已有研究表明,渗铝剂中的铝源和活化剂对涂层的物相成分有较大的影响。铝源的Al含量越高,制备涂层时越容易生成脆性相。Rohr等[29]用纯Al粉作为Al源对P91钢渗铝,制备出厚度约为50 μm的Fe2Al5相涂层。而用活性较低的Cr5Al8合金粉作为Al源渗铝,可制备出厚度约5 μm的韧性FeAl相涂层。BATES等[30]分别用Cr-25Al和Cr-15Al粉作为铝源对T91钢渗铝,前者生成的涂层由Fe2Al5相和FeAl相组成,后者生成的涂层为FeAl相。KE等[31]分别用NH4Cl和AlCl3作为活化剂对P02钢渗铝,前者的涂层由Ni3Al相组成,后者的涂层由Ni2Al3、NiAl和Ni3Al相组成。这表明,为了制备韧性相涂层,需调控渗铝剂和活化剂并进行低活性渗铝。
1 实验方法
1.1 涂层的制备
实验用316L不锈钢基材的化学成分,列于表1。管状样品的直径为25 mm长度为80 mm,块状样品的尺寸为10 mm × 10 mm × 6 mm。依次用240#、400#、800#、1200#、2000#金相砂纸将其打磨,然后用丙酮和乙醇超声清洗5 min后晾干。
表1 316L不锈钢基材的化学成分(质量分数,%)
Table 1
| Element | Fe | Cr | Ni | Mo | Mn | Total |
|---|---|---|---|---|---|---|
| % | 68.93 | 17.21 | 10.30 | 2.33 | 1.23 | 100 |
为了制备韧性相涂层进行低活性渗铝,铝源为Fe-Al粉(70%Al,30%Fe),活化剂为NH4Cl粉。三种渗铝剂配比,列于表2,渗铝剂的组成为Fe-Al粉(铝源)、Al2O3 (惰性填充剂)和NH4Cl (活化剂)。将粉末渗铝剂混合均匀后,在60℃烘干3 h。
表2 三种渗铝剂配比组成(质量分数,%)
Table 2
| Fe-Al | Al2O3 | NH4Cl | |
|---|---|---|---|
| P1 | 65 | 32 | 3 |
| P2 | 75 | 22 | 3 |
| P3 | 85 | 12 | 3 |
在316L钢管内装入渗铝剂并放入间距约为2 cm的3个块状样品,将渗铝剂压实后放入密封的渗铝腔室中进行渗铝。渗铝过程:将渗铝腔室抽真空至< 3 × 10-3 Pa后充入氩气至大气压,加热至实验温度后保温。最佳渗铝工艺参数:渗铝温度为700、750和800℃,保温时间为3、6、9 h。
1.2 涂层的表征
使用型号为FEI APREO型扫描电子显微镜和能谱仪(EDS)观察涂层的表面形貌和截面形貌,以及相的化学成分。测试涂层XRD谱,以分析其物相组成,Cu Kα靶,特征波长λ = 0.154148 nm,扫描速度为1 (°)/min。
2 结果和讨论
2.1 渗铝剂对Fe-Al涂层组织结构的影响
为了制备FeAl韧性相涂层,选择低活性的FeAl粉作为铝源。采用表1中三种配比的渗铝剂在800℃保温渗铝6 h。
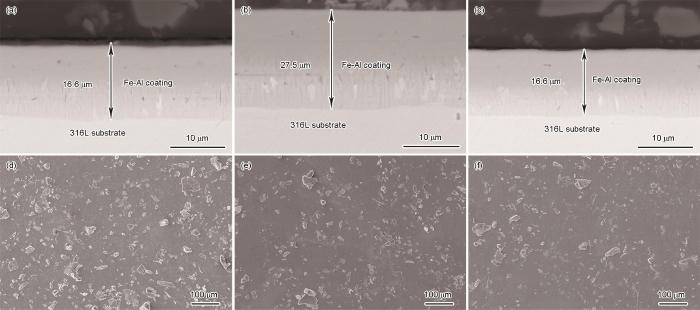
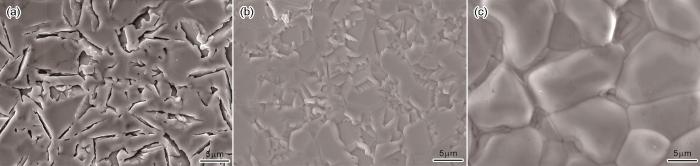
制备出的Fe-Al涂层的表面和截面形貌如图1所示。可以看出,用三种配比渗铝剂制备的涂层其厚度均匀且致密性良好,涂层的表面有渗剂粒子粘附或镶嵌。渗铝剂中FeAl粉的比例(质量分数,下同)分别为65%、75%和85%,制备出的Fe-Al涂层厚度分别为16.6 μm、27.5 μm和15.1 μm。渗铝剂中FeAl粉比例较低(65%)的涂层其厚度较小,因为铝源中FeAl粉的含量较低,与催化剂反应生成活性Al原子的速率较低,使膜层的生长速率较低;渗铝剂中FeAl粉的含量85%的涂层厚度最小,因为渗铝剂中的Al2O3分散剂较少且粉末的粘结严重。渗铝结束后含85%FeAl粉的渗铝剂粘结严重,而使用另外两种渗铝剂则没有出现明显的粘结。总之,渗铝剂配比对涂层的生长速率有重要的影响。
图1
图1
不同渗铝剂配比的Fe-Al涂层的表面和截面形貌
Fig.1
Surface and cross-sectional morphology of Fe-Al coatings prepared with different aluminizing agent ratios (a, e) P1; (b, d) P2; (c, f) P3
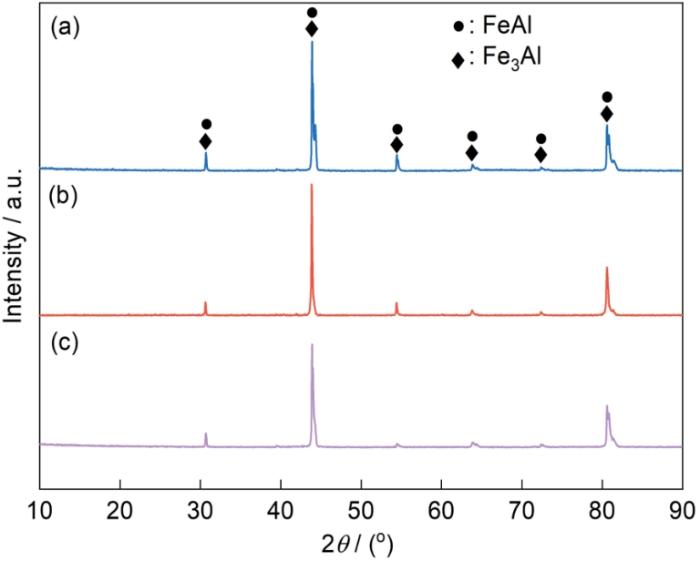
图2
图2
不同渗铝剂配比的Fe-Al涂层的XRD谱
Fig.2
XRD spectra of Fe-Al coatings prepared with different ratios of aluminizing agents
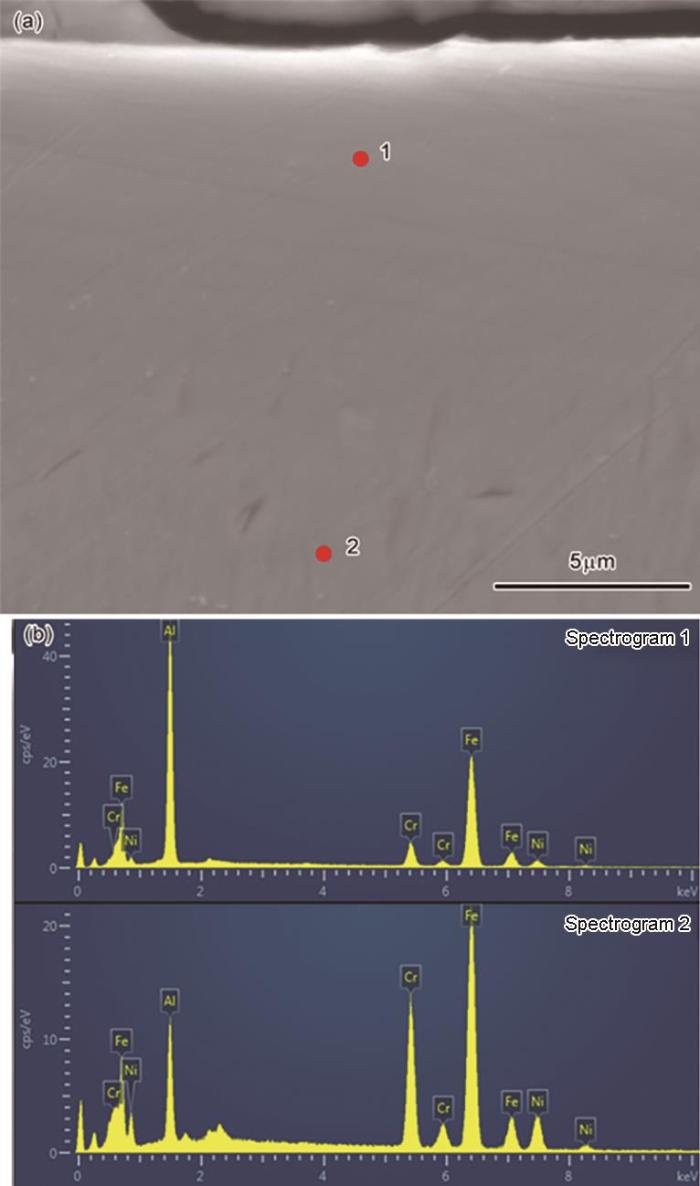
图3
表3 谱1和谱2的EDS结果
Table 3
| Spectrogram | Al | Cr | Fe | Ni |
|---|---|---|---|---|
| 1 | 35.18 | 7.01 | 53.38 | 4.44 |
| 2 | 10.31 | 21.22 | 55.64 | 12.83 |
综上所述,当渗铝剂中Fe-Al粉的含量为75%,惰性填充剂Al2O3粉的含量为22%,催化剂NH4Cl粉的含量为3%时,涂层的生长效率最高,制备出的涂层为韧性相FeAl和Fe3Al相,涂层均匀平整且致密性良好。鉴于此,后续实验采用这种渗铝剂配比。
2.2 渗铝温度对Fe-Al涂层组织结构的影响
渗铝温度是影响涂层生长的重要因素[35]。本文分别在700、750、800℃保温渗铝6 h制备涂层,然后分析涂层的微观组织结构,以找出最佳的渗铝温度范围。
图4
图4
在不同温度渗铝的Fe-Al涂层的表面形貌
Fig.4
SEM images of surface morphology of Fe-Al coatings prepared at different aluminizing temperatures (a) 700oC; (b) 750oC; (c) 800oC
图5给出了平面试样在不同温度渗铝后的Fe-Al涂层截面形貌和能谱分析结果。可以看出,分别在三个温度渗铝的Fe-Al涂层都连续均匀的覆盖在基体表面,涂层与基体之间的界面清晰,没有孔洞,涂层与基体形成了良好的冶金结合。
图5
图5
在不同温度渗铝6 h的Fe-Al涂层的截面形貌BSE图片和能谱分析
Fig.5
BSE pictures and energy spectrum analysis results of the cross-sectional morphology of Fe-Al coatings aluminized at different aluminizing temperatures for 6 h (a, d) 700oC; (b, e) 750oC; (c, f) 800oC
截面BSE结果表明,在三种温度制备的涂层均为双层结构,两层的元素分布有明显的不同,但是两层间没有明显的界面。能谱分析结果表明,涂层的表层是富铝层,由Fe和Al元素和少量Cr元素组成;内层为元素扩散层,Al元素含量逐渐降低到0,Fe元素的含量呈现逐渐提高,Cr、Ni元素局部富集。Cr、Ni元素局部富集的原因,可能是与Al生成了金属间化合物相[20,36],本文实验中部分样品的XRD谱中检测到铬铝化合物相,Pm3m (221)结构的NiAl相与FeAl相的衍射峰极为接近,无法区分。Al元素的含量降低到0而Fe的含量提高到稳定值的位置,定义为Fe-Al涂层与基体的分界线。可以确定,在700℃、750℃、800℃渗铝的Fe-Al涂层其厚度分别为4.4 μm、18.8 μm和27.5 μm。富铝层的厚度分别为2.4 μm和8.1 μm、13.1 μm,扩散层的厚度分别为2.0 μm、10.7 μm和14.4 μm。在700℃渗铝原子的扩散速度较低,涂层较薄但是均匀致密。随着渗铝温度的提高活性Al原子的沉积速率和原子扩散速率随之提高,涂层的富Al层和扩散层的厚度都增加。在750℃和800℃渗铝的Fe-Al涂层其扩散层内出现了弥散分布的孔洞。能谱分析结果表明,在涂层的生长过程中,沉积在基体表面的活性Al元素与基体元素产生了浓度差,基体中的Fe、Cr等元素向外扩散而Al元素向内扩散,扩散层中的Fe元素扩散速率高且其原子半径大,导致局部元素流失形成孔洞。总之,渗铝温度对Al原子的沉积和Fe、Al元素的互扩散有重要的影响,对涂层的生长速率和微观组织结构也有较大的影响。
从图6可以看出,在700~800℃渗铝的Fe-Al涂层主要由FeAl相和Fe3Al相组成,表明在此温度区间渗铝对物相组成的影响不大。
图6
图6
不同温度渗铝Fe-Al涂层的XRD谱
Fig.6
XRD spectrum of Fe-Al coatings prepared at different aluminizing temperatures
在包埋渗铝过程中,活性铝原子沉积在基体表面,然后与基体中的原子互扩散,扩散系数反应了渗铝过程中原子的扩散速度。从图5可见,随着渗铝温度的提高Fe-Al涂层的厚度增加。由于扩散的深度远小于试样的厚度,引入半无限固体扩散模型。在不同渗铝温度Al原子的扩散系数D与扩散物质的体积浓度等参数的关系为
初始条件:t = 0时 x ≥ 0,C=C0;
边界条件:t > 0时 x = 0,C=Cs;x=∞,C=C0。
式中C为扩散物质的体积浓度(原子数/m3或kg/m3);Cs为表面渗铝气氛所控制的铝原子浓度;C0为固体表面的原始含铝量;t为扩散时间;x为扩散距离(cm)。
若初始条件C0=0,则得
在已知x的情况下C和Cs为一个定值,则得
式中x为扩散距离,近似为Fe-Al涂层扩散层厚度;K为常数(取1)。
表4 不同温度的Al原子扩散系数(D)
Table 4
| Temperature / oC | 700 | 750 | 800 |
|---|---|---|---|
| Diffusion coefficient / m2·s-1 | 4.63 × 10-17 | 7.60 × 10-16 | 2.40 × 10-15 |
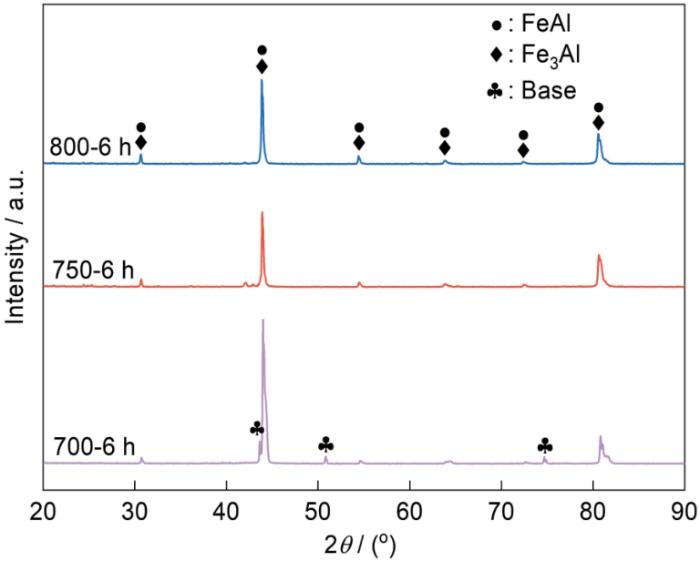
2.3 渗铝时间对Fe-Al涂层组织结构的影响
图7
图7
渗铝不同时间Fe-Al涂层的表面形貌
Fig.7
SEM images of surface morphology of Fe-Al coatings prepared at different aluminizing times (a) 3 h; (b) 6 h; (c) 9 h
图8给出了在316L不锈钢平面试样渗铝不同时间的Fe-Al涂层的截面形貌和能谱分析。可以看到,在800℃渗铝不同时间的涂层与316L基体的界面结合紧密、没有孔洞。随着渗铝时间的增加Al和Fe原子之间扩散更充分,Fe-Al涂层的厚度增加。从图8的涂层截面形貌和EDS线扫结果可以看出,渗铝3、6、9 h的Fe-Al涂层其厚度分别为19.5 μm、27.5 μm和46.4 μm,富铝层分别为9.7、13.1、20.0 μm,扩散层分别为9.8、14.4、26.4 μm,扩散层与富铝层厚度比逐渐增大,分别为1.01、1.07、1.32。同时,随着渗铝时间的增加富Al层与扩散层界面区域的孔洞数量增多,孔洞的尺寸约为1~2 μm。涂层的生长过程,是界面处Al原子向基体内部扩散、基体元素向涂层表面扩散和界面向基体方向移动的过程。这表明,元素扩散层中孔洞的形成,是元素扩散使界面处空位缺陷增多和合并的结果。
图8
图8
在800℃渗铝不同时间Fe-Al涂层的截面形貌的BSE图片和能谱分析
Fig.8
BSE pictures and energy spectrum analysis results of the cross-sectional morphology of Fe-Al coatings aluminied at 800oC for different times (a, d) 3 h; (b, e) 6 h; (c, f) 9 h
从图9可见,渗铝3 h和6 h的Fe-Al涂层其物相由FeAl和Fe3Al相组成,渗铝9 h的Fe-Al涂层中生成了大量的Al8Cr5相。渗铝时间的延长使渗铝反应更充分,Cr元素在扩散层中浓度梯度的提高使扩散到富Al层的Cr增加,与Al原子发生反应生成了大量的Al8Cr5相。
图9
图9
不同渗铝时间Fe-Al涂层的XRD谱
Fig.9
XRD spectrum of Fe-Al coatings aluminized for different times
2.4 块样和管内壁表面Fe-Al涂层的生长
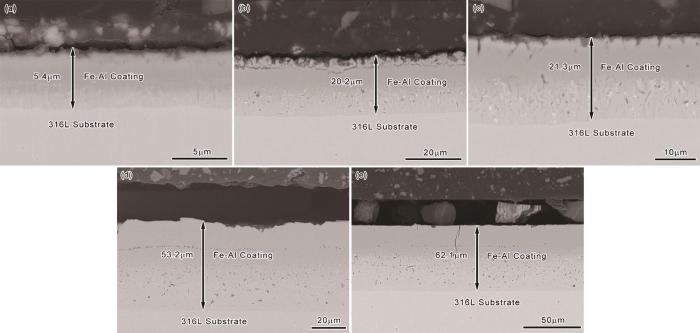
图10
图10
在不同温度渗铝不同时间316L管内壁的Fe-Al涂层的截面形貌的BSE图片
Fig.10
BSE images of the cross-sectional morphology of Fe-Al coatings aluminized on the inner wall of 316L tubes at different temperature for different time (a) 700oC/6 h; (b) 750oC/6 h; (c) 800oC/3 h; (d) 800oC/6 h; (e) 800oC/9 h
图11
图11
316L不锈钢平面试样和管试样Fe-Al涂层的厚度与温度和时间的拟合
Fig.11
Fitted plots of Fe-Al coating thickness and temperature (a) and time (b) for 316L stainless steel planar sample and tube sample
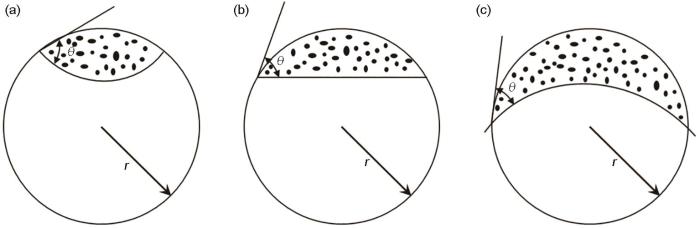
图12
图12
在不同形状的固体杂质表面形核的晶核体积
Fig.12
Nucleation volume at solid impurity surface of different shape (a) concave surface; (b) planar surface; (c) convex surface
3 结论
(1) 渗铝剂配比对涂层的生长速率有重要影响,在700~800℃渗铝Fe-Al粉含量为75%的涂层其生长率最高,且均匀平整、致密性良好。
(2) 在700~800℃渗铝的Fe-Al涂层均匀、连续、与基体形成冶金结合。涂层的外层是FeAl韧性富铝相;内层是Fe3Al相元素扩散层。随着渗铝温度的提高Al原子的扩散性增强,渗铝6h的涂层其厚度从4.4 μm增加到27.5 μm。
(3) 在800℃渗铝3 h-9 h,涂层的厚度明显增加。元素扩散使富Al层与扩散层界面的空位缺陷增多并合并形成微孔洞(尺寸约1~2 μm),随着渗铝时间的延长微孔洞数量增多。
(4) 在700~800℃渗铝316L不锈钢管内壁表面的Fe-Al涂层与基体界面结合紧密无孔洞。在曲面上形核的临界晶核体积小于在平面形核的临界晶核体积,在曲面形核的效能高,形核更快。在渗铝工艺参数相同的条件下管内壁表面的涂层其厚度是平面样品表面涂层厚度的1.1~2.1倍。
参考文献
Materials challenges in nuclear energy
[J].
Potential contribution of fusion power generation to low-carbon development under the Paris Agreement and associated uncertainties
[J].
Preparation technologies and performance studies of tritium permeation barriers for future nuclear fusion reactors
[J].
Tritium permeation behavior through pyrolytic carbon in tritium production using high-temperature gas-cooled reactor for fusion reactors
[J].
Current research and development activities on tritium permeation barriers for fusion reactors in China
[J].
我国聚变堆结构材料表面阻氚涂层的研究进展
[J].阻氚涂层是聚变堆实现氚自持及氚安全的关键科学与技术问题之一。我国通过国家磁约束聚变能发展研究专项依托国内优势单位部署了阻氚涂层基础问题及工程化技术研发工作。本文介绍了国内外聚变堆结构材料表面阻氚涂层研究进展,重点评述了近几年我国在阻氚涂层的材料选择、制备技术及阻滞氢渗透机制三个科学技术问题的研究进展,提出今后的研究方向。目前我国阻氚涂层材料类型以氧化物涂层为主,涂层制备工艺技术在不断优化和更新。Al<sub>2</sub>O<sub>3</sub>/FeAl阻氚涂层的电化学沉积铝(ECA)、粉末包埋渗铝(PC)及热浸铝(HDA)等方法的工艺处理规模及涂层阻氚性能在国际上均相对领先。发展了研究阻氚涂层阻滞氢渗透作用机理的方法,将通常基于Fick定律的表象研究方法向原子级方法前推了一步。未来需在考虑涂层制备工艺与基体材料成分、性能的关系及其在复杂形状结构件的适用性基础上,开发长寿命、高阻氚性能的阻氚涂层材料及制备工艺。
Crystallization and deuterium permeation behaviors of yttrium oxide coating prepared by metal organic decomposition
[J].
Deuterium permeation behavior of tritium permeation barrier coating containing carbide nanoparticles
[J].
Tritium permeation characterization of Al2O3/FeAl coatings as tritium permeation barriers on 321 type stainless steel containers
[J].
Design status and development strategy of China liquid lithium-lead blankets and related material technology
[J].
Grazing X-ray diffraction of surface oxide films on Fe-Al/ Al2O3 composite coating
[J].
Fe-Al/ Al2O3涂层表面氧化膜的掠入射X射线衍射研究
[J].
Experimental investigation of alumina coating as tritium permeation barrier for molten salt nuclear reactors
[J].
Review on preparation techniques of FeAl/Al2O3 composite tritium permeation barriers
[J].
Effect of ce on microstruvture and properties of hot dipaluminized tritium permeation barrier
[J].
Preparation and properties of FeAl/Al2O3 composite tritium permeation barrier coating on surface of 316L stainless steel
[J].
Study on pack cementation process for preparation of low activity pack aluminizing layer on RAFM steel
[J].
RAFM钢表面粉末包埋法制备低活性渗铝层工艺研究
[J].
Microstructure, growth kinetics and mechanical properties of interface layer for roll bonded aluminum-steel clad sheet annealed under argon gas protection
[J].
Review of preparation and corrosion resistance of Fe-Al coatings in molten salt
[J].
Fe-Al涂层制备及耐熔盐腐蚀性研究
[J].
Sol-gel prepared Al2O3 coatings for the application as tritium permeation barrier
[J].
Pack aluminizing process and characterization of aluminizen layer on stainless steels
[J].
不锈钢表面粉末包埋渗铝过程及渗铝层表征
[J].采用固体粉末包埋法对00Cr17Ni14Mo2和1Cr18Ni9Ti 不锈钢进行渗铝,形成富铝表层.阐述了不锈钢表面渗铝过程,并且对不锈钢渗铝层的成分、结构和形貌进行了表征.分析了合金中Ni元素对渗铝过程的影响.结果表明:渗铝层呈多层结构,渗层与基体及层间结合良好,界限明显、齐整.渗层组织主要由FeAl相组成,并含一定量的Ni3Al相.
High-temperature corrosion resistance of composite coatings prepared by microarc oxidation combined with pack cementation aluminum
[J].
微弧氧化及包埋渗铝法制备的复合涂层高温抗蚀性能
[J].采用包埋渗铝技术在C103铌合金基体上制备Al/C103,通过微弧氧化(MAO)处理获得Al<sub>2</sub>O<sub>3</sub>陶瓷膜外层。利用X射线衍射仪(XRD)和配有能谱仪(EDS)的扫描电镜(SEM),分析复合涂层高温腐蚀前后的成分和组织结构,并研究其高温氧化和热腐蚀行为与机理。结果表明:包埋渗铝处理的Al/C103经1000℃氧化10h后增重为6.98mg/cm<sup>2</sup>,微弧氧化结合包埋渗铝制备的MAO/Al/C103增重为2.89mg/cm<sup>2</sup>;氧化20h后,MAO/Al/C103增重为57.52mg/cm<sup>2</sup>,高于Al/C103的28.08mg/cm<sup>2</sup>。在900℃熔融混合盐(75% Na<sub>2</sub>SO<sub>4</sub>和25% NaCl,质量分数)中腐蚀50h后,Al/C103和MAO/Al/C103的增重分别为70.54,55.71mg/cm<sup>2</sup>,表面生成了Al<sub>2</sub>O<sub>3</sub>和钙钛矿结构NaNbO<sub>3</sub>相;部分NaNbO<sub>3</sub>堵塞MAO微孔,阻碍熔盐向内扩散,MAO/Al/C103试样表现出较优的抗热腐蚀性。
Formation of aluminide coatings on low alloy steels at 650 degrees C by pack cementation process
[J].
High temperature oxidation behavior of molybdenum borides by silicon pack cementation process
[J].
Microstructural investigation of the coatings prepared by simultsneous aluminizing and siliconizing process on gamma-TiAl
[J].
Corrosion resistance and application of martensitic stainless steels with an external Cr-N coating layer formed by pack cementation process
[J].
Deposition of Fe-Al intermetallic coatings on solid oxide fuel cell (SOFC) interconnects by pack cementation
[J].
Study of an iron-aluminide and alumina tritium barrier coating
[J].
A study of pack aluminizing technology on the surface of GCr15 steel
[J].
GCr15钢表面粉末包埋渗铝工艺研究
[J].
Development of novel diffusion coatings for 9-12%Cr ferritic-martensitic steels
[J].
Formation and oxidation performance of low-temperature pack aluminide coatings on ferritic-martensitic steels
[J].
On the selection of halide activators for the formation of hybrid Ni-aluminide/Ni coatings on creep resistant ferritic steels by low temperature pack cementation process
[J].
Hydrogen transport and solubility in 316L and 1.4914 steels for fusion reactor applications
[J].
Tritium permeation barriers for fusion technology
[J].
The pack-cementation process of iron-aluminide coating on china low activation martensitic and 316L austenitic stainless steel
[J].
Research progress of pack cementation aluminizing
[J].
粉末包埋渗铝研究进展
[J].
Formation of Al2O3/Fe-Al layers on SS 316 surface by pack aluminizing and heat treatment
[J].
Preparation method and diffusion mechanism of Fe-Al coating on Q235 low carbon steel by pack aluminizing
[J].The Fe-Al coating, with compactness, stiffness, and continuity, could be prepared on Q235 low carbon steel by pack aluminizing. The phase structure, morphology, composition, and hardness of the prepared coating were characterized by XRD, SEM, EDS, and micro-hardness tester respectively. Results indicate that the Fe-Al coating is composed of Fe2Al5 and FeAl3 phases, whilst, the coating fabricated at 750℃ is particularly rich in Fe2Al5 phase. With the rising temperature, the thickness of Fe-Al coating increases, whereas the micro-hardness decreases. As a result of aluminizing for different time, the formed coatings are composed of the two phases Fe2Al5 and FeAl3 as well. However, with the increasing aluminizing time, the content of FeAl3 phase decreases, while the micro-hardness of the coating decreases slightly. Finally, a diffusion mechanism related with the formation of Fe-Al coating is proposed based on the comprehensive analysis on the thermodynamics and kinetics of pack aluminizing process.
Structure-property correlation in Al-diffusion coated steels
[J].
Low temperature aluminising of Fe-Cr-Ni super alloy by pack cementation
[J].
The effects of processing time on the microstructure and composition of plasma pack-aluminized and oxidized surface layers on low carbon steel
[J].
Low temperature aluminisation of alloy steels by pack cementation process
[J].
Process and high-temperature oxidation resistance of pack-aluminized layers on cast iron
[J].