | 0.031 | 0.001 | 10.162 | 16.821 | 0.019 | 2.102 | Bal. |
长方体试样的尺寸为10 mm × 5 mm × 2 mm,去除表面油污。工作面的截面积为10 mm × 5 mm,在其背面焊接铜导线。将其余各面用环氧树脂密封。依次用800#、1000#、1500#、2000#的SiC水砂纸逐级打磨后,依次用去离子水、无水乙醇冲洗后用冷风吹干,再用指甲油封住工作面与环氧树脂的结合处,晾干后待用。
1.2 性能表征和组织观察
使用Gamry600+电化学工作站进行电化学测试,测试电极体系为三电极体系,Pt为辅助电极(CE),饱和甘汞电极(SCE)为参比电极(RE),待测样品为工作电极(WE),测试溶液为0.05 mol/L H2SO4 + 0.2 mol/L NaCl溶液,由质量分数为97%的浓H2SO4 (分析纯)和NaCl盐(分析纯)加入二次去离子水中配制而成。
为了使体系达到稳态,开路电位的测试时间为1800 s。在开路电位下测试电化学阻抗图谱(EIS),其频率测试范围为104~10-2 Hz,振幅为10 mV;动电位极化曲线的扫描速率为0.333 mV/s,扫描范围为-0.5~1.0 V (vs. SCE)。测试动电位极化曲线前,先将试样在测试溶液中浸泡1800 s;测试恒电位极化的电位为0.1 V (vs. SCE),极化时间为1200 s。在恒电位极化之后立即测试Mott-Schottky曲线,以保证试样表面生成钝化膜,测试范围为-0.25~1.0 V,幅值为10 mV,步长为20 mV,固定频率为1000 Hz。
所有实验都在室温下进行((25 ± 1)℃),所有试验均重复三次以上。
在室温下,采用草酸电解方法电解出两种不锈钢的金相显微组织形貌。用Regulus810场发射扫描电子显微镜(SEM)观察两种不锈钢的微观组织形貌。用Gemini SEM300扫描电镜测试了两种不锈钢的相成分、晶粒尺寸以及形状,使用Chinnel5和Image-Pro-Plus软件分析数据。用X-射线衍射仪(XRD)分析两种不锈钢相成分,使用jade6软件处理数据。采用Thermo Scientific Escalab 250Xi X射线光电子能谱(XPS)分析两种不锈钢在恒电位极化(0.1 V)1200 s后生成的钝化膜表面的化学成分,测试了Fe2p、Cr2p、Mo3d、Ni2p和O1s这五种能谱图。XPS处理时C1s峰的结合能被校准在248.8 eV能量刻度,并用Ar+离子束溅射表面以对元素进行深度剖析。使用Thermo Avantage软件处理实验数据。
2 结果和讨论
2.1 样品的相组成
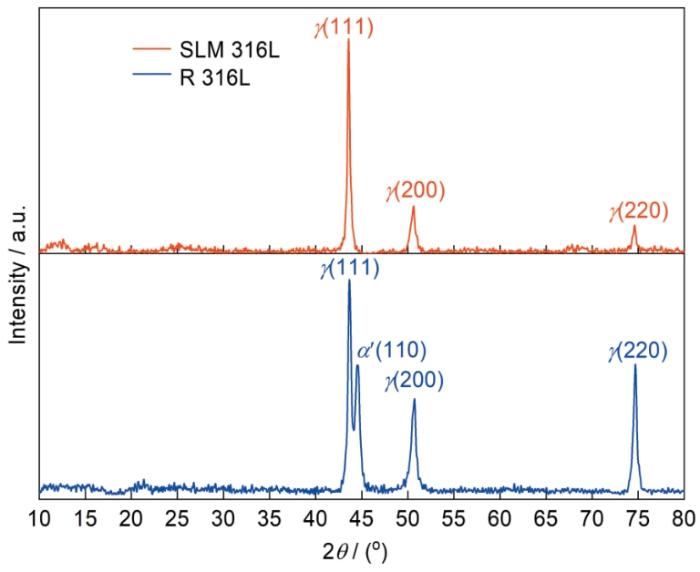
图1
图1
SLM 316L和R 316L的XRD谱
Fig.1
X-ray diffraction pattern of SLM 316L and R 316L
2.2 微观形貌
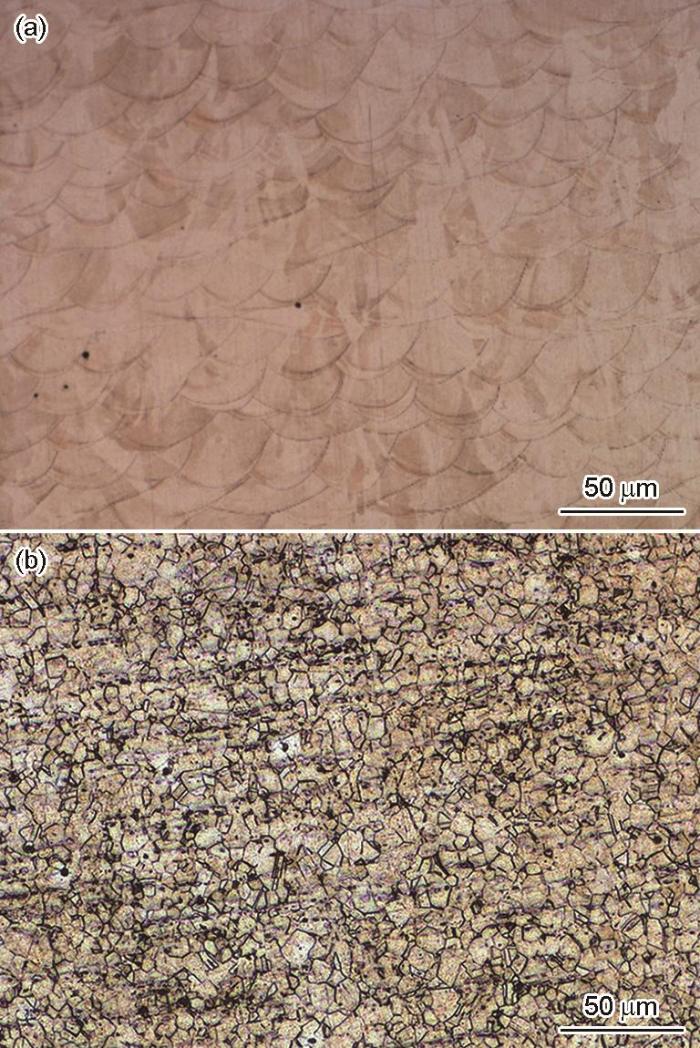
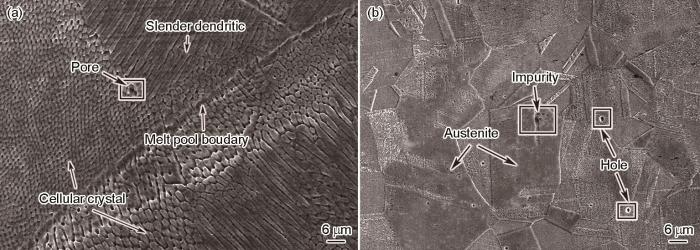
图2给出了两种不锈钢的金相照片,可见SLM 316L不锈钢呈典型的熔池特征(图2a),而R316L不锈钢呈等轴晶特征(图2b)。图3给出了两种不锈钢的扫描电镜微观形貌。可以看出,SLM 316L不锈钢呈典型的熔池特征,熔池边界内有很多的孔隙缺陷,熔池边界四周有不同形状的枝状晶和胞状晶结构[5],熔池边界四周不同区域的枝状晶形状、尺寸和方向不同(图3a)。其原因是热膨胀收缩应力引起的塑性变形使晶粒内产生不同的亚结构[15]。枝状晶沿着微区的温度梯度择优生长,也就是在激光选区熔化过程中材料冷却的速度不同所致,其中细长条的枝状晶其冷却速度比胞状晶和较短的长条枝状晶低[16]。R 316L不锈钢为典型的等轴多边形晶粒组成的奥氏体(图3b)。
图2
图2
SLM 316L和R 316L的金相组织
Fig.2
Metallographic Structure of SLM 316L (a) and R 316L (b)
图3
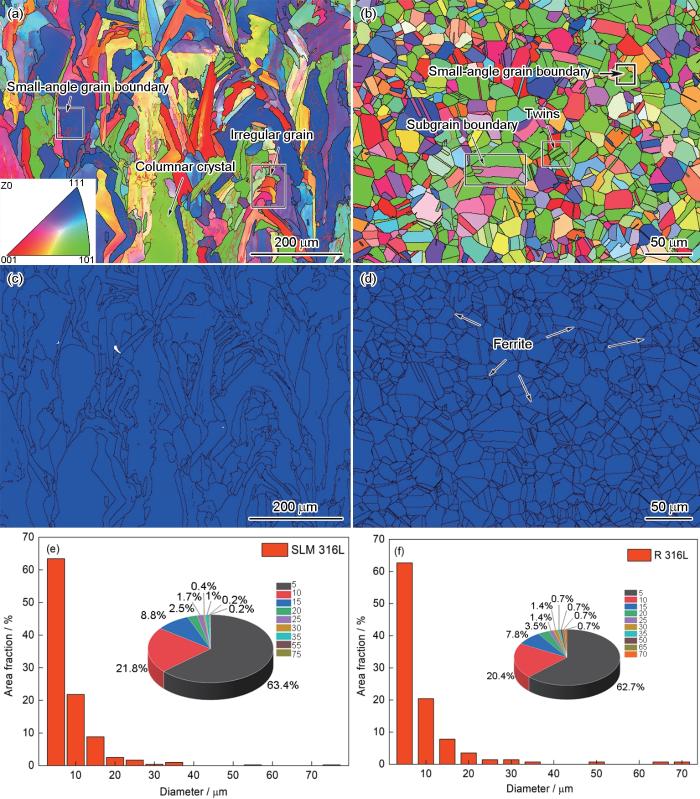
图4分别给出了两种钢的反极图(4a,4b)、Phase图(4c,4d)和晶粒尺寸统计图(4e,4f)。图4a表明,SLM 316L不锈钢主要由柱状晶粒和不规则晶粒组成,在柱状晶和其它不规则晶粒内含有大量的小角度晶界(2°~15°),小角度晶界的能量更低,腐蚀敏感性较弱。图4a和4c表明,SLM 316L不锈钢的晶面构建方向与XRD谱(图1)给出的结果一致,只有奥氏体相。图4b表明,R 316L反极图(IPF)给出的晶粒取向与XRD谱(图1)给出的晶面构建方向基本一致,而且在等轴晶粒内观察到少量的孪晶和亚晶界。从图4d的Phase图可见,R 316L不锈钢由奥氏体和少量的铁素体组成,与XRD谱(图1)给出的结果相同。体心立方(bcc)结构的铁素体主要出现在奥氏体晶界附近,铁素体的存在使R 316L不锈钢的耐腐蚀性降低[14]。图4e和4f表明,SLM 316L和R 316L的晶粒尺寸为0~15 μm,分别占全部晶粒的94%和90.9%。在两种材料中还有少量晶粒尺寸大于50 μm的超大晶粒,其含量分别为0.4%和2.1%。分析两种不锈钢的晶粒尺寸可知,SLM 316L的晶粒更小。晶粒越小,则晶界的密度越高,材料的耐蚀性越好[17]。
图4
图4
两种不锈钢材料的EBSD分析
Fig.4
EBSD analysis diagram for two stainless steel materials (a, c, e) SLM 316L stainless steel, (b, d, f) R316L stainless steel
2.3 电化学性能
2.3.1 开路电位
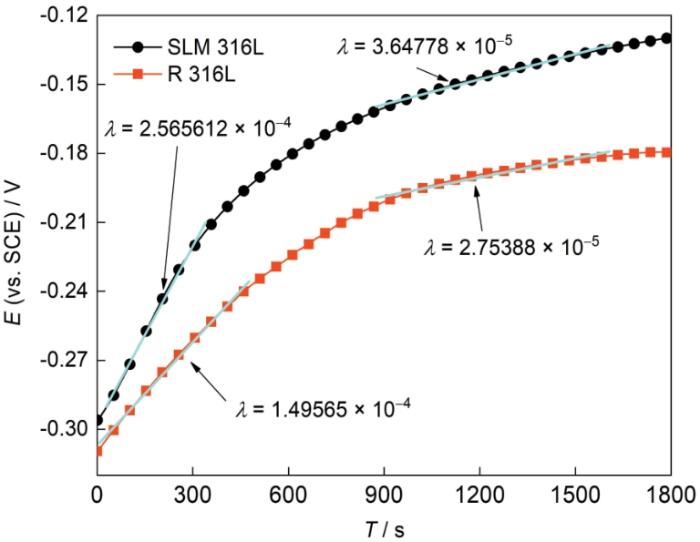
图5
图5
SLM 316L不锈钢和R 316L不锈钢的开路电位
Fig.5
Open circuit potential of SLM 316L stainless steel and R 316L stainless steel
2.3.2 动电位极化
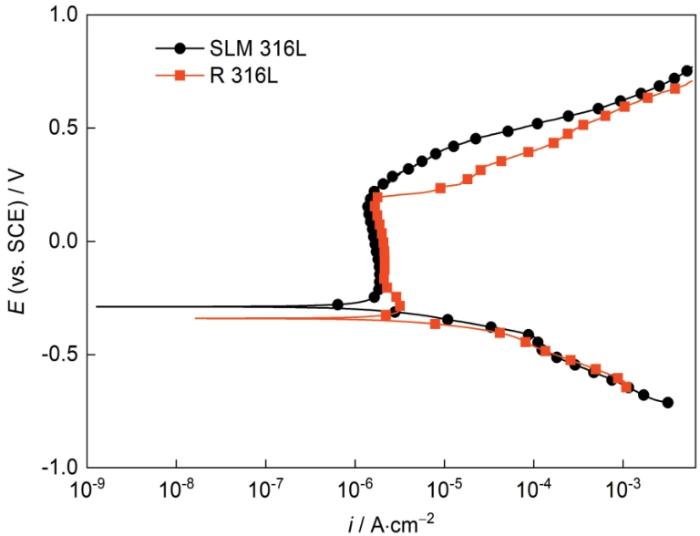
图6
图6
SLM 316L和R 316L在0.05 mol/L H2SO4 + 0.2 mol/L NaCl溶液中的动电位极化曲线
Fig.6
Potentiodynamic polarization curve of SLM 316L and R 316L in 0.05 mol/L H2SO4 + 0.2 mol/L NaCl solution
2.3.3 电化学阻抗谱
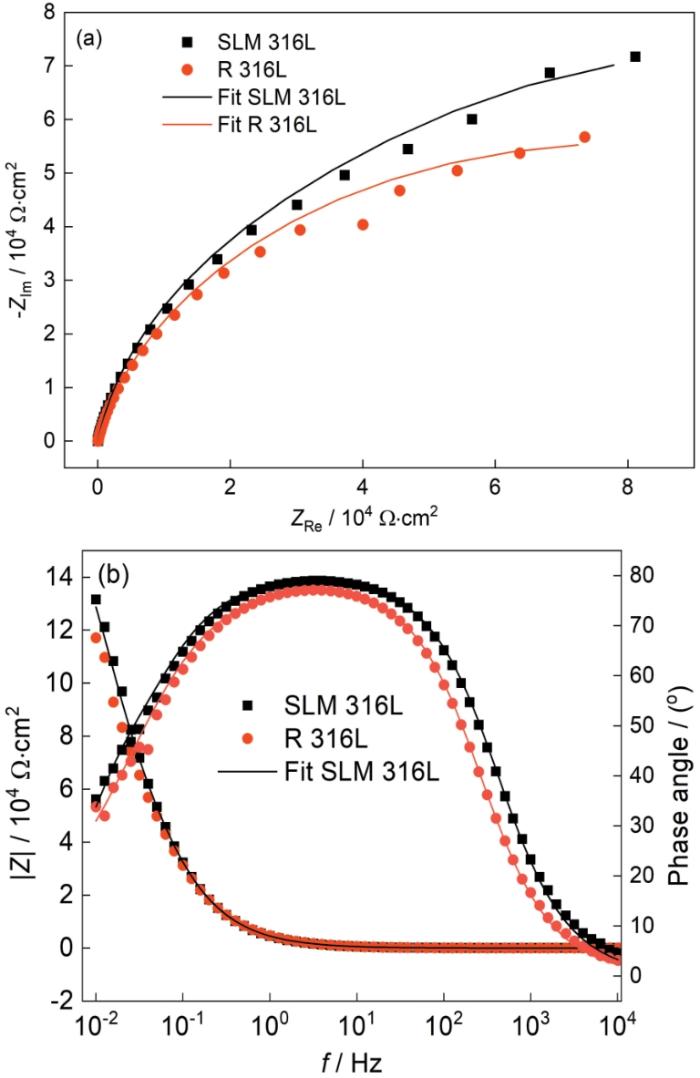
图7给出了SLM 316L和R 316L在0.05 mol/L H2SO4 + 0.2 mol/L NaCl溶液中的Nyquist图和Bode图。可以看出,两种不锈钢的Nyquist图均呈现一个压扁的不完整圆弧(图7a)。从图7b可见,两种不锈钢的Bode图均为一个不对称的峰且波峰较宽,表明电化学阻抗谱有两个时间常数,分别对应钝化膜电容和双电层电容。从图7b还可以看出,SLM 316L不锈钢和R 316L不锈钢在0.1 Hz对应的|Z|值分别为3.23 × 104 Ω·cm2和3.12 × 104 Ω·cm2。极化电阻与Bode图在0.1 Hz对应的|Z|值有关[20],SLM 316L不锈钢在0.1 Hz对应的|Z|值略高于R 316L不锈钢,表明SLM 316L不锈钢的极化电阻值略大,耐蚀性略优。
图7
图7
SLM 316L和R 316L在0.05 mol/L H2SO4 + 0.2 mol/L NaCl溶液中的电化学阻抗谱(EIS)
Fig.7
Electrochemical impedance spectroscopic (EIS) of SLM 316L and R 316L in 0.05 mol/L H2SO4 + 0.2 mol/L NaCl solution
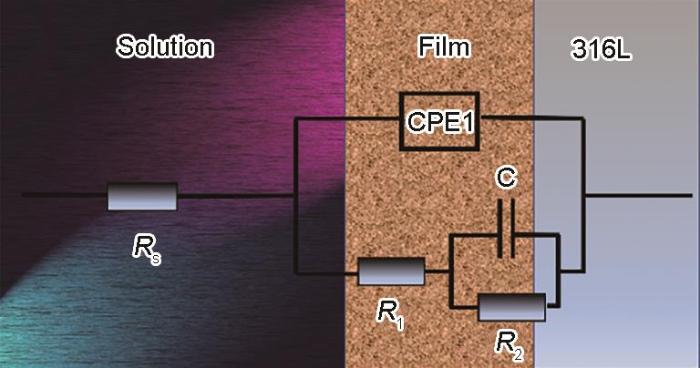
图8
图8
R 316L不锈钢和SLM 316L不锈钢的EIS等效电路图
Fig.8
Equivalent circuit diagram EIS of R 316L and SLM 316L
表2 SLM 316L和R 316L的等效电路参数
Table 2
| Rs / Ω·cm2 | CPE1 / F·cm-2 | n | R1 / Ω·cm2 | C / F·cm-2 | R2 / Ω·cm2 | |
|---|---|---|---|---|---|---|
| SLM 316L | 12.89 | 4.21 × 10-5 | 0.87 | 6.31 × 104 | -1.97 × 10-5 | 2.33 × 105 |
| R 316L | 13.67 | 4.53 × 10-5 | 0.86 | 5.88 × 104 | -2.44 × 10-5 | 1.71 × 105 |
2.3.4 钝化膜的形核机制和生长速率
按形核机制的不同,可将钝化膜的形核分为连续形核和瞬时形核。钝化膜形核的理论模型[21]为
和
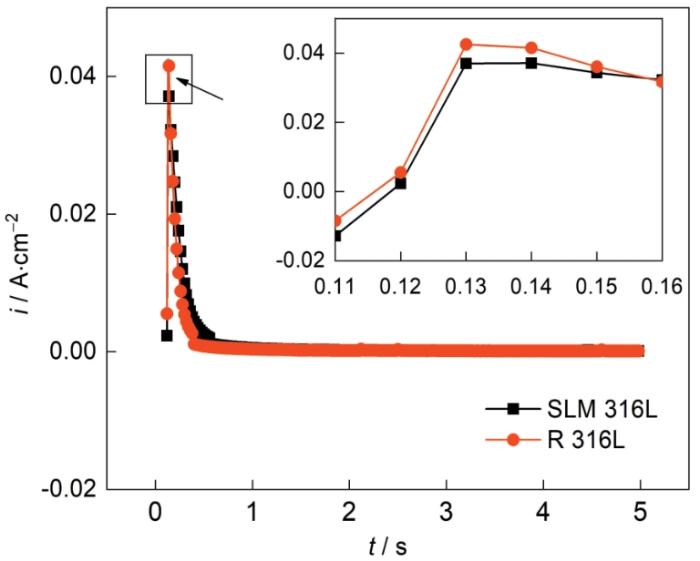
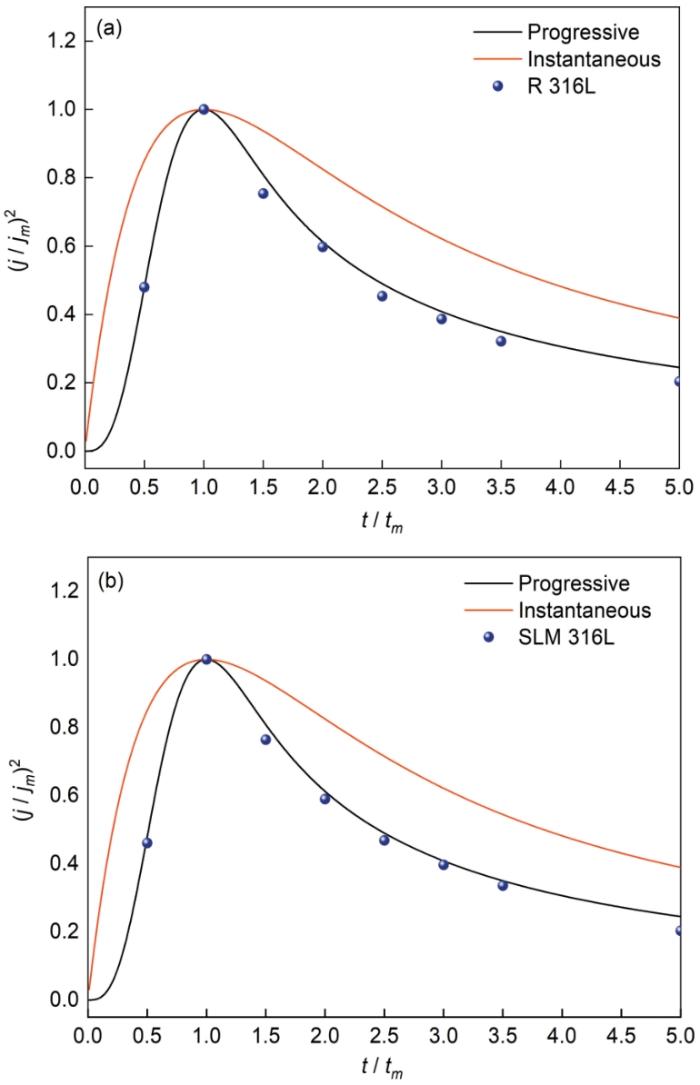
式中tm 和jm 为曲线的峰位对应的时间和电流密度。图9给出了两种材料在0.05 mol/L H2SO4 + 0.2 mol/L NaCl溶液中的暂态电流随时间的变化。在0.1VSCE,电极系统对外界电压的响应导致电流急剧上升,随后钝化膜的形核导致电流急速下降。SLM 316L不锈钢的暂态电流-时间曲线峰值对应的时间和电流均小于R 316L不锈钢,表明SLM 316L的钝化速度更高[22]。图10给出了SLM 316L不锈钢和R 316L不锈钢的暂态实验曲线与标准形核的对比。可以看出,SLM 316L和R 316L的形核机制均为连续形核,即在极化过程中不锈钢表面的活性点逐渐激活。这表明,激光选区熔化技术不改变316L不锈钢表面钝化膜的形核机制。
图9
图9
SLM 316L和R 316L在0.05 mol/L H2SO4 + 0.2 mol/L NaCl溶液中的暂态电流随时间的变化
Fig.9
SLM 316L and R 316L in 0.05 mol/L H2SO4 + 0.2 mol/L NaCl solution transient current versus time
图10
图10
SLM 316L不锈钢和R 316L不锈钢的暂态实验曲线与标准形核的对比
Fig.10
transient experimental curve of SLM 316L stainless steel and R 316L stainless steel and check comparison with standard shape
在钝化过程中,在不锈钢表面钝化膜的形成速度为
2.4 钝化膜的性能
2.4.1 钝化膜的半导体特性
式中C为钝化膜的空间电容,ε0为真空介电常数(8.85 × 10-14 F·cm-1),ε为室温下钝化膜的介电常数(取值15.6[28]),Nq为载流子密度。Efb为平带电位,k为波尔兹曼常数(1.38 × 10-23),T为绝对温度,e为电子电量(1.6 × 10-19 C)。根据不同电压范围内1/C
图11
图11
SLM 316L不锈钢和R 316L不锈钢在0.05 mol/L H2SO4 + 0.2 mol/L NaCl溶液中的Mott-Schottky曲线
Fig.11
Mott-Schottky curves of SLM 316L stainless steel and R 316L stainless steel in 0.05 mol/L H2SO4 + 0.2 mol/L NaCl solution
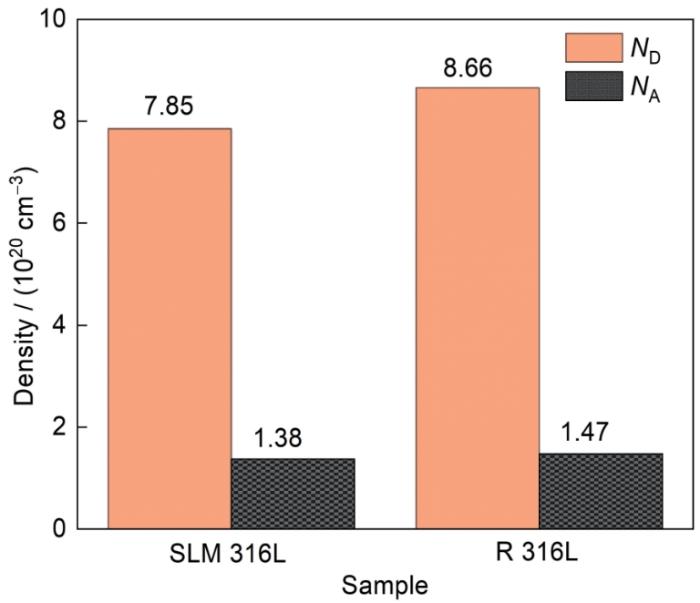
图12
图12
两种不锈钢在0.05 mol/L H2SO4 + 0.2 mol/L NaCl溶液中形成的钝化膜的载流子密度
Fig.12
Carrier density of passivation films formed in 0.05 mol/L H2SO4 + 0.2 mol/L NaCl solution for two stainless steels
2.4.2 钝化膜的致密性
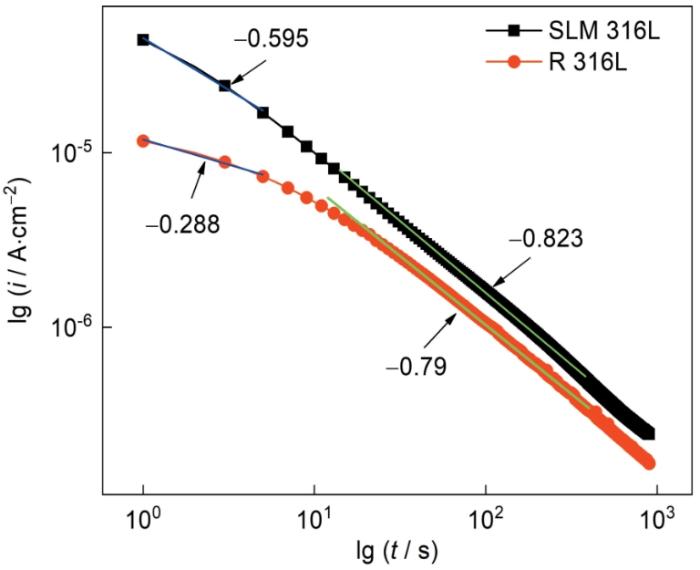
钝化膜能保护材料免于腐蚀,钝化膜越致密则其保护效果越好。钝化膜的致密性,可用电流密度随时间变化的双对数关系[31]
图13
图13
两种不锈钢的电流密度随时间变化的双对数曲线
Fig.13
Double logarithmic curves of current density with time for two stainless steels
2.5 钝化膜的成分
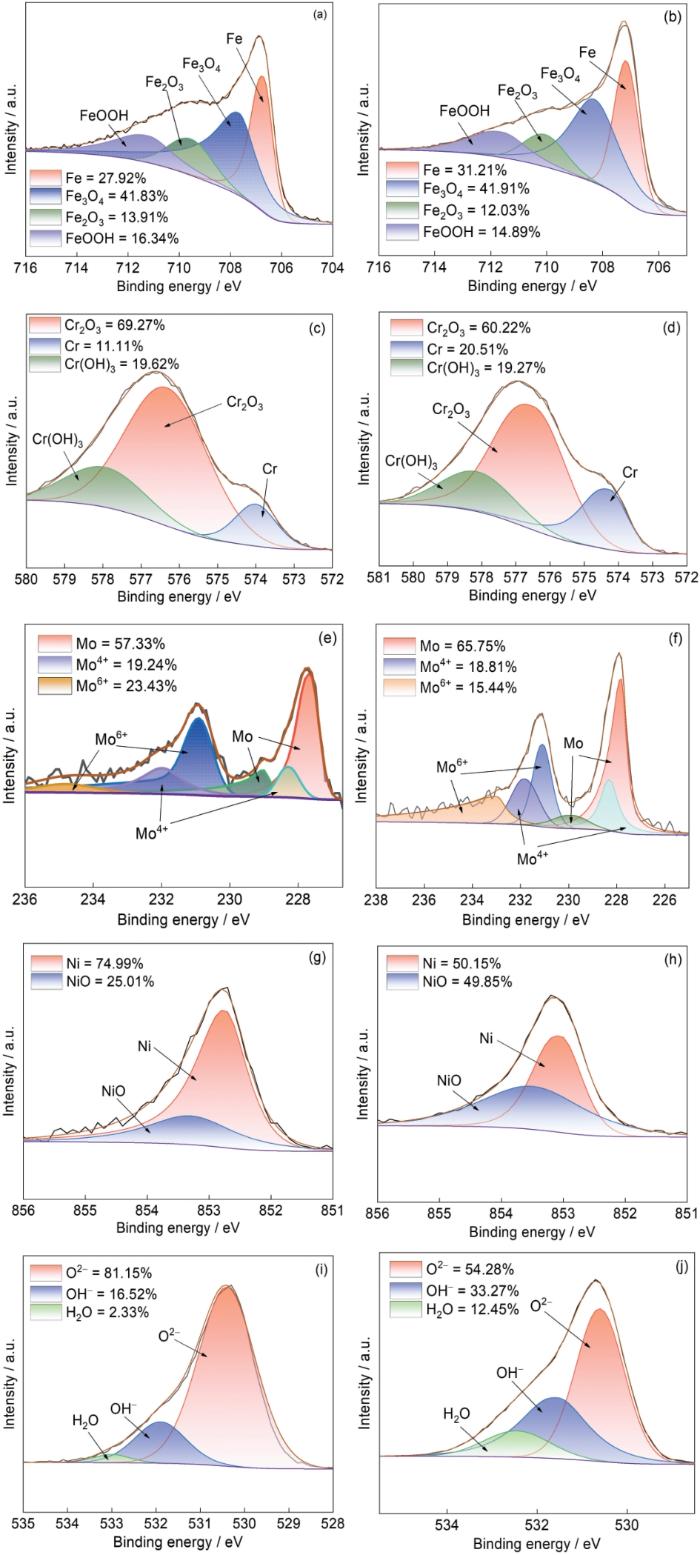
图14给出了钝化膜的XPS光谱及其拟合结果。在Fe 2p3/2光谱(图14a,14b)中,Fe 2p3/2光谱划分为四个峰,分别属于FeOOH、Fe2O3、Fe3O4和金属Fe[34]。SLM 316L不锈钢和R 316L不锈钢中构成钝化膜的FeOOH、Fe2O3和Fe3O4的含量很高。Cr 2p3/2光谱划分为Cr(OH)3、Cr2O3、金属Cr三个峰(图14c,14d),其中SLM 316L不锈钢Cr2O3/Cr(OH)3的比值为3.53,高于R 316L不锈钢的3.13。从图(14c,14d)可见,Mo 3d光谱有Mo 3d5/2和Mo 3d3/2两个轨道共六个峰,分别属于金属Mo、Mo4+和Mo6+。Mo6+是钝化膜中Mo的主要价态,Mo6+的存在降低了钝化膜的施主浓度和受主浓度[35]。SLM 316L不锈钢中Mo6+的含量高于Mo4+的含量。从Ni 2p3/2光谱(图14e,14f)可见,Ni在钝化膜中以金属Ni和NiO的形式存在。从Ni的峰面积比可知,SLM 316L不锈钢的NiO含量远低于金属Ni。R 316L不锈钢中NiO含量略低于金属Ni的含量。从O 1s光谱(图14i,14g)可知,O 1s可划分为H2O、OH-和O2-三个峰。其中OH-和O2-与钝化膜中生成的氢氧化物和氧化物的有关,钝化膜中的结合水H2O能捕获溶解的金属离子,对钝化膜有良好的修复作用[36]。从O 1s的不同离子峰的面积比可知,O2-的峰值面积大于OH-,说明钝化膜中的Cr和Fe氧化物的含量更高。Cheng等的[37]研究表明,O2-/OH-比值越低则发生点蚀的可能性越大。SLM 316L不锈钢中O2-/OH-的比值(为4.31)大于R 316L不锈钢O2-/OH-的比值(为1.63)。这表明,SLM 316L不锈钢的点蚀风险比R 316L不锈钢低。
图14
图14
两种不锈钢表面钝化膜的XPS分析
Fig.14
XPS analysis results of the surface passivation films of two stainless steels (a, c, e, g, i) SLM 316L stainless steel, (b, d, f, h, j) R 316L stainless steel
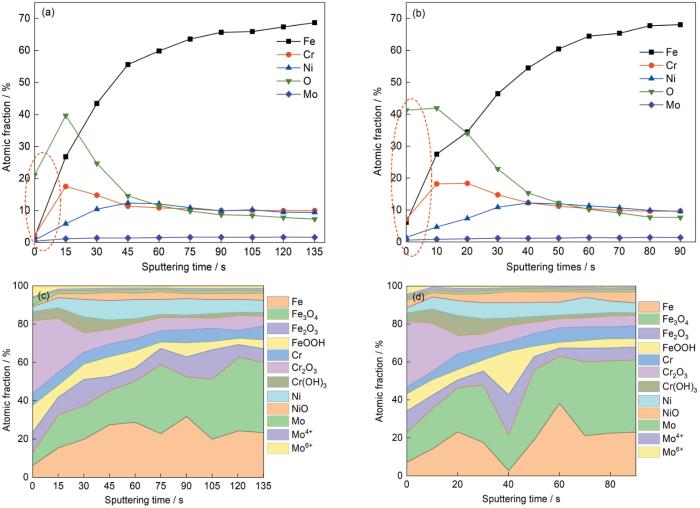
图15给出了两种不锈钢表面钝化膜中Fe、Cr、Ni、O和Mo元素的深度分布(15a,15b),以及对钝化膜中原子百分比深度的分布拟合结果(15c,15d)。如图15a和15b所示,随着溅射时间的延长Fe和Ni的含量随之提高,Cr和O的含量先提高后降低最后趋于平稳,Mo的含量基本上不变。SLM 316L不锈钢表层的Fe、Cr和O含量都比R 316L不锈钢的低,且两种不锈钢中Cr的含量都比Fe的含量高。这表明,在酸性环境中钝化膜的外层由富Fe外层变成富Cr外层[38]。如图15c和15d所示,与R 316L不锈钢相比,SLM 316L不锈钢中Cr2O3的含量略高,NiO含量略低,表层中的情况更为明显。Cr2O3含量越高,表明钝化膜对金属基体的保护性能越高[39,40]。NiO的特点是多孔的[41],而金属Ni则有利于在金属表面生成薄而致密的钝化膜[42]。因此,与R 316L不锈钢相比,SLM 316L不锈钢生成的钝化膜致密性更高,保护性能更好。
图15
图15
两种不锈钢表面钝化膜成分的深度分布
Fig.15
Depth distribution of passivation film composition on the surface of two stainless steels (a, c) SLM 316L stainless steel and (b, d) R 316L stainless steel
3 结论
(1) SLM 316L不锈钢由奥氏体相组成,而R 316L不锈钢由奥氏体主相和少量的铁素体相组成。与R 316L不锈钢相比,SLM 316L不锈钢中小角度晶界的占比更高,晶粒更小。
(2) 这两种钢的钝化膜的形核机制均为连续形核,选区熔化增材制造并未改变316L不锈钢的表面钝化膜的形核机制,但是SLM 316L不锈钢表面钝化膜的生长速率更高。
(3) SLM 316L不锈钢发生过钝化溶解,而R 316L不锈钢发生点蚀。与R 316L不锈钢相比,SLM 316L不锈钢表面生成的钝化膜中的O2-/OH-比值更低,耐点蚀性能更优,且其钝化膜的载流子浓度更低,Cr2O3的含量更高、NiO含量更低,钝化膜的保护效果更好。
参考文献
Towards large parts manufacturing in additive technologies for aerospace and automotive applications
[J].
Evaluation and prediction of the tensile properties of continuous fiber-reinforced 3d printed structures
[J].
Fused deposition modeling-based additive manufacturing (3D printing): techniques for polymer material systems
[J].
In-situ characterization and quantification of melt pool variation under constant input energy density in laser powder bed fusion additive manufacturing process
[J].
Selective laser melting of an equiatomic CoCrFeMnNi high-entropy alloy: Processability, non-equilibrium microstructure and mechanical property
[J].
Microstructural features contributing to macroscopic corrosion: The role of oxide inclusions on the corrosion properties of additively manufactured 316L stainless steel
[J].
Electrochemical noise comparative study of pitting corrosion of 316L stainless steel fabricated by selective laser melting and wrought
[J].
Influence of scanning strategy and building direction on microstructure and corrosion behaviour of selective laser melted 316L stainless steel
[J].
Improved corrosion performance of selective laser melted stainless steel 316L in the deep-sea environment
[J].
Unexpected erosion-corrosion behaviour of 316L stainless steel produced by selective laser melting
[J].
Microstructure and corrosion resistance of laser additively manufactured 316L stainless steel
[J].
Corrosion behavior of 316L austenitic steel processed by selective laser melting, hot-isostatic pressing, and casting
[J].
Microstructural and selective dissolution analysis of 316L austenitic stainless steel
[J].
Effects of non-metallic inclusions on the initiation of pitting corrosion in 11%Cr ferritic stainless steel examined by micro-droplet cell
[J].
Anisotropic cellular structure and texture microstructure of 316L stainless steel fabricated by selective laser melting via rotation scanning strategy
[J].
Influence of solidification conditions on chemical heterogeneities and dislocations patterning in additively manufactured 316L stainless steel
[J].
Improved corrosion performance of selective laser melted stainless steel 316L in the deep-sea environment
[J].
Oxide film thickening on the surface of metals in aqueous solutions: A critique of the theory of open-circuit potential transients
[J].
The transpassive dissolution mechanism of 316L stainless steel
[J],
Characterization of passive films on shape memory stainless steels
[J].
The nucleation and growth of two-dimensional anodic films under galvanostatic conditions
[J], J.
Electrochemical behaviour of passive film formed on the surface of Ti-6Al-4V alloys fabricated by electron beam melting
[J].
Passive behavior of a bulk nanostructured 316L austenitic stainless steel consisting of nanometer-sized grains with embedded nano-twin bundles
[J].
Passive film growth mechanism of nanocrystalline 304 stainless steel prepared by magnetron sputtering and deep rolling techniques
[J].
Effect of trace water in ammonia on breaking passive film of stainless steel during gas nitriding
[J].
Semiconducting properties of passive films formed on stainless steels: Influence of the alloying elements
[J].
The electronic properties of sputtered chromium and iron oxide films
[J].
On the effect of build orientation and residual stress on the corrosion of 316L stainless steel prepared by selective laser melting
[J].
Mott-Schottky Plot of the Passive Film Formed on Iron in Neutral Borate and Phosphate Solutions
[J].
Capacitance behaviour of passive films on ferritic and austenitic stainless steel
[J].
Influence of nanocrystallization on passive behavior of Ni-based superalloy in acidic solutions
[J].
Corrosion behaviour of the amorphous Mg65Y10Cu15Ag10 alloy
[J].
Stability of the bulk glass-forming Mg65Y10Cu25 alloy in aqueous electrolytes
[J].
Passivation characteristics of ultra-thin 316L foil in NaCl solutions
[J].Electrochemical behaviour and passive film characteristics of an ultra-thin 316L foil with a thickness of 20 μm in 3.5 wt.% NaCl solution were investigated using multiple techniques, focusing on the effect of microstructure, the applied potential, and the pH of the solution. The microstructure contains mainly fine grains (∼4 μm) with high-angle boundaries and preferential orientation of (220), and no MnS inclusion was detected. The electrochemical measurements show a significantly higher breakdown potential and lower passive current density for the 316L foil than traditional wrought 316L. The surface analyses using angle-resolved X-ray photoelectron spectroscopy (ARXPS) and time-of-flight secondary ion mass spectroscopy (TOF-SIMS) reveal that, compared to the wrought material, both the inner and out parts of the passive film on the 316L foil are more enriched in Cr- and Mo-oxides. The microstructure favourable for elemental diffusion and the absence of MnS inclusion facilitate the formation of a continuous compact Cr- and Mo-rich passive film, which effectively retards corrosion in NaCl solution and remains stable in acidic solution (pH 2) or at high polarised potential up to 600 mV vs Ag/AgCl.
Effect of Mo and Mn additions on the corrosion behaviour of AISI 304 and 316 stainless steels in H2SO4
[J].
Electrochemical and passive behaviour of tin alloyed ferritic stainless steel in concrete environment
[J].
Electrochemical corrosion and passive behavior of a new high-nitrogen austenitic stainless steel in chloride environment
[J].
Influence of pH on the passivation behavior of 254SMO stainless steel in 3.5%NaCl solution
[J].
Enhancing pitting corrosion resistance of severely cold-worked high nitrogen austenitic stainless steel by nitric acid passivation
[J].
Effect of nitrogen alloying on the semiconducting properties of passive films and metastable pitting susceptibility of 316L and 316LN stainless steels
[J].
Influence of micro-structure on corrosion behavior of a Ni-based superalloy in 3.5%NaCl
[J].