对于尖晶石型钛酸锂Li4Ti5O12,锂嵌入电压为1.55 V (vs. Li+/Li),但是其理论容量小于175 mAh·g-1[9,10,11]。Chen [12]等和Goodenough[13]等分别将Ti2Nb10O29和TiNb2O7视为Li4Ti5O12的替代物。Ti2Nb10O29和TiNb2O7具有不同的空间群A2/m和C2/m,但是其晶体结构均为层状单斜结构,具有相同的氧化还原对Ti4+/Ti3+和Nb5+/Nb3+,嵌入电压约为1.6 V [14]。它们的理论容量分别为396 mAh·g-1和387.6 mAh·g-1,可逆容量分别为247 mAh·g-1 和 280 mAh·g-1。鉴于此,可选择Ti2Nb10O29[15,16]和TiNb2O7 [17,18,19]铌钛氧化物作为锂离子电池的负极材料。Ti2Nb10O29和TiNb2O7的Ti4+和Nb5+离子的摩尔比分别为1:5和1:2,因此价格昂贵的铌含量较低的负极材料TiNb2O7比Ti2Nb10O29具有更大的优势。TiNb2O7的合成温度,受原材料的种类、初始粒度、合成方法和气氛等多种因素的影响。Ram Avtar Jat等[20]在1000℃保温48 h制备出TiNb2O7,Xia等[21]在1350℃保温24 h制备出TiNb2O7,而Han等[22]在1100℃煅烧24 h也制备出TiNb2O7。本文研究TiNb2O7合成过程中各物相的转变过程和TiNb2O7的合成机理。
1 实验方法
1.1 材料的合成
实验用原材料:锐钛矿(纯度为99.5%,平均粒径为100 nm),Nb(OH)5(化学纯)和乙醇(化学纯)。将按照TiNb2O7的化学计量比称取的原材料球磨以使其混合均匀,乙醇为分散剂,然后将其进一步球磨、干燥和筛分。将筛分后的原材料置于马弗炉中在不同温度(400℃、800℃、900℃、1000℃和1100℃)空气气氛下煅烧8 h,冷却至室温得到不同的产物。将产物研磨过筛备用。
1.2 产物的表征
用型号为STA449F3的同步热分析仪分析TiNb2O7前驱体在升温过程中热量和质量的变化,温度范围为25~1300℃,氮气气氛,升温速率为2.5℃/min。用X射线衍射仪(XRD, D8 ADVANCE, Bruker, German)表征在不同温度制备的样品的物相,Cu靶,Ka射线(λ=0.15406 nm)。使用Bruker Topas 4.2软件对得到的XRD图谱进行Rietveld结构精修,并对样品中各物相进行无标定量。用场发射扫描电镜(FESEM, Hitachi S-4800,Japan)观测样品颗粒的形貌。
使用N-甲基-2-吡咯烷酮作为溶剂,将混合活性材料(90%)、碳黑(5%, Timcal, Super-P)和PVDF粘合剂(5%, Type 761A, ARKEMA)制成电极,铝箔作为集流体。将电极在120℃真空烘箱中干燥10 h后冲片,将其称重并在80℃真空干燥10 h。将干燥后的电极放入手套箱(MBraun, Germany)中,用金属锂片作为对电极,使用Celgard 2400微孔聚丙烯膜作为隔膜,1 mol/L LiPF6/EC+DEC+EMC(体积比为1:1:1)溶液为电解液,在充满氩气的手套箱中组装成CR2032型扣式电池。对CR2032纽扣电池进行电化学测量。使用CT2001A电池测试仪(5 V-10 mA)在25℃进行充放电测试,电压范围为0.8~2.5 V。
2 结果和讨论
2.1 TG-DSC分析
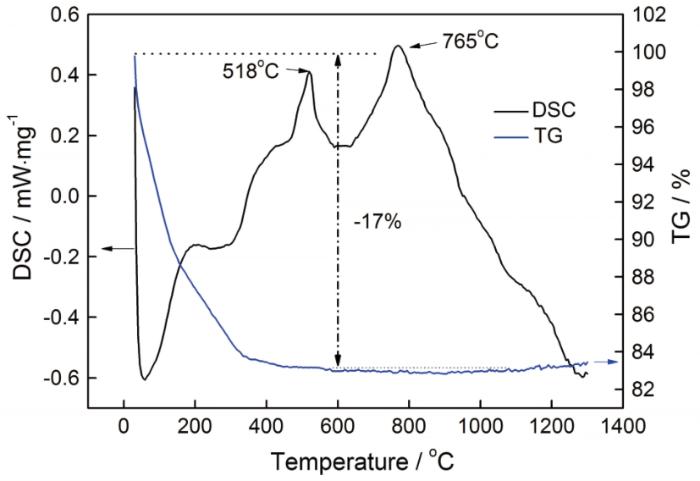
对混合均匀的TiNb2O7前驱体进行TG-DSC测试,结果如图1所示。TG曲线表明,TiNb2O7前驱体的总质量损失约为17%,主要与Nb(OH)5分解并蒸发掉部分H2O有关。温度低于200℃时,大部分H2O从Nb(OH)5中分解并蒸发掉。当温度升高至接近400℃时,Nb(OH)5中的结合水完全消失。温度高于600℃时TG曲线保持稳定,表明化合物的质量没有变化。由DSC曲线可见,在200℃以下有一个较大的吸热峰,与TG曲线中的H2O分解蒸发对应;在518℃和765℃均出现了放热峰,表明化合物中有新相生成。
图1
2.2 XRD分析
对不同温度(400℃、800℃、900℃、1000℃和1100℃)下制备的TiNb2O7样品进行了XRD分析,结果如图2所示。由图2可见,TiNb2O7的前驱体主要是锐钛矿和无定型结构的Nb(OH)5组成。温度为400℃时Nb(OH)5的馒头峰基本消失,出现了尖锐的Nb2O5衍射峰,说明Nb(OH)5分解转变为Nb2O5;在800℃出现了Ti2Nb10O29衍射峰,在900℃出现了金红石的衍射峰,表明在此温度下部分TiO2由锐钛矿结构转变成了金红石结构;在1000℃时Ti2Nb10O29衍射峰强度减弱,TiNb2O7衍射峰强度增强;在1100℃仅有TiNb2O7衍射峰,说明在此温度样品物相为纯净的TiNb2O7。
图2
图2
在不同温度合成的TiNb2O7的XRD图谱
Fig.2
X-ray diffraction powder patterns of TiNb2O7 obtained at different temperatures
TiNb2O7的全合成反应为
从图2a和b可见,在400℃煅烧8 h后原材料中的Nb(OH)5被Nb2O5取代,与TG-DSC分析结果一致,表明
表1 在不同温度合成的样品的XRD无标定量分析数据
Table 1
| Sintering parameter | Raw material | 400℃-8 h | 800℃-8 h | 900℃-8 h | 1000℃-8 h | 1100℃-8 h |
|---|---|---|---|---|---|---|
| Anatase | 23.10%* | 27.38% | 22.16% | 16.84% | - | 0 |
| Rutile | - | - | - | 4.18% | 6.13% | 0 |
| Nb2O5 | 76.90%* | 72.62% | 3.86% | - | - | 0 |
| Ti2Nb10O29 | - | - | 67.42% | 71.51% | 43.12% | 0 |
| TiNb2O7 | - | - | 6.56% | 7.47% | 50.75% | 100% |
| Rwp% | 10.57% | 14.50% | 15.68% | 12.87% | 15.03% |
一致,微量的TiNb2O7生成按
进行。温度升至900℃时Nb2O5衍射峰消失,锐钛矿的衍射峰减弱,同时出现了金红石的衍射峰,TiNb2O7衍射峰略有增加,此时Ti2Nb10O29含量为71.51%,达到最大值,表明反应
在1100℃保温8 h生成了纯的单斜相TiNb2O7,同时Ti2Nb10O29和金红石的衍射峰消失,1100℃时的反应主要是
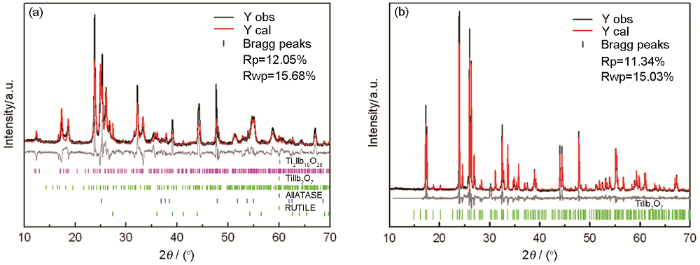
在900℃和1100℃烧结制备样品的Rietveld精修图谱如图3a和b所示。由图3a可知,900℃时样品的主要物相为Ti2Nb10O29,还有少量的TiNb2O7、锐钛矿和金红石。根据比对的JCPDS 72-0159和空间群A2/m(12),通过X射线Rietveld精修得出了Ti2Nb10O29的晶格参数(表2)。在900℃烧结的负极材料Ti2Nb10O29其晶格参数为a=1.5599 nm,b=0.3815 nm和c=2.0534 nm。由图3b可知,在1100℃烧结制备出了不含杂质的单斜相TiNb2O7。根据比对的JCPDS 77-1374和空间群C2/m(12),得到TiNb2O7的晶格参数。TiNb2O7的晶格参数为a=2.0376 nm,b=0.3802 nm,c=1.1894 nm。用固相法合成制备的Ti2Nb10O29和TiNb2O7负极材料的晶格参数,与文献[23]的结果基本一致。
图3
图3
在900℃保温8 h和在1100℃保温8 h样品的X射线Rietveld精修图谱
Fig.3
X-ray Rietveld refined patterns of sample sintered at 900℃ for 8 h (a) and 1100℃ for 8 h (b)
表2 TiNb2O7 和 Ti2Nb10O29的晶格参数
Table 2
| Compounds/Crystal | Lattice parameters (nm) | JCPDS file no. | |
|---|---|---|---|
| structure (space group) | This work | Literature[23] | |
| TiNb2O7monoclinic(C2/m(12)) | a = 2.0376 | a = 2.0351 | 77-1374 |
| b =0.3802 | b = 0.3801 | ||
| c = 1.1894 | c = 1.1882 | ||
| β (°) =120.19 | β (°) =120.19 | ||
Ti2Nb10O29monoclinic (A2/m(12)) | a = 1.5599 | a = 1.557 | 72-0159 |
| b = 0.3815 | b = 0.3814 | ||
| c = 2.0534 | c = 2.054 | ||
| β (°) = 113.683 | β (°) = 113.683 | ||
2.3 粉末前驱体和在不同温度煅烧的样品的形貌
对粉末前驱体和在不同温度下煅烧的样品进行了SEM观测,结果如图4所示。物料颗粒略有团聚,主要是球磨物料提高了颗粒表面的活性所致。由图4a和b可见,与前驱体相比,在400℃煅烧制备样品的形貌没有明显的变化。图4c表明,在800℃圆形颗粒略有长大,出现了大量棒状物,XRD分析结果表明棒状物为Ti2Nb10O29。图4d表明,与800℃相比900℃时颗粒均有长大,棒状物增多,圆形颗粒减少。图4e表明,在1000℃圆形颗粒继续长大,棒状颗粒边界逐渐变得不清晰。图4f表明,在1100℃呈棒状的Ti2Nb10O29完全消失,所有颗粒呈圆形,粒径约为1 μm。这些结果表明,在空气气氛中用固相法制备的Ti2Nb10O29为棒状结构,TiNb2O7为较均匀的圆形颗粒。
图4
图4
前驱体、在不同温度煅烧的TiNb2O7粉末的扫描电子显微镜图像及其相应的放大图像
Fig.4
Scanning electron microscopy images of precursor and TiNb2O7 powders calcined at different temperature and the corresponding magnified images (a, a’) precursor; (b, b’) 400℃; (c, c’) 800℃; (d, d’) 900℃; (e, e’) 1000℃; and (f, f’) 1100℃
2.4 充放电曲线
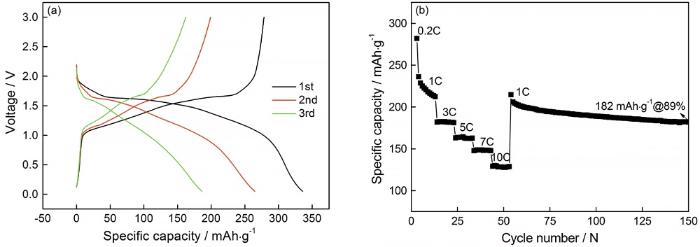
对在1100℃空气气氛中煅烧8 h制备的TiNb2O7进行了电化学性能检测,TiNb2O7的Li嵌入电压约为1.6 V,测试电压区间为0.8~2.5 V(vs. Li+/Li),结果如图5所示。图5a给出了TiNb2O7负极材料在0.2C电流密度下的充放电曲线。首次循环时TiNb2O7负极材料嵌锂容量为335.7 mAh/g,充电时脱锂容量为278.4 mAh/g,充放电的库仑效率为82.91%。主要原因是,TiNb2O7负极材料本征离子和电子导电性低,使部分嵌入的锂离子在充电过程中不能完全脱出;第二次循环时容量衰减,库仑效率为75.11%;第三次循环容量衰减减少,库仑效率增加到87.16%。图5b给出了TiNb2O7负极材料从1C到10C各循环10次再回到1C的倍率性能测试图,在1C、3C、5C、7C和10C电流密度下平均放电容量大致为204.5、181.6、162.3、147.2和128 mAh/g,表明TiNb2O7负极材料有良好的倍率容量。当电流密度重新回到1C循环100次后放电比容量为182 mAh/g,与1C初始循环放电比容量相比,Li的嵌入/脱出容量保持率达89%。这表明,TiNb2O7负极材料在循环后的容量恢复较好,具有较好的电化学可逆性。
图5
图5
TiNb2O7电极的0.8-2.5 V电化学性能
Fig.5
Electrochemical performance of TiNb2O7 electrode in a voltage range from 0.8 to 2.5 V (a) Initial charge/discharge curves at 0.2C and (b) Discharge capacity during cycling at different current rates
3 结论
(1) 用固相法合成制备TiNb2O7负极材料,Ti2Nb10O29棒状颗粒与金红石反应生成TiNb2O7,在1100℃煅烧6 h能制备出纯净的均匀圆形颗粒单斜相TiNb2O7,粒径约为1 μm。
(2) 在0.2C电流密度条件下TiNb2O7负极材料的初始容量为278.4 mAh/g,初始库伦效率为82.91%。TiNb2O7具有良好的倍率容量,在1C循环100次后容量保持率为89%。
参考文献
Research progress of preparation and application of cathode material for lithium ion battery
[J].
锂离子电池负极材料的制备及应用进展
[J].
Research progress of composite anode materials with high-capacity for lithium-ion batteries
[J].
锂离子电池用高容量复合负极材料的研究进展
[J].
The Li-ion rechargeable battery: a perspective
[J].
A review of advanced and practical lithium battery materials
[J].
Titanium-based anode materials for safe lithium-ion batteries
[J].
Zero-strain insertion material of Li[Li1/3Ti5/3]O4 for rechargeable lithium cells
[J].
Ti-based compounds an anode materials for Li-ion batteries
[J].
Flexible graphene-based lithium ion batteries with ultrafast charge and discharge rates
[J].
Nanosize storage properties in spinel Li4Ti5O12 explained by anisotropic surface lithium insertion
[J].
A novel Li4Ti5O12-based high-performance lithium-ion electrode at elevated temperature
[J].
Investigation on Ti2Nb10O29 anode material for lithium-ion batteries
[J].
New anode framework for rechargeable lithium batteries
[J].
Mixed Oxides of titanium and niobium.Ⅱ. the crystal structures of the dimorphic forms of Ti2Nb10O29
[J].
Solid-state synthesis of Ti2Nb10O29/reduced graphene oxide composites with enhanced lithium storage capability
[J].
Synthesis of Ti2Nb10O29/C composite as an anode material for lithium-ion batteries
[J].
“Nano-Pearl-String” TiNb2O7 as Anodes for Rechargeable Lithium Batteries
[J].
Porous TiNb2O7 Nanospheres as ultra Long-life and High-power Anodes for Lithium-ion Batteries
[J].
Cu0.02Ti0.94Nb2.04O7:an advanced anode material for lithium-ion batteries of electric vehicles
[J].
Synthesis, characterization and heat capacities of ternary oxides in the Ti-Nb-O system
[J].
Atomic-scale investigation on lithium storage mechanism in TiNb2O7
[J].
Goodenough. 3-V Full Cell Performance of Anode Framework TiNb2O7/Spinel LiNi0.5Mn1.5O4
[J].
POWD-an Interactive Powder Diffraction Data Interpretation and Indexing Program, Version 2.