Synthesis and Li-storage performance of hierarchical spheroid composites of MgFe2O4/C
1
2018
... 锂离子电池(LIBs)具有能量密度高、无记忆效应和工作温度范围宽等优点,已广泛应用在便携式电子设备和新能源汽车等领域[1].目前应用的石墨类负极材料其理论比容量较低(372 mAh·g-1),不能满足新一代锂离子电池的需求[2~4].过渡金属氧化物(Mx O y,M=Fe、Ni、Mn、Cu等)有较高的理论比容量(600~1200 mAh·g-1)、低成本和环境友好等优点[5,6].与过渡金属氧化物相比,过渡金属氢氧化物的制备成本更低,在储锂反应过程中生成的LiOH在低电位下还可与Li进一步可逆生成Li2O和LiH,提供更高的理论比容量,极有应用前景[7~9].例如,Ni(OH)2的理论储锂比容量高达1735 mAh·g-1,约为NiO理论比容量(718 mAh·g-1)的2倍[10].但是,过渡金属氢氧化物在充放电过程中的动力学缓慢和显著的体积效应使其倍率性能和循环性能降低[11].将电极材料纳米化可缩短Li+在材料中的扩散路径、提高材料的比表面积和缓解材料的体积效应,进而改善电极材料的动力学性能和循环稳定性[12].在各种纳米结构材料中,3D微/纳分级结构(由低维纳米结构单元构成的微米级二次结构)材料具有纳米材料优势的同时还具有协同效应(即有效避免低维纳米材料的自团聚、提高振实密度)[13,14].Lim等[9]制备的花状微/纳分级结构Ni(OH)2Cl,用作锂离子电池负极材料,在0.2 A·g-1电流密度下经过150次循环其比容量为1236 mAh·g-1;Inamdar等[15]以Cu箔为基体用表面化学氧化法制备的由Cu(OH)2纳米棒构成的3D微/纳分级网状结构材料,在0.1 A·g-1电流密度下可逆比容量高达1472 mAh·g-1,在0.2 A·g-1电流密度下循环100次后比容量保持为506 mAh·g-1. ...
分级结构类球形MgFe2O4/C复合材料的制备及其储锂性能
1
2018
... 锂离子电池(LIBs)具有能量密度高、无记忆效应和工作温度范围宽等优点,已广泛应用在便携式电子设备和新能源汽车等领域[1].目前应用的石墨类负极材料其理论比容量较低(372 mAh·g-1),不能满足新一代锂离子电池的需求[2~4].过渡金属氧化物(Mx O y,M=Fe、Ni、Mn、Cu等)有较高的理论比容量(600~1200 mAh·g-1)、低成本和环境友好等优点[5,6].与过渡金属氧化物相比,过渡金属氢氧化物的制备成本更低,在储锂反应过程中生成的LiOH在低电位下还可与Li进一步可逆生成Li2O和LiH,提供更高的理论比容量,极有应用前景[7~9].例如,Ni(OH)2的理论储锂比容量高达1735 mAh·g-1,约为NiO理论比容量(718 mAh·g-1)的2倍[10].但是,过渡金属氢氧化物在充放电过程中的动力学缓慢和显著的体积效应使其倍率性能和循环性能降低[11].将电极材料纳米化可缩短Li+在材料中的扩散路径、提高材料的比表面积和缓解材料的体积效应,进而改善电极材料的动力学性能和循环稳定性[12].在各种纳米结构材料中,3D微/纳分级结构(由低维纳米结构单元构成的微米级二次结构)材料具有纳米材料优势的同时还具有协同效应(即有效避免低维纳米材料的自团聚、提高振实密度)[13,14].Lim等[9]制备的花状微/纳分级结构Ni(OH)2Cl,用作锂离子电池负极材料,在0.2 A·g-1电流密度下经过150次循环其比容量为1236 mAh·g-1;Inamdar等[15]以Cu箔为基体用表面化学氧化法制备的由Cu(OH)2纳米棒构成的3D微/纳分级网状结构材料,在0.1 A·g-1电流密度下可逆比容量高达1472 mAh·g-1,在0.2 A·g-1电流密度下循环100次后比容量保持为506 mAh·g-1. ...
One step hydrothermal preparation of SnO2@C composite and its lithium storage performance
1
2020
... 锂离子电池(LIBs)具有能量密度高、无记忆效应和工作温度范围宽等优点,已广泛应用在便携式电子设备和新能源汽车等领域[1].目前应用的石墨类负极材料其理论比容量较低(372 mAh·g-1),不能满足新一代锂离子电池的需求[2~4].过渡金属氧化物(Mx O y,M=Fe、Ni、Mn、Cu等)有较高的理论比容量(600~1200 mAh·g-1)、低成本和环境友好等优点[5,6].与过渡金属氧化物相比,过渡金属氢氧化物的制备成本更低,在储锂反应过程中生成的LiOH在低电位下还可与Li进一步可逆生成Li2O和LiH,提供更高的理论比容量,极有应用前景[7~9].例如,Ni(OH)2的理论储锂比容量高达1735 mAh·g-1,约为NiO理论比容量(718 mAh·g-1)的2倍[10].但是,过渡金属氢氧化物在充放电过程中的动力学缓慢和显著的体积效应使其倍率性能和循环性能降低[11].将电极材料纳米化可缩短Li+在材料中的扩散路径、提高材料的比表面积和缓解材料的体积效应,进而改善电极材料的动力学性能和循环稳定性[12].在各种纳米结构材料中,3D微/纳分级结构(由低维纳米结构单元构成的微米级二次结构)材料具有纳米材料优势的同时还具有协同效应(即有效避免低维纳米材料的自团聚、提高振实密度)[13,14].Lim等[9]制备的花状微/纳分级结构Ni(OH)2Cl,用作锂离子电池负极材料,在0.2 A·g-1电流密度下经过150次循环其比容量为1236 mAh·g-1;Inamdar等[15]以Cu箔为基体用表面化学氧化法制备的由Cu(OH)2纳米棒构成的3D微/纳分级网状结构材料,在0.1 A·g-1电流密度下可逆比容量高达1472 mAh·g-1,在0.2 A·g-1电流密度下循环100次后比容量保持为506 mAh·g-1. ...
一步水热法制备纳米SnO2@C复合材料及其储锂性能研究
1
2020
... 锂离子电池(LIBs)具有能量密度高、无记忆效应和工作温度范围宽等优点,已广泛应用在便携式电子设备和新能源汽车等领域[1].目前应用的石墨类负极材料其理论比容量较低(372 mAh·g-1),不能满足新一代锂离子电池的需求[2~4].过渡金属氧化物(Mx O y,M=Fe、Ni、Mn、Cu等)有较高的理论比容量(600~1200 mAh·g-1)、低成本和环境友好等优点[5,6].与过渡金属氧化物相比,过渡金属氢氧化物的制备成本更低,在储锂反应过程中生成的LiOH在低电位下还可与Li进一步可逆生成Li2O和LiH,提供更高的理论比容量,极有应用前景[7~9].例如,Ni(OH)2的理论储锂比容量高达1735 mAh·g-1,约为NiO理论比容量(718 mAh·g-1)的2倍[10].但是,过渡金属氢氧化物在充放电过程中的动力学缓慢和显著的体积效应使其倍率性能和循环性能降低[11].将电极材料纳米化可缩短Li+在材料中的扩散路径、提高材料的比表面积和缓解材料的体积效应,进而改善电极材料的动力学性能和循环稳定性[12].在各种纳米结构材料中,3D微/纳分级结构(由低维纳米结构单元构成的微米级二次结构)材料具有纳米材料优势的同时还具有协同效应(即有效避免低维纳米材料的自团聚、提高振实密度)[13,14].Lim等[9]制备的花状微/纳分级结构Ni(OH)2Cl,用作锂离子电池负极材料,在0.2 A·g-1电流密度下经过150次循环其比容量为1236 mAh·g-1;Inamdar等[15]以Cu箔为基体用表面化学氧化法制备的由Cu(OH)2纳米棒构成的3D微/纳分级网状结构材料,在0.1 A·g-1电流密度下可逆比容量高达1472 mAh·g-1,在0.2 A·g-1电流密度下循环100次后比容量保持为506 mAh·g-1. ...
Advanced electrode materials in lithium batteries: Retrospect and prospect
0
2021
Regulating bifunctional flower-like NiFe2O4/graphene for green EMI shielding and lithium ion storage
1
2022
... 锂离子电池(LIBs)具有能量密度高、无记忆效应和工作温度范围宽等优点,已广泛应用在便携式电子设备和新能源汽车等领域[1].目前应用的石墨类负极材料其理论比容量较低(372 mAh·g-1),不能满足新一代锂离子电池的需求[2~4].过渡金属氧化物(Mx O y,M=Fe、Ni、Mn、Cu等)有较高的理论比容量(600~1200 mAh·g-1)、低成本和环境友好等优点[5,6].与过渡金属氧化物相比,过渡金属氢氧化物的制备成本更低,在储锂反应过程中生成的LiOH在低电位下还可与Li进一步可逆生成Li2O和LiH,提供更高的理论比容量,极有应用前景[7~9].例如,Ni(OH)2的理论储锂比容量高达1735 mAh·g-1,约为NiO理论比容量(718 mAh·g-1)的2倍[10].但是,过渡金属氢氧化物在充放电过程中的动力学缓慢和显著的体积效应使其倍率性能和循环性能降低[11].将电极材料纳米化可缩短Li+在材料中的扩散路径、提高材料的比表面积和缓解材料的体积效应,进而改善电极材料的动力学性能和循环稳定性[12].在各种纳米结构材料中,3D微/纳分级结构(由低维纳米结构单元构成的微米级二次结构)材料具有纳米材料优势的同时还具有协同效应(即有效避免低维纳米材料的自团聚、提高振实密度)[13,14].Lim等[9]制备的花状微/纳分级结构Ni(OH)2Cl,用作锂离子电池负极材料,在0.2 A·g-1电流密度下经过150次循环其比容量为1236 mAh·g-1;Inamdar等[15]以Cu箔为基体用表面化学氧化法制备的由Cu(OH)2纳米棒构成的3D微/纳分级网状结构材料,在0.1 A·g-1电流密度下可逆比容量高达1472 mAh·g-1,在0.2 A·g-1电流密度下循环100次后比容量保持为506 mAh·g-1. ...
Transition metal oxide anodes for electrochemical energy storage in lithium- and sodium-ion batteries
1
2020
... 锂离子电池(LIBs)具有能量密度高、无记忆效应和工作温度范围宽等优点,已广泛应用在便携式电子设备和新能源汽车等领域[1].目前应用的石墨类负极材料其理论比容量较低(372 mAh·g-1),不能满足新一代锂离子电池的需求[2~4].过渡金属氧化物(Mx O y,M=Fe、Ni、Mn、Cu等)有较高的理论比容量(600~1200 mAh·g-1)、低成本和环境友好等优点[5,6].与过渡金属氧化物相比,过渡金属氢氧化物的制备成本更低,在储锂反应过程中生成的LiOH在低电位下还可与Li进一步可逆生成Li2O和LiH,提供更高的理论比容量,极有应用前景[7~9].例如,Ni(OH)2的理论储锂比容量高达1735 mAh·g-1,约为NiO理论比容量(718 mAh·g-1)的2倍[10].但是,过渡金属氢氧化物在充放电过程中的动力学缓慢和显著的体积效应使其倍率性能和循环性能降低[11].将电极材料纳米化可缩短Li+在材料中的扩散路径、提高材料的比表面积和缓解材料的体积效应,进而改善电极材料的动力学性能和循环稳定性[12].在各种纳米结构材料中,3D微/纳分级结构(由低维纳米结构单元构成的微米级二次结构)材料具有纳米材料优势的同时还具有协同效应(即有效避免低维纳米材料的自团聚、提高振实密度)[13,14].Lim等[9]制备的花状微/纳分级结构Ni(OH)2Cl,用作锂离子电池负极材料,在0.2 A·g-1电流密度下经过150次循环其比容量为1236 mAh·g-1;Inamdar等[15]以Cu箔为基体用表面化学氧化法制备的由Cu(OH)2纳米棒构成的3D微/纳分级网状结构材料,在0.1 A·g-1电流密度下可逆比容量高达1472 mAh·g-1,在0.2 A·g-1电流密度下循环100次后比容量保持为506 mAh·g-1. ...
Hollow nanoparticle-assembled hierarchical NiCo2O4 nanofibers with enhanced electrochemical performance for lithium-ion batteries
1
2020
... 锂离子电池(LIBs)具有能量密度高、无记忆效应和工作温度范围宽等优点,已广泛应用在便携式电子设备和新能源汽车等领域[1].目前应用的石墨类负极材料其理论比容量较低(372 mAh·g-1),不能满足新一代锂离子电池的需求[2~4].过渡金属氧化物(Mx O y,M=Fe、Ni、Mn、Cu等)有较高的理论比容量(600~1200 mAh·g-1)、低成本和环境友好等优点[5,6].与过渡金属氧化物相比,过渡金属氢氧化物的制备成本更低,在储锂反应过程中生成的LiOH在低电位下还可与Li进一步可逆生成Li2O和LiH,提供更高的理论比容量,极有应用前景[7~9].例如,Ni(OH)2的理论储锂比容量高达1735 mAh·g-1,约为NiO理论比容量(718 mAh·g-1)的2倍[10].但是,过渡金属氢氧化物在充放电过程中的动力学缓慢和显著的体积效应使其倍率性能和循环性能降低[11].将电极材料纳米化可缩短Li+在材料中的扩散路径、提高材料的比表面积和缓解材料的体积效应,进而改善电极材料的动力学性能和循环稳定性[12].在各种纳米结构材料中,3D微/纳分级结构(由低维纳米结构单元构成的微米级二次结构)材料具有纳米材料优势的同时还具有协同效应(即有效避免低维纳米材料的自团聚、提高振实密度)[13,14].Lim等[9]制备的花状微/纳分级结构Ni(OH)2Cl,用作锂离子电池负极材料,在0.2 A·g-1电流密度下经过150次循环其比容量为1236 mAh·g-1;Inamdar等[15]以Cu箔为基体用表面化学氧化法制备的由Cu(OH)2纳米棒构成的3D微/纳分级网状结构材料,在0.1 A·g-1电流密度下可逆比容量高达1472 mAh·g-1,在0.2 A·g-1电流密度下循环100次后比容量保持为506 mAh·g-1. ...
Exceptional lithium storage in a Co(OH)2 Anode: Hydride formation
1
2018
... 锂离子电池(LIBs)具有能量密度高、无记忆效应和工作温度范围宽等优点,已广泛应用在便携式电子设备和新能源汽车等领域[1].目前应用的石墨类负极材料其理论比容量较低(372 mAh·g-1),不能满足新一代锂离子电池的需求[2~4].过渡金属氧化物(Mx O y,M=Fe、Ni、Mn、Cu等)有较高的理论比容量(600~1200 mAh·g-1)、低成本和环境友好等优点[5,6].与过渡金属氧化物相比,过渡金属氢氧化物的制备成本更低,在储锂反应过程中生成的LiOH在低电位下还可与Li进一步可逆生成Li2O和LiH,提供更高的理论比容量,极有应用前景[7~9].例如,Ni(OH)2的理论储锂比容量高达1735 mAh·g-1,约为NiO理论比容量(718 mAh·g-1)的2倍[10].但是,过渡金属氢氧化物在充放电过程中的动力学缓慢和显著的体积效应使其倍率性能和循环性能降低[11].将电极材料纳米化可缩短Li+在材料中的扩散路径、提高材料的比表面积和缓解材料的体积效应,进而改善电极材料的动力学性能和循环稳定性[12].在各种纳米结构材料中,3D微/纳分级结构(由低维纳米结构单元构成的微米级二次结构)材料具有纳米材料优势的同时还具有协同效应(即有效避免低维纳米材料的自团聚、提高振实密度)[13,14].Lim等[9]制备的花状微/纳分级结构Ni(OH)2Cl,用作锂离子电池负极材料,在0.2 A·g-1电流密度下经过150次循环其比容量为1236 mAh·g-1;Inamdar等[15]以Cu箔为基体用表面化学氧化法制备的由Cu(OH)2纳米棒构成的3D微/纳分级网状结构材料,在0.1 A·g-1电流密度下可逆比容量高达1472 mAh·g-1,在0.2 A·g-1电流密度下循环100次后比容量保持为506 mAh·g-1. ...
Origin of additional capacities in metal oxide lithium-ion battery electrodes
0
2013
Towards an efficient anode material for Li-ion batteries: understanding the conversion mechanism of nickel hydroxy chloride with Li-ions
3
2020
... 锂离子电池(LIBs)具有能量密度高、无记忆效应和工作温度范围宽等优点,已广泛应用在便携式电子设备和新能源汽车等领域[1].目前应用的石墨类负极材料其理论比容量较低(372 mAh·g-1),不能满足新一代锂离子电池的需求[2~4].过渡金属氧化物(Mx O y,M=Fe、Ni、Mn、Cu等)有较高的理论比容量(600~1200 mAh·g-1)、低成本和环境友好等优点[5,6].与过渡金属氧化物相比,过渡金属氢氧化物的制备成本更低,在储锂反应过程中生成的LiOH在低电位下还可与Li进一步可逆生成Li2O和LiH,提供更高的理论比容量,极有应用前景[7~9].例如,Ni(OH)2的理论储锂比容量高达1735 mAh·g-1,约为NiO理论比容量(718 mAh·g-1)的2倍[10].但是,过渡金属氢氧化物在充放电过程中的动力学缓慢和显著的体积效应使其倍率性能和循环性能降低[11].将电极材料纳米化可缩短Li+在材料中的扩散路径、提高材料的比表面积和缓解材料的体积效应,进而改善电极材料的动力学性能和循环稳定性[12].在各种纳米结构材料中,3D微/纳分级结构(由低维纳米结构单元构成的微米级二次结构)材料具有纳米材料优势的同时还具有协同效应(即有效避免低维纳米材料的自团聚、提高振实密度)[13,14].Lim等[9]制备的花状微/纳分级结构Ni(OH)2Cl,用作锂离子电池负极材料,在0.2 A·g-1电流密度下经过150次循环其比容量为1236 mAh·g-1;Inamdar等[15]以Cu箔为基体用表面化学氧化法制备的由Cu(OH)2纳米棒构成的3D微/纳分级网状结构材料,在0.1 A·g-1电流密度下可逆比容量高达1472 mAh·g-1,在0.2 A·g-1电流密度下循环100次后比容量保持为506 mAh·g-1. ...
... [9]制备的花状微/纳分级结构Ni(OH)2Cl,用作锂离子电池负极材料,在0.2 A·g-1电流密度下经过150次循环其比容量为1236 mAh·g-1;Inamdar等[15]以Cu箔为基体用表面化学氧化法制备的由Cu(OH)2纳米棒构成的3D微/纳分级网状结构材料,在0.1 A·g-1电流密度下可逆比容量高达1472 mAh·g-1,在0.2 A·g-1电流密度下循环100次后比容量保持为506 mAh·g-1. ...
... / V vs. Li/Li
+Reference | | NS-20 | 2.0 | 800 mAh·g-1 after 40 cycles | 0~3.0 | This work |
| α-Ni(OH)2 | 1.0 | 743 mAh·g-1 after 50 cycles | 0~3.0 | [35] |
| Co-Ni-LDH | 0.05 | 450.4 mAh·g-1 after 40 cycles | 0~3.0 | [36] |
| Ni-Co-LDH | 0.1 | 335.4 mAh·g-1 after 50 cycles | 0~3.0 | [37] |
| Ni(OH)2-CTAB | 0.5 | 952 mAh·g-1 after 25 cycles | 0~3.0 | [38] |
| Fe-Ni-LDH | 0.2 | 1080 mAh·g-1 after 30 cycles | 0~3.0 | [39] |
| Ni- Fe-OH | 0.85 | 540 mAh·g-1 after 50 cycles | 0~3.0 | [40] |
| Ni(OH)Cl | 0.2 | 1236 mAh·g-1 after 150 cycles | 0~3.0 | [9] |
图4a给出了NS-0、NS-10、NS-20和NS-30四个样品的倍率性能对比.可以看出,随着电流密度的增大样品的放电比容量逐渐降低.NS-0的倍率性能最差,在3 A·g-1电流密度下其比容量已降低至231 mAh·g-1.相比之下,SDS辅助制备的三个样品均表现出更好倍率性能;尤其是NS-20的倍率性能最佳,在3 A·g-1高电流密度下其比容量仍高达662 mAh·g-1,电流密度回到较低的0.5 A·g-1时其比容量恢复到980 mAh·g-1并保持良好的循环稳定性.图4b~d给出了NS-0、NS-20和NS-30三个样品在不同电流密度下的充放电曲线.可以看出,电流密度为0.2 A·g-1时三个样品的充放电平台均较为明显,随着电流密度的提高三个样品电极的充放电平台逐渐变短表明电极的极化逐渐变大.相比之下,NS-20样品的极化明显低于其他两个样品. ...
Crucial role of water content on the electrochemical performance of α-Ni(OH)2 as an anode material for lithium-ion batteries
1
2021
... 锂离子电池(LIBs)具有能量密度高、无记忆效应和工作温度范围宽等优点,已广泛应用在便携式电子设备和新能源汽车等领域[1].目前应用的石墨类负极材料其理论比容量较低(372 mAh·g-1),不能满足新一代锂离子电池的需求[2~4].过渡金属氧化物(Mx O y,M=Fe、Ni、Mn、Cu等)有较高的理论比容量(600~1200 mAh·g-1)、低成本和环境友好等优点[5,6].与过渡金属氧化物相比,过渡金属氢氧化物的制备成本更低,在储锂反应过程中生成的LiOH在低电位下还可与Li进一步可逆生成Li2O和LiH,提供更高的理论比容量,极有应用前景[7~9].例如,Ni(OH)2的理论储锂比容量高达1735 mAh·g-1,约为NiO理论比容量(718 mAh·g-1)的2倍[10].但是,过渡金属氢氧化物在充放电过程中的动力学缓慢和显著的体积效应使其倍率性能和循环性能降低[11].将电极材料纳米化可缩短Li+在材料中的扩散路径、提高材料的比表面积和缓解材料的体积效应,进而改善电极材料的动力学性能和循环稳定性[12].在各种纳米结构材料中,3D微/纳分级结构(由低维纳米结构单元构成的微米级二次结构)材料具有纳米材料优势的同时还具有协同效应(即有效避免低维纳米材料的自团聚、提高振实密度)[13,14].Lim等[9]制备的花状微/纳分级结构Ni(OH)2Cl,用作锂离子电池负极材料,在0.2 A·g-1电流密度下经过150次循环其比容量为1236 mAh·g-1;Inamdar等[15]以Cu箔为基体用表面化学氧化法制备的由Cu(OH)2纳米棒构成的3D微/纳分级网状结构材料,在0.1 A·g-1电流密度下可逆比容量高达1472 mAh·g-1,在0.2 A·g-1电流密度下循环100次后比容量保持为506 mAh·g-1. ...
Effect of Al substitution on the microstructure and lithium storage performance of nickel hydroxide
1
2016
... 锂离子电池(LIBs)具有能量密度高、无记忆效应和工作温度范围宽等优点,已广泛应用在便携式电子设备和新能源汽车等领域[1].目前应用的石墨类负极材料其理论比容量较低(372 mAh·g-1),不能满足新一代锂离子电池的需求[2~4].过渡金属氧化物(Mx O y,M=Fe、Ni、Mn、Cu等)有较高的理论比容量(600~1200 mAh·g-1)、低成本和环境友好等优点[5,6].与过渡金属氧化物相比,过渡金属氢氧化物的制备成本更低,在储锂反应过程中生成的LiOH在低电位下还可与Li进一步可逆生成Li2O和LiH,提供更高的理论比容量,极有应用前景[7~9].例如,Ni(OH)2的理论储锂比容量高达1735 mAh·g-1,约为NiO理论比容量(718 mAh·g-1)的2倍[10].但是,过渡金属氢氧化物在充放电过程中的动力学缓慢和显著的体积效应使其倍率性能和循环性能降低[11].将电极材料纳米化可缩短Li+在材料中的扩散路径、提高材料的比表面积和缓解材料的体积效应,进而改善电极材料的动力学性能和循环稳定性[12].在各种纳米结构材料中,3D微/纳分级结构(由低维纳米结构单元构成的微米级二次结构)材料具有纳米材料优势的同时还具有协同效应(即有效避免低维纳米材料的自团聚、提高振实密度)[13,14].Lim等[9]制备的花状微/纳分级结构Ni(OH)2Cl,用作锂离子电池负极材料,在0.2 A·g-1电流密度下经过150次循环其比容量为1236 mAh·g-1;Inamdar等[15]以Cu箔为基体用表面化学氧化法制备的由Cu(OH)2纳米棒构成的3D微/纳分级网状结构材料,在0.1 A·g-1电流密度下可逆比容量高达1472 mAh·g-1,在0.2 A·g-1电流密度下循环100次后比容量保持为506 mAh·g-1. ...
Hierarchical three-dimensional flower-like Co3O4 architectures with a mesocrystal structure as high capacity anode materials for long-lived lithium-ion batteries
1
2018
... 锂离子电池(LIBs)具有能量密度高、无记忆效应和工作温度范围宽等优点,已广泛应用在便携式电子设备和新能源汽车等领域[1].目前应用的石墨类负极材料其理论比容量较低(372 mAh·g-1),不能满足新一代锂离子电池的需求[2~4].过渡金属氧化物(Mx O y,M=Fe、Ni、Mn、Cu等)有较高的理论比容量(600~1200 mAh·g-1)、低成本和环境友好等优点[5,6].与过渡金属氧化物相比,过渡金属氢氧化物的制备成本更低,在储锂反应过程中生成的LiOH在低电位下还可与Li进一步可逆生成Li2O和LiH,提供更高的理论比容量,极有应用前景[7~9].例如,Ni(OH)2的理论储锂比容量高达1735 mAh·g-1,约为NiO理论比容量(718 mAh·g-1)的2倍[10].但是,过渡金属氢氧化物在充放电过程中的动力学缓慢和显著的体积效应使其倍率性能和循环性能降低[11].将电极材料纳米化可缩短Li+在材料中的扩散路径、提高材料的比表面积和缓解材料的体积效应,进而改善电极材料的动力学性能和循环稳定性[12].在各种纳米结构材料中,3D微/纳分级结构(由低维纳米结构单元构成的微米级二次结构)材料具有纳米材料优势的同时还具有协同效应(即有效避免低维纳米材料的自团聚、提高振实密度)[13,14].Lim等[9]制备的花状微/纳分级结构Ni(OH)2Cl,用作锂离子电池负极材料,在0.2 A·g-1电流密度下经过150次循环其比容量为1236 mAh·g-1;Inamdar等[15]以Cu箔为基体用表面化学氧化法制备的由Cu(OH)2纳米棒构成的3D微/纳分级网状结构材料,在0.1 A·g-1电流密度下可逆比容量高达1472 mAh·g-1,在0.2 A·g-1电流密度下循环100次后比容量保持为506 mAh·g-1. ...
Nanostructured anode materials for lithium ion batteries: Progress, challenge and perspective
1
2016
... 锂离子电池(LIBs)具有能量密度高、无记忆效应和工作温度范围宽等优点,已广泛应用在便携式电子设备和新能源汽车等领域[1].目前应用的石墨类负极材料其理论比容量较低(372 mAh·g-1),不能满足新一代锂离子电池的需求[2~4].过渡金属氧化物(Mx O y,M=Fe、Ni、Mn、Cu等)有较高的理论比容量(600~1200 mAh·g-1)、低成本和环境友好等优点[5,6].与过渡金属氧化物相比,过渡金属氢氧化物的制备成本更低,在储锂反应过程中生成的LiOH在低电位下还可与Li进一步可逆生成Li2O和LiH,提供更高的理论比容量,极有应用前景[7~9].例如,Ni(OH)2的理论储锂比容量高达1735 mAh·g-1,约为NiO理论比容量(718 mAh·g-1)的2倍[10].但是,过渡金属氢氧化物在充放电过程中的动力学缓慢和显著的体积效应使其倍率性能和循环性能降低[11].将电极材料纳米化可缩短Li+在材料中的扩散路径、提高材料的比表面积和缓解材料的体积效应,进而改善电极材料的动力学性能和循环稳定性[12].在各种纳米结构材料中,3D微/纳分级结构(由低维纳米结构单元构成的微米级二次结构)材料具有纳米材料优势的同时还具有协同效应(即有效避免低维纳米材料的自团聚、提高振实密度)[13,14].Lim等[9]制备的花状微/纳分级结构Ni(OH)2Cl,用作锂离子电池负极材料,在0.2 A·g-1电流密度下经过150次循环其比容量为1236 mAh·g-1;Inamdar等[15]以Cu箔为基体用表面化学氧化法制备的由Cu(OH)2纳米棒构成的3D微/纳分级网状结构材料,在0.1 A·g-1电流密度下可逆比容量高达1472 mAh·g-1,在0.2 A·g-1电流密度下循环100次后比容量保持为506 mAh·g-1. ...
Hierarchically nanostructured transition metal oxides for lithium-ion batteries
1
2018
... 锂离子电池(LIBs)具有能量密度高、无记忆效应和工作温度范围宽等优点,已广泛应用在便携式电子设备和新能源汽车等领域[1].目前应用的石墨类负极材料其理论比容量较低(372 mAh·g-1),不能满足新一代锂离子电池的需求[2~4].过渡金属氧化物(Mx O y,M=Fe、Ni、Mn、Cu等)有较高的理论比容量(600~1200 mAh·g-1)、低成本和环境友好等优点[5,6].与过渡金属氧化物相比,过渡金属氢氧化物的制备成本更低,在储锂反应过程中生成的LiOH在低电位下还可与Li进一步可逆生成Li2O和LiH,提供更高的理论比容量,极有应用前景[7~9].例如,Ni(OH)2的理论储锂比容量高达1735 mAh·g-1,约为NiO理论比容量(718 mAh·g-1)的2倍[10].但是,过渡金属氢氧化物在充放电过程中的动力学缓慢和显著的体积效应使其倍率性能和循环性能降低[11].将电极材料纳米化可缩短Li+在材料中的扩散路径、提高材料的比表面积和缓解材料的体积效应,进而改善电极材料的动力学性能和循环稳定性[12].在各种纳米结构材料中,3D微/纳分级结构(由低维纳米结构单元构成的微米级二次结构)材料具有纳米材料优势的同时还具有协同效应(即有效避免低维纳米材料的自团聚、提高振实密度)[13,14].Lim等[9]制备的花状微/纳分级结构Ni(OH)2Cl,用作锂离子电池负极材料,在0.2 A·g-1电流密度下经过150次循环其比容量为1236 mAh·g-1;Inamdar等[15]以Cu箔为基体用表面化学氧化法制备的由Cu(OH)2纳米棒构成的3D微/纳分级网状结构材料,在0.1 A·g-1电流密度下可逆比容量高达1472 mAh·g-1,在0.2 A·g-1电流密度下循环100次后比容量保持为506 mAh·g-1. ...
Macroporous Cu(OH)2 nanorod network fabricated directly on Cu foil as binder-free Lithium-ion battery anode with ultrahigh capacity
1
2020
... 锂离子电池(LIBs)具有能量密度高、无记忆效应和工作温度范围宽等优点,已广泛应用在便携式电子设备和新能源汽车等领域[1].目前应用的石墨类负极材料其理论比容量较低(372 mAh·g-1),不能满足新一代锂离子电池的需求[2~4].过渡金属氧化物(Mx O y,M=Fe、Ni、Mn、Cu等)有较高的理论比容量(600~1200 mAh·g-1)、低成本和环境友好等优点[5,6].与过渡金属氧化物相比,过渡金属氢氧化物的制备成本更低,在储锂反应过程中生成的LiOH在低电位下还可与Li进一步可逆生成Li2O和LiH,提供更高的理论比容量,极有应用前景[7~9].例如,Ni(OH)2的理论储锂比容量高达1735 mAh·g-1,约为NiO理论比容量(718 mAh·g-1)的2倍[10].但是,过渡金属氢氧化物在充放电过程中的动力学缓慢和显著的体积效应使其倍率性能和循环性能降低[11].将电极材料纳米化可缩短Li+在材料中的扩散路径、提高材料的比表面积和缓解材料的体积效应,进而改善电极材料的动力学性能和循环稳定性[12].在各种纳米结构材料中,3D微/纳分级结构(由低维纳米结构单元构成的微米级二次结构)材料具有纳米材料优势的同时还具有协同效应(即有效避免低维纳米材料的自团聚、提高振实密度)[13,14].Lim等[9]制备的花状微/纳分级结构Ni(OH)2Cl,用作锂离子电池负极材料,在0.2 A·g-1电流密度下经过150次循环其比容量为1236 mAh·g-1;Inamdar等[15]以Cu箔为基体用表面化学氧化法制备的由Cu(OH)2纳米棒构成的3D微/纳分级网状结构材料,在0.1 A·g-1电流密度下可逆比容量高达1472 mAh·g-1,在0.2 A·g-1电流密度下循环100次后比容量保持为506 mAh·g-1. ...
Stacking faults in the structure of nickel hydroxide: a rationale of its high electrochemical activity
1
1997
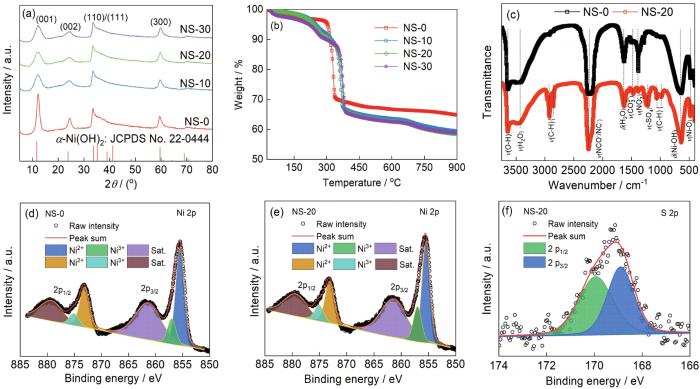
... 图1a给出了不同SDS用量样品的XRD谱.可以看出,四个样品的衍射峰均与α-Ni(OH)2 (JCPDS No. 22-0444)相符,没有出现杂质相的峰,表明制备出的样品都是α-Ni(OH)2.相对而言,无SDS辅助的NS-0样品其(001)衍射峰最强,说明其结晶性较好;而SDS辅助的3个样品其(001)衍射峰明显宽化且强度变低,表明样品的结晶性降低[16].利用Scherrer公式计算出样品的晶格常数和平均晶粒尺寸,结果列于表1.可以看出,随着SDS用量的增多样品的层间距由NS-0的0.7243 nm增大到NS-30的0.7338 nm,平均晶粒尺寸由5.6 nm降低到3.0 nm.这表明,SDS的加入影响了α-Ni(OH)2结晶和生长过程,在α-Ni(OH)2内引入了更多的结构缺陷、细化了晶粒并拓宽了层间距.电极材料中的结构缺陷可为锂离子存储提供更多的活性位点,并改善材料的电子/离子传输性能,因此有利于提升材料的储锂活性和反应动力学[17]. ...
Defect engineering on electrode materials for rechargeable batteries
1
2020
... 图1a给出了不同SDS用量样品的XRD谱.可以看出,四个样品的衍射峰均与α-Ni(OH)2 (JCPDS No. 22-0444)相符,没有出现杂质相的峰,表明制备出的样品都是α-Ni(OH)2.相对而言,无SDS辅助的NS-0样品其(001)衍射峰最强,说明其结晶性较好;而SDS辅助的3个样品其(001)衍射峰明显宽化且强度变低,表明样品的结晶性降低[16].利用Scherrer公式计算出样品的晶格常数和平均晶粒尺寸,结果列于表1.可以看出,随着SDS用量的增多样品的层间距由NS-0的0.7243 nm增大到NS-30的0.7338 nm,平均晶粒尺寸由5.6 nm降低到3.0 nm.这表明,SDS的加入影响了α-Ni(OH)2结晶和生长过程,在α-Ni(OH)2内引入了更多的结构缺陷、细化了晶粒并拓宽了层间距.电极材料中的结构缺陷可为锂离子存储提供更多的活性位点,并改善材料的电子/离子传输性能,因此有利于提升材料的储锂活性和反应动力学[17]. ...
Facile preparation of α-Ni(OH)2/graphene nanosheet composite as a cathode material for alkaline secondary batteries
1
2019
... 图1b给出了不同SDS用量的α-Ni(OH)2的TGA图.可以看出,NS-0样品有3个失重区间,25~300℃区间的失重(~3.7%)对应吸附水和层间水的去除,300~340℃区间的失重(~23.0%)对应Ni(OH)2分解为NiO和H2O,340~600℃区间的失重对应样品吸附的阴离子(如NO、CO)的除去[18].NS-10、NS-20和NS-30三个样品都有4个失重区间,其中25~230℃区间的失重对应吸附水和层间水的去除,230~350℃区间的失重(~7.1%、8.5%和9.0%)对应DS-中烷基长链的分解[19],350~380℃区间的失重对应Ni(OH)2的分解,380~600℃区间的失重对应吸附的阴离子(如NO、CO)的除去,600~900℃区间的失重对应DS-中-SO4的分解[20].上述结果表明,在NS-10、NS-20和NS-30三个样品中含有一定量的DS-阴离子.根据TGA曲线可计算出NS-20样品的分子式:Ni(OH)1.847-x-2y M x D y (SO4)0.056(DS)0.041(其中M和D分别表示NO和CO,DS表示十二烷基硫酸根).根据XRD分析结果可以推断,DS-阴离子已进入α-Ni(OH)2的层间而使层间距增大. ...
Halloysite nanotubes noncovalently functionalised with SDS anionic surfactant and PS-b-P4VP block copolymer for their effective dispersion in polystyrene as UV-blocking nanocomposite films
1
2017
... 图1b给出了不同SDS用量的α-Ni(OH)2的TGA图.可以看出,NS-0样品有3个失重区间,25~300℃区间的失重(~3.7%)对应吸附水和层间水的去除,300~340℃区间的失重(~23.0%)对应Ni(OH)2分解为NiO和H2O,340~600℃区间的失重对应样品吸附的阴离子(如NO、CO)的除去[18].NS-10、NS-20和NS-30三个样品都有4个失重区间,其中25~230℃区间的失重对应吸附水和层间水的去除,230~350℃区间的失重(~7.1%、8.5%和9.0%)对应DS-中烷基长链的分解[19],350~380℃区间的失重对应Ni(OH)2的分解,380~600℃区间的失重对应吸附的阴离子(如NO、CO)的除去,600~900℃区间的失重对应DS-中-SO4的分解[20].上述结果表明,在NS-10、NS-20和NS-30三个样品中含有一定量的DS-阴离子.根据TGA曲线可计算出NS-20样品的分子式:Ni(OH)1.847-x-2y M x D y (SO4)0.056(DS)0.041(其中M和D分别表示NO和CO,DS表示十二烷基硫酸根).根据XRD分析结果可以推断,DS-阴离子已进入α-Ni(OH)2的层间而使层间距增大. ...
Thermogravimetric analysis of layered double hydroxides intercalated with sulfate and alkaline cations [M6 2+Al3(OH)18] [A +(SO4)2] 12H2O (M 2+=Mn, Mg, Zn; A +=Li, Na, K)
1
2020
... 图1b给出了不同SDS用量的α-Ni(OH)2的TGA图.可以看出,NS-0样品有3个失重区间,25~300℃区间的失重(~3.7%)对应吸附水和层间水的去除,300~340℃区间的失重(~23.0%)对应Ni(OH)2分解为NiO和H2O,340~600℃区间的失重对应样品吸附的阴离子(如NO、CO)的除去[18].NS-10、NS-20和NS-30三个样品都有4个失重区间,其中25~230℃区间的失重对应吸附水和层间水的去除,230~350℃区间的失重(~7.1%、8.5%和9.0%)对应DS-中烷基长链的分解[19],350~380℃区间的失重对应Ni(OH)2的分解,380~600℃区间的失重对应吸附的阴离子(如NO、CO)的除去,600~900℃区间的失重对应DS-中-SO4的分解[20].上述结果表明,在NS-10、NS-20和NS-30三个样品中含有一定量的DS-阴离子.根据TGA曲线可计算出NS-20样品的分子式:Ni(OH)1.847-x-2y M x D y (SO4)0.056(DS)0.041(其中M和D分别表示NO和CO,DS表示十二烷基硫酸根).根据XRD分析结果可以推断,DS-阴离子已进入α-Ni(OH)2的层间而使层间距增大. ...
Synthesis and electrochemical performance of mixed phase α/β nickel hydroxide
2
2012
... 图1c给出了NS-0和NS-20两个样品的FT-IR谱.可以看出,两个样品在3450和1632 cm-1处的吸收峰分别对应层间水分子中-OH伸缩振动和吸附水分子的弯曲振动,表明两个峰都是α-Ni(OH)2的特征吸收峰[21];在2230 cm-1处的强吸收峰对应尿素在均相反应过程中分解生成的NCO-/NC-的伸缩振动[22];在1462和1370cm-1附近出现的是CO和NO的振动峰[23];在638和482 cm-1附近出现的吸收峰分别对应Ni-OH和Ni-O的振动[21, 24].在NS-20的谱中2917和2853 cm-1附近出现的两个较强的吸收峰,对应DS-烷基长链基团中C-H的对称和不对称伸缩振动[25,26];在1206和1065 cm-1处出现的吸收峰对应DS-中S=O的伸缩振动[26],进一步证明材料中含有一定量的SDS.以上结果与XRD和TGA分析结果相符. ...
... [21, 24].在NS-20的谱中2917和2853 cm-1附近出现的两个较强的吸收峰,对应DS-烷基长链基团中C-H的对称和不对称伸缩振动[25,26];在1206和1065 cm-1处出现的吸收峰对应DS-中S=O的伸缩振动[26],进一步证明材料中含有一定量的SDS.以上结果与XRD和TGA分析结果相符. ...
Multiple anionic Ni(SO4)0.3(OH)1.4 nanobelts/reduced graphene oxide enabled by enhanced multielectron reactions with superior lithium storage capacity
1
2021
... 图1c给出了NS-0和NS-20两个样品的FT-IR谱.可以看出,两个样品在3450和1632 cm-1处的吸收峰分别对应层间水分子中-OH伸缩振动和吸附水分子的弯曲振动,表明两个峰都是α-Ni(OH)2的特征吸收峰[21];在2230 cm-1处的强吸收峰对应尿素在均相反应过程中分解生成的NCO-/NC-的伸缩振动[22];在1462和1370cm-1附近出现的是CO和NO的振动峰[23];在638和482 cm-1附近出现的吸收峰分别对应Ni-OH和Ni-O的振动[21, 24].在NS-20的谱中2917和2853 cm-1附近出现的两个较强的吸收峰,对应DS-烷基长链基团中C-H的对称和不对称伸缩振动[25,26];在1206和1065 cm-1处出现的吸收峰对应DS-中S=O的伸缩振动[26],进一步证明材料中含有一定量的SDS.以上结果与XRD和TGA分析结果相符. ...
Lattice water for the enhanced performance of amorphous iron phosphate in sodium-ion batteries
1
2017
... 图1c给出了NS-0和NS-20两个样品的FT-IR谱.可以看出,两个样品在3450和1632 cm-1处的吸收峰分别对应层间水分子中-OH伸缩振动和吸附水分子的弯曲振动,表明两个峰都是α-Ni(OH)2的特征吸收峰[21];在2230 cm-1处的强吸收峰对应尿素在均相反应过程中分解生成的NCO-/NC-的伸缩振动[22];在1462和1370cm-1附近出现的是CO和NO的振动峰[23];在638和482 cm-1附近出现的吸收峰分别对应Ni-OH和Ni-O的振动[21, 24].在NS-20的谱中2917和2853 cm-1附近出现的两个较强的吸收峰,对应DS-烷基长链基团中C-H的对称和不对称伸缩振动[25,26];在1206和1065 cm-1处出现的吸收峰对应DS-中S=O的伸缩振动[26],进一步证明材料中含有一定量的SDS.以上结果与XRD和TGA分析结果相符. ...
Electrochemical performances of Ni/Al-LDHs/rGO composites prepared by homogeneous precipitation method
1
2020
... 图1c给出了NS-0和NS-20两个样品的FT-IR谱.可以看出,两个样品在3450和1632 cm-1处的吸收峰分别对应层间水分子中-OH伸缩振动和吸附水分子的弯曲振动,表明两个峰都是α-Ni(OH)2的特征吸收峰[21];在2230 cm-1处的强吸收峰对应尿素在均相反应过程中分解生成的NCO-/NC-的伸缩振动[22];在1462和1370cm-1附近出现的是CO和NO的振动峰[23];在638和482 cm-1附近出现的吸收峰分别对应Ni-OH和Ni-O的振动[21, 24].在NS-20的谱中2917和2853 cm-1附近出现的两个较强的吸收峰,对应DS-烷基长链基团中C-H的对称和不对称伸缩振动[25,26];在1206和1065 cm-1处出现的吸收峰对应DS-中S=O的伸缩振动[26],进一步证明材料中含有一定量的SDS.以上结果与XRD和TGA分析结果相符. ...
均相沉淀法制备Ni/Al-LDHs/rGO复合电极材料的电化学性能
1
2020
... 图1c给出了NS-0和NS-20两个样品的FT-IR谱.可以看出,两个样品在3450和1632 cm-1处的吸收峰分别对应层间水分子中-OH伸缩振动和吸附水分子的弯曲振动,表明两个峰都是α-Ni(OH)2的特征吸收峰[21];在2230 cm-1处的强吸收峰对应尿素在均相反应过程中分解生成的NCO-/NC-的伸缩振动[22];在1462和1370cm-1附近出现的是CO和NO的振动峰[23];在638和482 cm-1附近出现的吸收峰分别对应Ni-OH和Ni-O的振动[21, 24].在NS-20的谱中2917和2853 cm-1附近出现的两个较强的吸收峰,对应DS-烷基长链基团中C-H的对称和不对称伸缩振动[25,26];在1206和1065 cm-1处出现的吸收峰对应DS-中S=O的伸缩振动[26],进一步证明材料中含有一定量的SDS.以上结果与XRD和TGA分析结果相符. ...
Interlayer expanded nickel-iron layered double hydroxide by intercalation with sodium dodecyl sulfate for enhanced oxygen evolution reaction
1
2021
... 图1c给出了NS-0和NS-20两个样品的FT-IR谱.可以看出,两个样品在3450和1632 cm-1处的吸收峰分别对应层间水分子中-OH伸缩振动和吸附水分子的弯曲振动,表明两个峰都是α-Ni(OH)2的特征吸收峰[21];在2230 cm-1处的强吸收峰对应尿素在均相反应过程中分解生成的NCO-/NC-的伸缩振动[22];在1462和1370cm-1附近出现的是CO和NO的振动峰[23];在638和482 cm-1附近出现的吸收峰分别对应Ni-OH和Ni-O的振动[21, 24].在NS-20的谱中2917和2853 cm-1附近出现的两个较强的吸收峰,对应DS-烷基长链基团中C-H的对称和不对称伸缩振动[25,26];在1206和1065 cm-1处出现的吸收峰对应DS-中S=O的伸缩振动[26],进一步证明材料中含有一定量的SDS.以上结果与XRD和TGA分析结果相符. ...
Effect of pH on the morphology and optical properties of modified ZnO particles by SDS via a precipitation method
2
2010
... 图1c给出了NS-0和NS-20两个样品的FT-IR谱.可以看出,两个样品在3450和1632 cm-1处的吸收峰分别对应层间水分子中-OH伸缩振动和吸附水分子的弯曲振动,表明两个峰都是α-Ni(OH)2的特征吸收峰[21];在2230 cm-1处的强吸收峰对应尿素在均相反应过程中分解生成的NCO-/NC-的伸缩振动[22];在1462和1370cm-1附近出现的是CO和NO的振动峰[23];在638和482 cm-1附近出现的吸收峰分别对应Ni-OH和Ni-O的振动[21, 24].在NS-20的谱中2917和2853 cm-1附近出现的两个较强的吸收峰,对应DS-烷基长链基团中C-H的对称和不对称伸缩振动[25,26];在1206和1065 cm-1处出现的吸收峰对应DS-中S=O的伸缩振动[26],进一步证明材料中含有一定量的SDS.以上结果与XRD和TGA分析结果相符. ...
... [26],进一步证明材料中含有一定量的SDS.以上结果与XRD和TGA分析结果相符. ...
Heteroelement Y-doped α-Ni(OH)2 nanosheets with excellent pseudocapacitive performance
1
2017
... 图1d和图1e分别给出了NS-0和NS-20样品的Ni 2p XPS谱.可以看出,两样品的Ni 2p XPS谱都由6个峰组成,位于855.51和873.17 eV处的峰对应Ni2+;位于857.07和875.19 eV处的峰对应Ni3+;位于861.56和879.44 eV处的峰分别对应Ni 2p3/2和Ni 2p1/2卫星峰[27,28].NS-20样品的S 2p XPS谱由位于168.92和169.94 eV的两个峰组成(图1f),分别对应DS-阴离子中S6+的S 2p3/2和S 2p3/2峰[29]. ...
Lithium storage performance of α-Ni(OH)2 regulated by partial interlayer anion exchange
1
2021
... 图1d和图1e分别给出了NS-0和NS-20样品的Ni 2p XPS谱.可以看出,两样品的Ni 2p XPS谱都由6个峰组成,位于855.51和873.17 eV处的峰对应Ni2+;位于857.07和875.19 eV处的峰对应Ni3+;位于861.56和879.44 eV处的峰分别对应Ni 2p3/2和Ni 2p1/2卫星峰[27,28].NS-20样品的S 2p XPS谱由位于168.92和169.94 eV的两个峰组成(图1f),分别对应DS-阴离子中S6+的S 2p3/2和S 2p3/2峰[29]. ...
Electrochemical performance of Fe2(SO4)3 as a novel anode material for lithium-ion batteries
1
2021
... 图1d和图1e分别给出了NS-0和NS-20样品的Ni 2p XPS谱.可以看出,两样品的Ni 2p XPS谱都由6个峰组成,位于855.51和873.17 eV处的峰对应Ni2+;位于857.07和875.19 eV处的峰对应Ni3+;位于861.56和879.44 eV处的峰分别对应Ni 2p3/2和Ni 2p1/2卫星峰[27,28].NS-20样品的S 2p XPS谱由位于168.92和169.94 eV的两个峰组成(图1f),分别对应DS-阴离子中S6+的S 2p3/2和S 2p3/2峰[29]. ...
Crystal water-assisted additional capacity for nickel hydroxide anode materials
0
2022
Enhancing the lithium storage performance of α-Ni(OH)2 by Zn2+ doping
0
2022
Electrochemical properties of ultrasonically prepared Ni(OH)2 nanosheets in lithium cells
1
2013
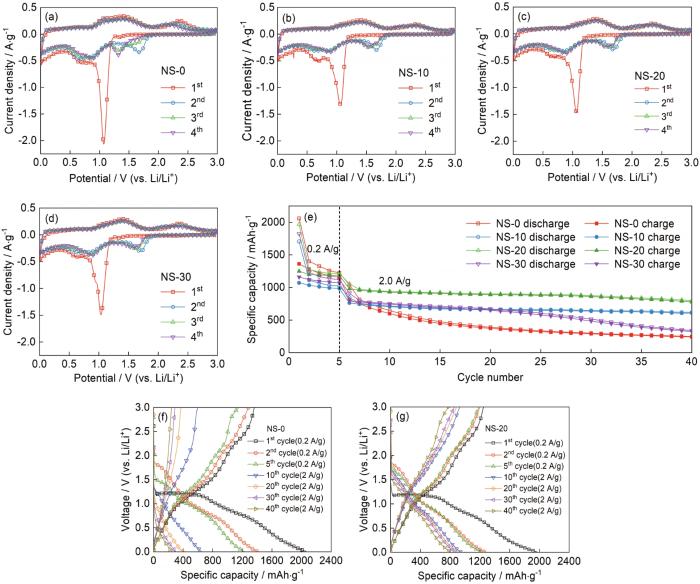
... 图3a~d给出了NS-0、NS-10、NS-20和NS-30样品的CV曲线,扫描速度为0.1 mV·s-1.从图中可见,四个样品的CV曲线的特征相似.在首圈CV负向扫描过程中,在1.15 V附近出现的强还原峰对应Ni(OH)2还原为Ni0(Ni(OH)2+2Li++2e-→Ni0+2LiOH)和固体电解质界面(SEI)膜的生成,在0.75 V附近出现的较宽还原峰对应LiOH进一步还原为LiH(LiOH+2Li++2e-→Li2O+LiH);在首圈CV正向扫描过程中,在0.9~1.8 V的宽氧化峰对应LiH可逆氧化为LiOH,在2.3 V附近出现的氧化峰对应Ni0氧化为Ni2+[30,31].从第二圈CV开始,样品的在1.15 V附近的还原峰强度明显降低并正移,其主要原因是在首圈负扫过程中SEI膜的生成产生了不可逆Li+消耗.仔细对比可以发现,随着循环次数的增加NS-0样品的CV曲线面积明显变小,表明电化学活性降低;而NS-10、NS-20和NS-30样品的第三、四圈CV曲线几乎重叠,表明其较高的循环稳定性.图3e给出了NS-0、NS-10、NS-20和NS-30四个样品在电流密度为0.2 A·g-1时活化5圈后又在电流密度为2 A·g-1条件下循环35圈的循环性能比较.结果表明,NS-0的循环性能最差,40次循环后其比容量已衰减至248 mAh·g-1;使用SDS辅助制备的三个样品均表现出更好循环性能,尤其是NS-20的循环性能最好,40次循环后其比容量仍然保持在800 mAh·g-1.图3f,g分别给出了NS-0和NS-20样品不同循环圈数的放电/充电曲线.可以看出,在充放电曲线的前5圈活化过程中放电曲线有两段不同斜率的近直线容量衰减,分别对应材料的两个嵌锂反应过程.在0.9~1.3 V附近的放电平台对应Ni(OH)2还原为Ni0(Ni(OH)2+2Li++2e-→Ni0+2LiOH)和固体电解质界面(SEI)膜的生成,在0.6~0.8 V附近出现的放电平台对应LiOH进一步还原为LiH(LiOH+2Li++2e-→Li2O+LiH)[32].同时,NS-0和NS-20的首圈放电/充电比容量分别为2059/1316和1967/1245 mAh·g-1,对应的库伦效率分别为63.9%和63.3%.其主要原因是,材料在首次放电过程中生成SEI膜消耗了部分锂离子,引起了不可逆的容量损失[33],这与首圈CV测试结果(图3a~d)一致.随着循环次数的增加两样品的充电电位逐渐升高、放电电位逐渐降低和可逆比容量逐渐降低.NS-0样品经过10次循环后已没有明显的充放电平台,而NS-20样品即使经过40次循环后仍出现较好的充放电平台.以上结果表明,随着SDS用量的增加样品的的电化学性能先提高后降低,其中NS-20的性能最佳.NS-20样品突出的电化学性能,与其自身的微观结构密切相关:一方面NS-20样品的结晶性较差(图1a),结构缺陷使其含有更多的配位不饱和原子,有利于提高材料的电化学反应活性[34];另一方面,NS-20样品更加开放的三维结构有利于暴露更多的反应位点、缓解材料在充放电过程中的体积效应、促进电解液在电极中的渗透,进而改善电极的循环性能和动力学性能.表2对比了NS-20样品与文献报道的氢氧化镍基负极材料的循环性能.从表中可以看出,本文制备的NS-20样品其电化学性能较为优异. ...
Review on comprehending and enhancing the initial coulombic efficiency of anode materials in lithium-ion/sodium-ion batteries
1
2020
... 图3a~d给出了NS-0、NS-10、NS-20和NS-30样品的CV曲线,扫描速度为0.1 mV·s-1.从图中可见,四个样品的CV曲线的特征相似.在首圈CV负向扫描过程中,在1.15 V附近出现的强还原峰对应Ni(OH)2还原为Ni0(Ni(OH)2+2Li++2e-→Ni0+2LiOH)和固体电解质界面(SEI)膜的生成,在0.75 V附近出现的较宽还原峰对应LiOH进一步还原为LiH(LiOH+2Li++2e-→Li2O+LiH);在首圈CV正向扫描过程中,在0.9~1.8 V的宽氧化峰对应LiH可逆氧化为LiOH,在2.3 V附近出现的氧化峰对应Ni0氧化为Ni2+[30,31].从第二圈CV开始,样品的在1.15 V附近的还原峰强度明显降低并正移,其主要原因是在首圈负扫过程中SEI膜的生成产生了不可逆Li+消耗.仔细对比可以发现,随着循环次数的增加NS-0样品的CV曲线面积明显变小,表明电化学活性降低;而NS-10、NS-20和NS-30样品的第三、四圈CV曲线几乎重叠,表明其较高的循环稳定性.图3e给出了NS-0、NS-10、NS-20和NS-30四个样品在电流密度为0.2 A·g-1时活化5圈后又在电流密度为2 A·g-1条件下循环35圈的循环性能比较.结果表明,NS-0的循环性能最差,40次循环后其比容量已衰减至248 mAh·g-1;使用SDS辅助制备的三个样品均表现出更好循环性能,尤其是NS-20的循环性能最好,40次循环后其比容量仍然保持在800 mAh·g-1.图3f,g分别给出了NS-0和NS-20样品不同循环圈数的放电/充电曲线.可以看出,在充放电曲线的前5圈活化过程中放电曲线有两段不同斜率的近直线容量衰减,分别对应材料的两个嵌锂反应过程.在0.9~1.3 V附近的放电平台对应Ni(OH)2还原为Ni0(Ni(OH)2+2Li++2e-→Ni0+2LiOH)和固体电解质界面(SEI)膜的生成,在0.6~0.8 V附近出现的放电平台对应LiOH进一步还原为LiH(LiOH+2Li++2e-→Li2O+LiH)[32].同时,NS-0和NS-20的首圈放电/充电比容量分别为2059/1316和1967/1245 mAh·g-1,对应的库伦效率分别为63.9%和63.3%.其主要原因是,材料在首次放电过程中生成SEI膜消耗了部分锂离子,引起了不可逆的容量损失[33],这与首圈CV测试结果(图3a~d)一致.随着循环次数的增加两样品的充电电位逐渐升高、放电电位逐渐降低和可逆比容量逐渐降低.NS-0样品经过10次循环后已没有明显的充放电平台,而NS-20样品即使经过40次循环后仍出现较好的充放电平台.以上结果表明,随着SDS用量的增加样品的的电化学性能先提高后降低,其中NS-20的性能最佳.NS-20样品突出的电化学性能,与其自身的微观结构密切相关:一方面NS-20样品的结晶性较差(图1a),结构缺陷使其含有更多的配位不饱和原子,有利于提高材料的电化学反应活性[34];另一方面,NS-20样品更加开放的三维结构有利于暴露更多的反应位点、缓解材料在充放电过程中的体积效应、促进电解液在电极中的渗透,进而改善电极的循环性能和动力学性能.表2对比了NS-20样品与文献报道的氢氧化镍基负极材料的循环性能.从表中可以看出,本文制备的NS-20样品其电化学性能较为优异. ...
Studies on electrochemical activity of amorphous anode materials Ni(OH)2
1
2006
... 图3a~d给出了NS-0、NS-10、NS-20和NS-30样品的CV曲线,扫描速度为0.1 mV·s-1.从图中可见,四个样品的CV曲线的特征相似.在首圈CV负向扫描过程中,在1.15 V附近出现的强还原峰对应Ni(OH)2还原为Ni0(Ni(OH)2+2Li++2e-→Ni0+2LiOH)和固体电解质界面(SEI)膜的生成,在0.75 V附近出现的较宽还原峰对应LiOH进一步还原为LiH(LiOH+2Li++2e-→Li2O+LiH);在首圈CV正向扫描过程中,在0.9~1.8 V的宽氧化峰对应LiH可逆氧化为LiOH,在2.3 V附近出现的氧化峰对应Ni0氧化为Ni2+[30,31].从第二圈CV开始,样品的在1.15 V附近的还原峰强度明显降低并正移,其主要原因是在首圈负扫过程中SEI膜的生成产生了不可逆Li+消耗.仔细对比可以发现,随着循环次数的增加NS-0样品的CV曲线面积明显变小,表明电化学活性降低;而NS-10、NS-20和NS-30样品的第三、四圈CV曲线几乎重叠,表明其较高的循环稳定性.图3e给出了NS-0、NS-10、NS-20和NS-30四个样品在电流密度为0.2 A·g-1时活化5圈后又在电流密度为2 A·g-1条件下循环35圈的循环性能比较.结果表明,NS-0的循环性能最差,40次循环后其比容量已衰减至248 mAh·g-1;使用SDS辅助制备的三个样品均表现出更好循环性能,尤其是NS-20的循环性能最好,40次循环后其比容量仍然保持在800 mAh·g-1.图3f,g分别给出了NS-0和NS-20样品不同循环圈数的放电/充电曲线.可以看出,在充放电曲线的前5圈活化过程中放电曲线有两段不同斜率的近直线容量衰减,分别对应材料的两个嵌锂反应过程.在0.9~1.3 V附近的放电平台对应Ni(OH)2还原为Ni0(Ni(OH)2+2Li++2e-→Ni0+2LiOH)和固体电解质界面(SEI)膜的生成,在0.6~0.8 V附近出现的放电平台对应LiOH进一步还原为LiH(LiOH+2Li++2e-→Li2O+LiH)[32].同时,NS-0和NS-20的首圈放电/充电比容量分别为2059/1316和1967/1245 mAh·g-1,对应的库伦效率分别为63.9%和63.3%.其主要原因是,材料在首次放电过程中生成SEI膜消耗了部分锂离子,引起了不可逆的容量损失[33],这与首圈CV测试结果(图3a~d)一致.随着循环次数的增加两样品的充电电位逐渐升高、放电电位逐渐降低和可逆比容量逐渐降低.NS-0样品经过10次循环后已没有明显的充放电平台,而NS-20样品即使经过40次循环后仍出现较好的充放电平台.以上结果表明,随着SDS用量的增加样品的的电化学性能先提高后降低,其中NS-20的性能最佳.NS-20样品突出的电化学性能,与其自身的微观结构密切相关:一方面NS-20样品的结晶性较差(图1a),结构缺陷使其含有更多的配位不饱和原子,有利于提高材料的电化学反应活性[34];另一方面,NS-20样品更加开放的三维结构有利于暴露更多的反应位点、缓解材料在充放电过程中的体积效应、促进电解液在电极中的渗透,进而改善电极的循环性能和动力学性能.表2对比了NS-20样品与文献报道的氢氧化镍基负极材料的循环性能.从表中可以看出,本文制备的NS-20样品其电化学性能较为优异. ...
电极材料非晶态氢氧化镍的电化学活性
1
2006
... 图3a~d给出了NS-0、NS-10、NS-20和NS-30样品的CV曲线,扫描速度为0.1 mV·s-1.从图中可见,四个样品的CV曲线的特征相似.在首圈CV负向扫描过程中,在1.15 V附近出现的强还原峰对应Ni(OH)2还原为Ni0(Ni(OH)2+2Li++2e-→Ni0+2LiOH)和固体电解质界面(SEI)膜的生成,在0.75 V附近出现的较宽还原峰对应LiOH进一步还原为LiH(LiOH+2Li++2e-→Li2O+LiH);在首圈CV正向扫描过程中,在0.9~1.8 V的宽氧化峰对应LiH可逆氧化为LiOH,在2.3 V附近出现的氧化峰对应Ni0氧化为Ni2+[30,31].从第二圈CV开始,样品的在1.15 V附近的还原峰强度明显降低并正移,其主要原因是在首圈负扫过程中SEI膜的生成产生了不可逆Li+消耗.仔细对比可以发现,随着循环次数的增加NS-0样品的CV曲线面积明显变小,表明电化学活性降低;而NS-10、NS-20和NS-30样品的第三、四圈CV曲线几乎重叠,表明其较高的循环稳定性.图3e给出了NS-0、NS-10、NS-20和NS-30四个样品在电流密度为0.2 A·g-1时活化5圈后又在电流密度为2 A·g-1条件下循环35圈的循环性能比较.结果表明,NS-0的循环性能最差,40次循环后其比容量已衰减至248 mAh·g-1;使用SDS辅助制备的三个样品均表现出更好循环性能,尤其是NS-20的循环性能最好,40次循环后其比容量仍然保持在800 mAh·g-1.图3f,g分别给出了NS-0和NS-20样品不同循环圈数的放电/充电曲线.可以看出,在充放电曲线的前5圈活化过程中放电曲线有两段不同斜率的近直线容量衰减,分别对应材料的两个嵌锂反应过程.在0.9~1.3 V附近的放电平台对应Ni(OH)2还原为Ni0(Ni(OH)2+2Li++2e-→Ni0+2LiOH)和固体电解质界面(SEI)膜的生成,在0.6~0.8 V附近出现的放电平台对应LiOH进一步还原为LiH(LiOH+2Li++2e-→Li2O+LiH)[32].同时,NS-0和NS-20的首圈放电/充电比容量分别为2059/1316和1967/1245 mAh·g-1,对应的库伦效率分别为63.9%和63.3%.其主要原因是,材料在首次放电过程中生成SEI膜消耗了部分锂离子,引起了不可逆的容量损失[33],这与首圈CV测试结果(图3a~d)一致.随着循环次数的增加两样品的充电电位逐渐升高、放电电位逐渐降低和可逆比容量逐渐降低.NS-0样品经过10次循环后已没有明显的充放电平台,而NS-20样品即使经过40次循环后仍出现较好的充放电平台.以上结果表明,随着SDS用量的增加样品的的电化学性能先提高后降低,其中NS-20的性能最佳.NS-20样品突出的电化学性能,与其自身的微观结构密切相关:一方面NS-20样品的结晶性较差(图1a),结构缺陷使其含有更多的配位不饱和原子,有利于提高材料的电化学反应活性[34];另一方面,NS-20样品更加开放的三维结构有利于暴露更多的反应位点、缓解材料在充放电过程中的体积效应、促进电解液在电极中的渗透,进而改善电极的循环性能和动力学性能.表2对比了NS-20样品与文献报道的氢氧化镍基负极材料的循环性能.从表中可以看出,本文制备的NS-20样品其电化学性能较为优异. ...
Electrochemical property of hierarchical flower-like α-Ni(OH)2 as an anode material for lithium-ion batteries
1
2021
... / V vs. Li/Li
+Reference | | NS-20 | 2.0 | 800 mAh·g-1 after 40 cycles | 0~3.0 | This work |
| α-Ni(OH)2 | 1.0 | 743 mAh·g-1 after 50 cycles | 0~3.0 | [35] |
| Co-Ni-LDH | 0.05 | 450.4 mAh·g-1 after 40 cycles | 0~3.0 | [36] |
| Ni-Co-LDH | 0.1 | 335.4 mAh·g-1 after 50 cycles | 0~3.0 | [37] |
| Ni(OH)2-CTAB | 0.5 | 952 mAh·g-1 after 25 cycles | 0~3.0 | [38] |
| Fe-Ni-LDH | 0.2 | 1080 mAh·g-1 after 30 cycles | 0~3.0 | [39] |
| Ni- Fe-OH | 0.85 | 540 mAh·g-1 after 50 cycles | 0~3.0 | [40] |
| Ni(OH)Cl | 0.2 | 1236 mAh·g-1 after 150 cycles | 0~3.0 | [9] |
图4a给出了NS-0、NS-10、NS-20和NS-30四个样品的倍率性能对比.可以看出,随着电流密度的增大样品的放电比容量逐渐降低.NS-0的倍率性能最差,在3 A·g-1电流密度下其比容量已降低至231 mAh·g-1.相比之下,SDS辅助制备的三个样品均表现出更好倍率性能;尤其是NS-20的倍率性能最佳,在3 A·g-1高电流密度下其比容量仍高达662 mAh·g-1,电流密度回到较低的0.5 A·g-1时其比容量恢复到980 mAh·g-1并保持良好的循环稳定性.图4b~d给出了NS-0、NS-20和NS-30三个样品在不同电流密度下的充放电曲线.可以看出,电流密度为0.2 A·g-1时三个样品的充放电平台均较为明显,随着电流密度的提高三个样品电极的充放电平台逐渐变短表明电极的极化逐渐变大.相比之下,NS-20样品的极化明显低于其他两个样品. ...
Graphene-wrapped CoNi-layered double hydroxide microspheres as a new anode material for lithium-ion batteries
1
2018
... / V vs. Li/Li
+Reference | | NS-20 | 2.0 | 800 mAh·g-1 after 40 cycles | 0~3.0 | This work |
| α-Ni(OH)2 | 1.0 | 743 mAh·g-1 after 50 cycles | 0~3.0 | [35] |
| Co-Ni-LDH | 0.05 | 450.4 mAh·g-1 after 40 cycles | 0~3.0 | [36] |
| Ni-Co-LDH | 0.1 | 335.4 mAh·g-1 after 50 cycles | 0~3.0 | [37] |
| Ni(OH)2-CTAB | 0.5 | 952 mAh·g-1 after 25 cycles | 0~3.0 | [38] |
| Fe-Ni-LDH | 0.2 | 1080 mAh·g-1 after 30 cycles | 0~3.0 | [39] |
| Ni- Fe-OH | 0.85 | 540 mAh·g-1 after 50 cycles | 0~3.0 | [40] |
| Ni(OH)Cl | 0.2 | 1236 mAh·g-1 after 150 cycles | 0~3.0 | [9] |
图4a给出了NS-0、NS-10、NS-20和NS-30四个样品的倍率性能对比.可以看出,随着电流密度的增大样品的放电比容量逐渐降低.NS-0的倍率性能最差,在3 A·g-1电流密度下其比容量已降低至231 mAh·g-1.相比之下,SDS辅助制备的三个样品均表现出更好倍率性能;尤其是NS-20的倍率性能最佳,在3 A·g-1高电流密度下其比容量仍高达662 mAh·g-1,电流密度回到较低的0.5 A·g-1时其比容量恢复到980 mAh·g-1并保持良好的循环稳定性.图4b~d给出了NS-0、NS-20和NS-30三个样品在不同电流密度下的充放电曲线.可以看出,电流密度为0.2 A·g-1时三个样品的充放电平台均较为明显,随着电流密度的提高三个样品电极的充放电平台逐渐变短表明电极的极化逐渐变大.相比之下,NS-20样品的极化明显低于其他两个样品. ...
Template-sacrificing synthesis of Ni-Co layered double hydroxides polyhedron as advanced anode for lithium ions battery
1
2020
... / V vs. Li/Li
+Reference | | NS-20 | 2.0 | 800 mAh·g-1 after 40 cycles | 0~3.0 | This work |
| α-Ni(OH)2 | 1.0 | 743 mAh·g-1 after 50 cycles | 0~3.0 | [35] |
| Co-Ni-LDH | 0.05 | 450.4 mAh·g-1 after 40 cycles | 0~3.0 | [36] |
| Ni-Co-LDH | 0.1 | 335.4 mAh·g-1 after 50 cycles | 0~3.0 | [37] |
| Ni(OH)2-CTAB | 0.5 | 952 mAh·g-1 after 25 cycles | 0~3.0 | [38] |
| Fe-Ni-LDH | 0.2 | 1080 mAh·g-1 after 30 cycles | 0~3.0 | [39] |
| Ni- Fe-OH | 0.85 | 540 mAh·g-1 after 50 cycles | 0~3.0 | [40] |
| Ni(OH)Cl | 0.2 | 1236 mAh·g-1 after 150 cycles | 0~3.0 | [9] |
图4a给出了NS-0、NS-10、NS-20和NS-30四个样品的倍率性能对比.可以看出,随着电流密度的增大样品的放电比容量逐渐降低.NS-0的倍率性能最差,在3 A·g-1电流密度下其比容量已降低至231 mAh·g-1.相比之下,SDS辅助制备的三个样品均表现出更好倍率性能;尤其是NS-20的倍率性能最佳,在3 A·g-1高电流密度下其比容量仍高达662 mAh·g-1,电流密度回到较低的0.5 A·g-1时其比容量恢复到980 mAh·g-1并保持良好的循环稳定性.图4b~d给出了NS-0、NS-20和NS-30三个样品在不同电流密度下的充放电曲线.可以看出,电流密度为0.2 A·g-1时三个样品的充放电平台均较为明显,随着电流密度的提高三个样品电极的充放电平台逐渐变短表明电极的极化逐渐变大.相比之下,NS-20样品的极化明显低于其他两个样品. ...
Effect of CTAB addition on the lithium storage performance of Ni(OH)2 prepared by homogeneous precipitation method
1
2019
... / V vs. Li/Li
+Reference | | NS-20 | 2.0 | 800 mAh·g-1 after 40 cycles | 0~3.0 | This work |
| α-Ni(OH)2 | 1.0 | 743 mAh·g-1 after 50 cycles | 0~3.0 | [35] |
| Co-Ni-LDH | 0.05 | 450.4 mAh·g-1 after 40 cycles | 0~3.0 | [36] |
| Ni-Co-LDH | 0.1 | 335.4 mAh·g-1 after 50 cycles | 0~3.0 | [37] |
| Ni(OH)2-CTAB | 0.5 | 952 mAh·g-1 after 25 cycles | 0~3.0 | [38] |
| Fe-Ni-LDH | 0.2 | 1080 mAh·g-1 after 30 cycles | 0~3.0 | [39] |
| Ni- Fe-OH | 0.85 | 540 mAh·g-1 after 50 cycles | 0~3.0 | [40] |
| Ni(OH)Cl | 0.2 | 1236 mAh·g-1 after 150 cycles | 0~3.0 | [9] |
图4a给出了NS-0、NS-10、NS-20和NS-30四个样品的倍率性能对比.可以看出,随着电流密度的增大样品的放电比容量逐渐降低.NS-0的倍率性能最差,在3 A·g-1电流密度下其比容量已降低至231 mAh·g-1.相比之下,SDS辅助制备的三个样品均表现出更好倍率性能;尤其是NS-20的倍率性能最佳,在3 A·g-1高电流密度下其比容量仍高达662 mAh·g-1,电流密度回到较低的0.5 A·g-1时其比容量恢复到980 mAh·g-1并保持良好的循环稳定性.图4b~d给出了NS-0、NS-20和NS-30三个样品在不同电流密度下的充放电曲线.可以看出,电流密度为0.2 A·g-1时三个样品的充放电平台均较为明显,随着电流密度的提高三个样品电极的充放电平台逐渐变短表明电极的极化逐渐变大.相比之下,NS-20样品的极化明显低于其他两个样品. ...
CTAB对均相沉淀法制备Ni(OH)2电极材料储锂性能的影响
1
2019
... / V vs. Li/Li
+Reference | | NS-20 | 2.0 | 800 mAh·g-1 after 40 cycles | 0~3.0 | This work |
| α-Ni(OH)2 | 1.0 | 743 mAh·g-1 after 50 cycles | 0~3.0 | [35] |
| Co-Ni-LDH | 0.05 | 450.4 mAh·g-1 after 40 cycles | 0~3.0 | [36] |
| Ni-Co-LDH | 0.1 | 335.4 mAh·g-1 after 50 cycles | 0~3.0 | [37] |
| Ni(OH)2-CTAB | 0.5 | 952 mAh·g-1 after 25 cycles | 0~3.0 | [38] |
| Fe-Ni-LDH | 0.2 | 1080 mAh·g-1 after 30 cycles | 0~3.0 | [39] |
| Ni- Fe-OH | 0.85 | 540 mAh·g-1 after 50 cycles | 0~3.0 | [40] |
| Ni(OH)Cl | 0.2 | 1236 mAh·g-1 after 150 cycles | 0~3.0 | [9] |
图4a给出了NS-0、NS-10、NS-20和NS-30四个样品的倍率性能对比.可以看出,随着电流密度的增大样品的放电比容量逐渐降低.NS-0的倍率性能最差,在3 A·g-1电流密度下其比容量已降低至231 mAh·g-1.相比之下,SDS辅助制备的三个样品均表现出更好倍率性能;尤其是NS-20的倍率性能最佳,在3 A·g-1高电流密度下其比容量仍高达662 mAh·g-1,电流密度回到较低的0.5 A·g-1时其比容量恢复到980 mAh·g-1并保持良好的循环稳定性.图4b~d给出了NS-0、NS-20和NS-30三个样品在不同电流密度下的充放电曲线.可以看出,电流密度为0.2 A·g-1时三个样品的充放电平台均较为明显,随着电流密度的提高三个样品电极的充放电平台逐渐变短表明电极的极化逐渐变大.相比之下,NS-20样品的极化明显低于其他两个样品. ...
High-tap-density Fe-doped nickel hydroxide with enhanced lithium storage performance
1
2019
... / V vs. Li/Li
+Reference | | NS-20 | 2.0 | 800 mAh·g-1 after 40 cycles | 0~3.0 | This work |
| α-Ni(OH)2 | 1.0 | 743 mAh·g-1 after 50 cycles | 0~3.0 | [35] |
| Co-Ni-LDH | 0.05 | 450.4 mAh·g-1 after 40 cycles | 0~3.0 | [36] |
| Ni-Co-LDH | 0.1 | 335.4 mAh·g-1 after 50 cycles | 0~3.0 | [37] |
| Ni(OH)2-CTAB | 0.5 | 952 mAh·g-1 after 25 cycles | 0~3.0 | [38] |
| Fe-Ni-LDH | 0.2 | 1080 mAh·g-1 after 30 cycles | 0~3.0 | [39] |
| Ni- Fe-OH | 0.85 | 540 mAh·g-1 after 50 cycles | 0~3.0 | [40] |
| Ni(OH)Cl | 0.2 | 1236 mAh·g-1 after 150 cycles | 0~3.0 | [9] |
图4a给出了NS-0、NS-10、NS-20和NS-30四个样品的倍率性能对比.可以看出,随着电流密度的增大样品的放电比容量逐渐降低.NS-0的倍率性能最差,在3 A·g-1电流密度下其比容量已降低至231 mAh·g-1.相比之下,SDS辅助制备的三个样品均表现出更好倍率性能;尤其是NS-20的倍率性能最佳,在3 A·g-1高电流密度下其比容量仍高达662 mAh·g-1,电流密度回到较低的0.5 A·g-1时其比容量恢复到980 mAh·g-1并保持良好的循环稳定性.图4b~d给出了NS-0、NS-20和NS-30三个样品在不同电流密度下的充放电曲线.可以看出,电流密度为0.2 A·g-1时三个样品的充放电平台均较为明显,随着电流密度的提高三个样品电极的充放电平台逐渐变短表明电极的极化逐渐变大.相比之下,NS-20样品的极化明显低于其他两个样品. ...
Structural and chemical evolution of amorphous nickel iron complex hydroxide upon lithiation/delithiation
1
2015
... / V vs. Li/Li
+Reference | | NS-20 | 2.0 | 800 mAh·g-1 after 40 cycles | 0~3.0 | This work |
| α-Ni(OH)2 | 1.0 | 743 mAh·g-1 after 50 cycles | 0~3.0 | [35] |
| Co-Ni-LDH | 0.05 | 450.4 mAh·g-1 after 40 cycles | 0~3.0 | [36] |
| Ni-Co-LDH | 0.1 | 335.4 mAh·g-1 after 50 cycles | 0~3.0 | [37] |
| Ni(OH)2-CTAB | 0.5 | 952 mAh·g-1 after 25 cycles | 0~3.0 | [38] |
| Fe-Ni-LDH | 0.2 | 1080 mAh·g-1 after 30 cycles | 0~3.0 | [39] |
| Ni- Fe-OH | 0.85 | 540 mAh·g-1 after 50 cycles | 0~3.0 | [40] |
| Ni(OH)Cl | 0.2 | 1236 mAh·g-1 after 150 cycles | 0~3.0 | [9] |
图4a给出了NS-0、NS-10、NS-20和NS-30四个样品的倍率性能对比.可以看出,随着电流密度的增大样品的放电比容量逐渐降低.NS-0的倍率性能最差,在3 A·g-1电流密度下其比容量已降低至231 mAh·g-1.相比之下,SDS辅助制备的三个样品均表现出更好倍率性能;尤其是NS-20的倍率性能最佳,在3 A·g-1高电流密度下其比容量仍高达662 mAh·g-1,电流密度回到较低的0.5 A·g-1时其比容量恢复到980 mAh·g-1并保持良好的循环稳定性.图4b~d给出了NS-0、NS-20和NS-30三个样品在不同电流密度下的充放电曲线.可以看出,电流密度为0.2 A·g-1时三个样品的充放电平台均较为明显,随着电流密度的提高三个样品电极的充放电平台逐渐变短表明电极的极化逐渐变大.相比之下,NS-20样品的极化明显低于其他两个样品. ...
Achieving high energy density and high power density with pseudocapacitive materials
0
2020
Facile and efficient synthesis of α-Fe2O3 nanocrystals by glucose-assisted thermal decomposition method and its application in lithium ion batteries
1
2019
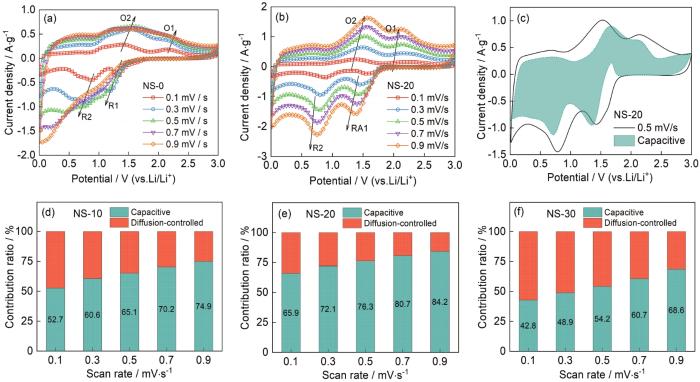
... 进行定量分析[42],式中i(V)为特定电位下的CV电流值;k1v和k2v1/2分别为赝电容效应电流和扩散控制电流.根据 式(2)和图5c中的CV数据可计算出NS-20样品的赝电容效应电流.以扫速为0.5 mV·s-1的CV为例,图5e给出了NS-20样品在CV过程中的赝电容效应贡献.图5d~f给出了不同扫速下NS-10、NS-20和NS-30三个样品赝电容效应的贡献.可以看出,赝电容效应的贡献占据主导地位且随着扫描速度的提高其贡献比例逐渐增大.NS-10和NS-20样品比NS-30样品赝电容效应的贡献更为显著,扫描速度为0.9 mV·s-1时赝电容效应的贡献比例分别为74.9%、84.2%和68.6%.这表明,NS-10和NS-20样品突出的倍率性能与其显著的赝电容贡献密切相关,其中NS-20样品的赝电容效应最显著. ...
Morphology, structure and electrochemical properties of single phase electrospun vanadium pentoxide nanofibers for lithium ion batteries
1
2011
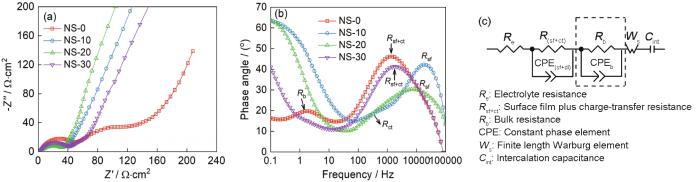
... 图6a、b给出了NS-0、NS-10、NS-20和NS-30四个样品经过20次循环后的Nyquist图和对应的Bode图.可以看出,NS-0样品的Nyquist图由高频区的半圆、中低频区半圆和低频区的斜线组成,高频区的半圆对应SEI膜电阻和电化学反应电阻容抗弧(Rsf+ct),中低频区的半圆对应材料的体相电阻(Rb,包括活性材料电阻、复合电极中孔道内部电解液的离子电阻、材料充放电结构变化引起的电阻)[43];低频区的直线对应Li+在材料中扩散引起的Warburg阻抗[44].对等效电路(含虚线框部分)的拟合结果表明,NS-0样品的Rsf+ct和Rb值分别为48.3和73.8 Ω·cm2.较大的Rb值表明其电化学活性低,与上文的循环性能(图3e)是相对应的.NS-10、NS-20和NS-30样品的Nyquist图都由高中频区的半圆和低频区的斜线组成,高中频区半圆对应SEI膜电阻和电化学反应电阻容抗弧,低频区斜线对应Li+在材料中扩散引起的Warburg阻抗.三个样品的EIS图均未出现明显的体相电阻容抗弧,表明其电化学活性较高.对等效电路图6c(不含虚线框部分)的拟合结果表明,NS-10、NS-20和NS-30样品的Rsf+ct值分别为33.3、29.4和33.6 Ω·cm2,明显低于NS-0的Rsf+ct值(48.3 Ω·cm2),表明其电化学反应更容易进行.其中NS-20的Rsf+ct值最低,表明其电化学反应活性最高,与其循环性能曲线(图3e)相对应. ...
An elaborate insight of lithiation behavior of V2O5 anode
1
2020
... 图6a、b给出了NS-0、NS-10、NS-20和NS-30四个样品经过20次循环后的Nyquist图和对应的Bode图.可以看出,NS-0样品的Nyquist图由高频区的半圆、中低频区半圆和低频区的斜线组成,高频区的半圆对应SEI膜电阻和电化学反应电阻容抗弧(Rsf+ct),中低频区的半圆对应材料的体相电阻(Rb,包括活性材料电阻、复合电极中孔道内部电解液的离子电阻、材料充放电结构变化引起的电阻)[43];低频区的直线对应Li+在材料中扩散引起的Warburg阻抗[44].对等效电路(含虚线框部分)的拟合结果表明,NS-0样品的Rsf+ct和Rb值分别为48.3和73.8 Ω·cm2.较大的Rb值表明其电化学活性低,与上文的循环性能(图3e)是相对应的.NS-10、NS-20和NS-30样品的Nyquist图都由高中频区的半圆和低频区的斜线组成,高中频区半圆对应SEI膜电阻和电化学反应电阻容抗弧,低频区斜线对应Li+在材料中扩散引起的Warburg阻抗.三个样品的EIS图均未出现明显的体相电阻容抗弧,表明其电化学活性较高.对等效电路图6c(不含虚线框部分)的拟合结果表明,NS-10、NS-20和NS-30样品的Rsf+ct值分别为33.3、29.4和33.6 Ω·cm2,明显低于NS-0的Rsf+ct值(48.3 Ω·cm2),表明其电化学反应更容易进行.其中NS-20的Rsf+ct值最低,表明其电化学反应活性最高,与其循环性能曲线(图3e)相对应. ...