非金属光催化材料石墨相氮化碳(g-C3N4)的化学性和热稳定性良好且其带隙合适和对环境友好,是一种理想的光催化材料[13]。但是,石墨相氮化碳的电子-空穴复合速度过高和电子迁移方向混乱而使其光催化效率较低。将石墨相氮化碳与二维材料复合,可构建g-C3N4基光催化材料异质结,例如g-C3N4/TiO2[14], g-C3N4/WO3[15], g-C3N4/CeO2[16],g-C3N4/ZnO[17],g-C3N4/Bi2O3[18], g-C3N4/Co3O4[19]等,使其光催化反应的效率显著提高。Gao等[14]将(001)-TiO2纳米颗粒沉积在g-C3N4表面制备出具有非均相的异质结复合材料,在光照60 min后对MB的降解率为95%,其降解速率是(001)-TiO2的7倍,是g-C3N4的4倍。Wang等[16]使用水热法合成的2D/1D g-C3N4/CeO2 S型异质结光催化剂具有良好的光降解性能,在120 min内对RhB的去除率为99.07%。与原始的CeO2纳米棒和CN纳米片相比,g-C3N4/CeO2 S型异质结的CH4产率分别是其22.4倍和20.9倍。石墨相氮化碳与二维材料可构建具有合适能带位置的S型异质结光催化剂,引入的内建电场能调节载流子的迁移方向和分离和传输载流子,从而具有更强的光催化活性和更高的产率[13]。因此,构建载流子分离能力高的S型异质结是提高光催化活性的重要策略之一[20~23]。本文采用水热法将非金属聚合物g-C3N4与合成的CdS复合制备g-C3N4/CdS S型光催化材料,研究其光催化性能并揭示其可能的光催化机理。
1 实验方法
1.1 光催化剂的制备
将5 g三聚氰胺(C3H6N6)放入马弗炉中以10 ℃/min的速率加热到500 ℃,恒温加热4 h后随炉冷却。取出块状样品并将其研磨成细粉末状,即得g-C3N4(CN)。
配制50 mL浓度为0.5 mol/L的二水柠檬酸三钠和0.2 mol/L的酒石酸。将0.02 mol/L氯化镉溶于120 mL去离子水中形成溶液A。在溶液A中加入5 mL的0.4 mol/L的氨水溶液并持续搅拌,然后再分别加入5 mL柠檬酸三钠和5 mL酒石酸混合液(作为络合剂),再将80 mL硫脲溶液(0.05 mol/L)匀速滴入并持续搅拌使药品完全溶解,随后放入90 ℃水浴锅中持续搅拌反应3 h。最后用去离子水和酒精将所得沉淀离心洗涤使其pH值变为中性,然后放入60 ℃干燥箱中干燥12 h得到CdS。
先配制50 mL的0.5 mol/L的二水柠檬酸三钠和0.2 mol/L的酒石酸。在溶液A中加入5 mL浓度为0.4 mol/L的氨水溶液并持续搅拌,然后分别加入5 mL的0.5 mol/L的柠檬酸三钠和5 mL的0.2 mol/L的酒石酸混合液(作为络合剂)。再将溶解在去离子水中的硫脲缓慢滴入持续搅拌的A溶液中,然后分别加入1 g、2 g和4 g的CN悬浊液,最后将其放入90 ℃的水浴锅中持续搅拌反应3 h。用去离子水和乙醇离心洗涤所得沉淀使其pH值变为中性。将所得样品放入60 ℃干燥箱中干燥12 h,再将其研磨成粉末即得到质量分数分别为0.5%、1%、2%的g-C3N4/CdS(GCS)复合材料。制备流程如图1a所示。
图1
图1
二元复合纳米材料GCS的合成流程和催化降解流程
Fig.1
Synthesis flow diagram (a), catalytic degradation flow diagram (b) of binary composite nanomaterials GCS
1.2 样品的表征
用扫描电子显微镜(SEM)观察样品的微观形貌。用X射线衍射仪(XRD)分析样品的晶体结构和物相组成,扫描时衍射角2θ范围设定为10°~80°。用X 射线光电子能谱(XPS)仪测试样品的表面的化学组成和化学态;用光致发光光谱(PL)仪测量样品受光激发后发射的光的波长和强度,来分析样品的发光特性。用带有滤光片的300 W的氙灯(MC-PF30)进行光催化降解实验。用紫外可见分光光度计(UV-vis)测定有机污染物溶液的吸光度,根据吸光度的变化研究其降解效率。
光催化实验中的催化流程图如图1b所示。在60 mL成浓度为10 mg/L的甲基蓝溶液中加入80 mg光催化剂,随后置于超声波清洗仪中在黑暗环境中超声30 min使催化剂均匀地分散在甲基蓝溶液中形成稳定的悬浊液,以使复合样品达到吸附平衡。在可见光试验箱中氙灯的正下方放入磁力搅拌仪,氙灯照射悬浊液使光催化反应进行。在照射过程中计时,每隔15 min用注射器收集8 mL上清液,用紫外-可见分光光度计测量其吸光度。
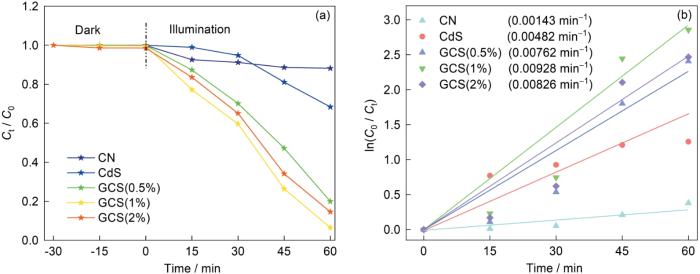
如图2所示,分别测量加入五种不同光催化剂溶液的吸光光谱。最大吸收波长峰下污染物的浓度表明,CdS的催化效率较低,而复合样品的催化效率并不随着石墨相氮化碳量的增加而增加。实验结果表明,GCS(1%)复合材料光催化效果最好。最后,根据Lambert-Beer定律
图2
图2
CN、CdS、GCS(0.5%)、GCS(1%)和GCS(2%)的吸收光谱
Fig.2
Absorption spectra of CN (a), CdS (b), GCS(0.5%) (c), GCS(1%) (d) and GCS(2%) (e)
计算降解过程中目标污染物溶液的浓度,画出有机污染物的标准曲线[24]。
确定有机污染物的降解效率,绘制出降解曲线[25]。
将80 mL的甲基蓝溶液(10 mg/L)倒入100 mL烧杯中,再加入60 mg光催化剂。将烧杯置于超声波清洗仪中超声30 min使催化剂均匀地分散在甲基蓝溶液中形成稳定的悬浊液。在可见光试验箱中氙灯的正下方放入磁力搅拌仪,开灯前将上面的烧杯放在磁力搅拌器上,在黑暗环境下搅拌5 min使甲基蓝在复合样品表面达到吸附平衡并暗反应30 min,然后打开氙灯照射悬浊液,开始光催化反应。在照射过程中计时,每隔15 min收集8 mL上清液,待完全降解完后将溶液洗涤数次使其pH值呈中性,然后放入60 ℃的干燥箱中干燥12 h,补齐实验中损失的光催化剂使其重量达到80 mg,再进行催化降解,如此循环三次。
2 结果和讨论
2.1 结构分析
2.1.1 SEM分析
从图3a可以看出,纯CN的团聚显著,且大多呈不规则的块状堆积结构。从图3b可见,CdS呈球形结构,是由更细的颗粒堆积成的。这种形貌结构使CdS纳米颗粒很容易沉积在CN的表面。从图3e可见,块状堆积结构上面附着有球状的颗粒,将其放大得到图3d。从图3d可见CN和CdS两组分交联构建的GCS复合材料的形貌。可以看出,CdS颗粒在CN的内外表面均有分布,这种紧密接触的结构有利于光生载流子在两个带隙之间迁移。粗糙的表面也有利于增大样品的比表面积。GCS(1%)复合材料映射图对应的样品的元素分布如图3c所示。可以看出,四种元素的分布均匀,表明CN和CdS两种成分是均匀结合的。图3f统计了GCS(1%)复合样品中各元素的含量,可见在复合样品中存在单个样品的所有元素,与本文实验方案中的实验数据吻合。
图3
图3
g-C3N4、CdS和复合材料GCS (5 μm且局部放大到1 μm)的SEM图像以及复合材料GCS中的元素分布和能谱
Fig.3
SEM image of g-C3N4 (a); SEM images with CdS (b); SEM image of composite GCS at 5 μm (e) and SEM image locally enlarged to 1 μm (d), and distribution of elements (c) and energy spectrum (f) in GCS of composite materials
2.1.2 材料的组成和晶体结构
图4
图4
CN,CdS,GCS (0.5%),GCS (1%)和GCS (2%)复合材料的XRD谱
Fig.4
XRD patterns of CN, CdS, GCS (0.5%),GCS (1%) and GCS (2%)
2.1.3 样品的光致发光光谱分析
图5
图5
不同波长激发GCS(1%)复合样品的荧光光谱和同一波长激发样品的荧光光谱
Fig.5
Fluorescence spectra of GCS(1%) composite samples at different excitation wavelengths (a) and each sample at the same excitation wavelength (b)
2.1.4 CN、CdS和GCS的化学状态
根据X射线光电子能谱(XPS)检查CN、CdS和GCS中的化学状态。如图6a所示,与单独的CdS和CN相比,GCS不仅含有CdS和CN的所有元素,还含有少量的O元素,表明其表面吸收了空气中少量的氧元素。图6b给出了GCS与CN的C 1s谱图,将其中一个C-C峰设置为284.8 eV校准其余谱图。进行高斯函数拟合以分析GCS和CN中官能团的数量和类型。在CN的C 1s谱图中的288.63、286.82和285.07 eV处的三个峰分别对应N-C=N、C=O和C=C键,GCS谱中三个峰的位置产生了一定的偏移,可归因于复合材料中生成了异质结。图6c给出了GCS与CN的N 1s谱,CN的N 1s核心能级光谱由位于401.06、400.12和398.74 eV的三个峰组成,可归因于C-N-H、N-(C)3和C-N=C键的作用[27,28]。图6d给出了GCS和CdS的Cd 3d谱,411.92和405.12 eV处的两个峰分别对应CdS键中Cd原子的Cd 3d3/2和Cd 3d5/2状态。对S 2p进行高斯拟合并将其解卷积为两个双峰,如图6e所示。两个位于162.83和161.35 eV的峰,分别对应S元素的S 2p1/2和S 2p3/2[29]。
图6
图6
GCS、CN、CdS、C 1s、N 1s、Cd 3d和S 2p的XPS总谱和轨道电子谱
Fig.6
Total XPS spectra (a) GCS, CN, CdS and their orbital electron spectra (b) C 1s; (c) N 1s; (d) Cd 3d and (e) S 2p
2.2 材料的光催化性能
图7
图7
催化剂对MB的降解曲线及其动力学曲线
Fig.7
Degradation curves (a) and dynamic curves (b) of MB under different catalysts
图8
图8
GCS (1%)催化剂的循环降解图和降解柱状图
Fig.8
Cyclic degradation diagram (a) and degradation column diagram (b) of GCS (1%) catalyst
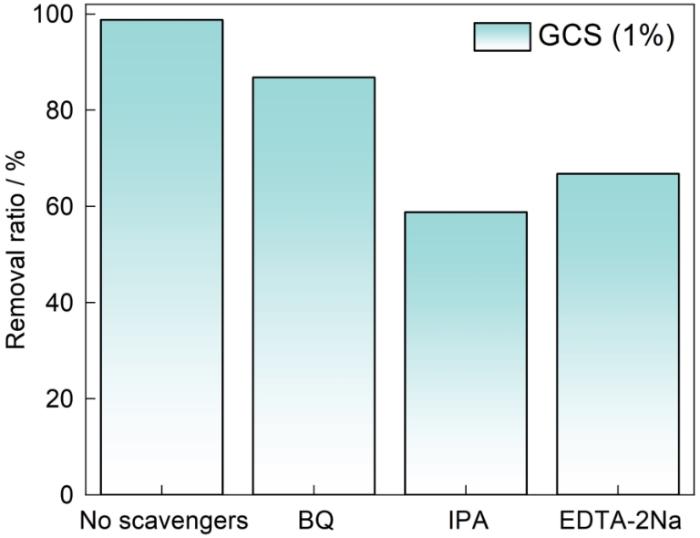
为了研究反应过程中生成的活性物质对反应效率的影响,进行了对不同活性物质的捕获实验。在该反应体系中,牺牲剂的浓度为10-4 mol/L,分别用对苯醌(BQ)捕获·O
图9
如图10a~c所示,根据对各样品的价带XPS光谱(VB-XPS)的测量,得到了CN、CdS和GCS的功函数。当两种半导体材料相互接触时电子被功函数较大的半导体材料吸引,直到两种材料的费米能级达到平衡。因此,功函数小的材料表面带正电,功函数大的材料表面带负电,从而在接触界面处形成内建电场。可根据
图10
计算半导体的功函数,式中Φ(4.55 eV)为样品的功函数,ΔV为IP1和IP2之间的距离,IP1为结合能相对于基线的变化点,IP2为费米边缘曲线的中点。计算结果表明,CN、CdS和GCS的两个IP点之间的距离分别为1.03、1.22和1.14 eV,进而求出CN、CdS和GCS的功函数分别为5.58、5.77和5.69 eV。CN的功函数小比CdS的小而容易失去电子,于是生成由CN指向CdS方向的内建电场并使能带弯曲。有XPS谱测处CN和CdS的价带电位分别1.38和1.68 eV,如图10a、b所示。根据带隙计算出CN和CdS的价带电位分别为1.53和1.83 eV,导带电位分别为-1.13和-0.44 eV [31]。
图11给出了二维光催化复合材料GCS的光活性机理,CdS作为边缘位置之间的敏化剂,CN作为衬底,通过两种半导体之间的带边对齐特性形成了典型的S型异质结。由于CdS和CN的导带和价带显著不同,在界面处产生了一个驱动载流子分离的内建电场。这一内建电场方向从CN指向CdS,有助于实现光生电子和空穴的高效分离。光照射到S型异质结表面,将CdS的导带中光生电子驱动到CN价带与空穴复合。CN导带中较强还原能力的光生电子与溶解氧反应生成·O
图11
图11
二元复合光催化剂(GCS)降解MB机理
Fig.11
Mechanism of MB degradation by binary composite photocatalyst (GCS)
3 结论
(1) 用热缩聚法方法可制备具有光催化性能的层状CN纳米片,用水热法可将CdS纳米颗粒与其复合制备稳定性良好的S型异质结GCS复合光催化材料。
(2) 这种光催化剂具有较好活性的原因是在CN和CdS之间构建了S型异质结,产生的内建电场使光生电子和空穴高速转移并阻止电子和空穴的重组。
参考文献
A novel dual-channel carbon nitride homojunction with nanofibrous carbon for significantly boosting photocatalytic hydrogen peroxide production
[J].
Heterostructuring noble-metal-free 1T' phase MoS2 with g-C3N4 hollow nanocages to improve the photocatalytic H2 evolution activity
[J].
Unveiling the bifunctional photo/electrocatalytic activity of in situ grown CdSe QDs on g-C3N4 nanosheet Z-scheme heterostructures for efficient hydrogen generation
[J].
Co-Cu-P nanosheet-based open architecture for high-performance oxygen evolution reaction
[J].
Novel urchin-like CoNiP as advanced pH-universal electrocatalysts toward hydrogen evolution reaction
[J].
Carbon layer derived carrier transport in Co/g-C3N4 nanosheet junctions for efficient H2O2 production and NO removal
[J].
Preparation of highly dispersed Ni single-atom doped ultrathin g-C3N4 nanosheets by metal vapor exfoliation for efficient photocatalytic CO2 reduction
[J].
Acid-induced topological morphology modulation of graphitic carbon nitride homojunctions as advanced metal-free catalysts for OER and pollutant degradation
[J].
Zinc vacancy-promoted photocatalytic activity and photostability of ZnS for efficient visible-light-driven hydrogen evolution
[J].
Isolated Cu sites in CdS hollow nanocubes with doping-location-dependent performance for photocatalytic CO2 reduction
[J].
Enhanced photocatalytic activity and stability of Zn x Cd1- x /TiO2 nanocomposites synthesized by chemical bath deposition
[J].
Visible light-driven photocatalytic degradation of methylene blue dye using a highly efficient Mg-Al LDH@g-C3N4@Ag3PO4 nanocomposite
[J].
g-C3N4 -based S-scheme heterojunction photocatalysts
[J].
Construction of (001)-TiO2/g-C3N4 heterojunction for enhanced photocatalytic degradation of methylene blue
[J].
Facile ball-milling synthesis of WO3/g-C3N4 heterojunction for photocatalytic degradation of Rhodamine B
[J].
A directional built-in electric field assisted 2D/1D g-C3N4/CeO2 S-scheme heterojunction for efficient RhB degradation and highly-selective CO2 photoreduction
[J].
Ternary nanocomposite ZnO-g-C3N4-Go for enhanced photocatalytic degradation of RhB
[J].
Photocatalytic degradation of tetracycline hydrochloride by g-C3N4 modified Bi2O3
[J].
g-C3N4改性Bi2O3对盐酸四环素的光催化降解
[J].使用液相沉淀法和热聚合法制备Bi<sub>2</sub>O<sub>3</sub>/g-C<sub>3</sub>N<sub>4</sub>复合催化剂,用SEM、XRD、XPS、FT-IR和紫外可见漫反射等手段对其微观形貌、晶体结构和光催化性能进行了表征。结果表明,这种Bi<sub>2</sub>O<sub>3</sub>/g-C<sub>3</sub>N<sub>4</sub>复合光催化剂的形貌较好、分布均匀,具有较高的光催化性能;复合催化剂Bi<sub>2</sub>O<sub>3</sub>/g-C<sub>3</sub>N<sub>4</sub>-30%的光催化性能最好,用300 W模拟可见光氙灯照射2 h后对盐酸四环素(TCH)的去除率为70%;捕获实验的结果表明,光催化降解盐酸四环素(TCH)的主要活性物种为超氧自由基(·O2-)。
Preparation and photocatalytic activity of Co3O4/g-C3N4 composite photocatalysts via one-pot synthesis
[J].
一锅法制备Co3O4/g-C3N4复合光催化剂及其光催化性能
[J].
Vertically implanting MoSe2 nanosheets on superior thin C-doped g-C3N4 nanosheets towards interface-enhanced electrochemical activities
[J].
Accelerating photocatalytic hydrogen production by anchoring Pt single atoms on few-layer g-C3N4 nanosheets with Pt-N coordination
[J].
Controllable preparation of Zn x Cd1 - x S films by chemical bath deposition for enhanced photocatalytic activity
[J].
Construction of Zn x Cd1- x S/CeO2 composites for enhanced photocatalytic activity and stability by chemical precipitation method
[J].
Enhanced photoelectrochemical water splitting, and photocatalytic and piezo-photocatalytic pollutant removal performance over CdS/g-C3N4/ZnO ternary heterojunctions
[J].
Facile fabrication of g-C3N4/CdS heterojunctions with enhanced visible-light photocatalytic degradation performances
[J].
Fabrication of CdS NRs/MoS2@g-C3N4 nanotubes for efficient photoelectrochemical hydrogen production
[J].
Constructing 0D/2D Z-scheme heterojunction of CdS/g-C3N4 with enhanced photocatalytic activity for H2 evolution
[J].
Enhanced photocatalytic hydrogen evolution based on ternary noble-metal-free Co3O4/CdS/g-C3N4 composite
[J].
CdS-modified one-dimensional g-C3N4 porous nanotubes for efficient visible-light photocatalytic conversion
[J].<p>A heterojunction photocatalyst based on porous tubular g-C<sub>3</sub>N<sub>4</sub> decorated with CdS nanoparticles was fabricated by a facile hydrothermal co-deposition method. The one-dimensional porous structure of g-C<sub>3</sub>N<sub>4</sub> provides a higher specific surface area, enhanced light absorption, and better separation and transport performance of charge carriers along the longitudinal direction, all of which synergistically contribute to the superior photocatalytic activity observed. The significantly enhanced catalytic efficiency is also a benefit originating from the fast transfer of photogenerated electrons and holes between g-C<sub>3</sub>N<sub>4</sub> and CdS through a built-in electric field, which was confirmed by investigating the morphology, structure, optical properties, electrochemical properties, and photocatalytic activities. Photocatalytic degradation of rhodamine B (RhB) and photocatalytic hydrogen evolution reaction were also carried out to investigate its photocatalytic performance. RhB can be degraded completely within 60 min, and the optimum H<sub>2</sub> evolution rate of tubular g-C<sub>3</sub>N<sub>4</sub>/CdS composite is as high as 71.6 μmol h<sup>-1</sup>, which is about 16.3 times higher than that of pure bulk g-C<sub>3</sub>N<sub>4</sub>. The as-prepared nanostructure would be suitable for treating environmental pollutants as well as for water splitting.</p>
Sodium gluconate assisted synthesis of nest-like Bi/β-Bi2O3 heterojunction and its visible-light driven photocatalytic activities
[J].
鸟巢状Bi/β-Bi2O3异质结的制备及其可见光催化性能
[J].
Rational construction of a direct Z-scheme g-C3N4/CdS photocatalyst with enhanced visible light photo-catalytic activity and degradation of erythromycin and tetracycline
[J].
The modification of graphite carbon nitride and its applications in photocatalysis
[J].
石墨相氮化碳的改性及其在光催化中的应用进展
[J].
Preparation and visible light catalytic performance of g-C3N4/POPs heterojunction
[J].
g-C3N4/POPs异质结制备及其可见光催化性能
[J].
Sulfur-doped g-C3N4/TiO2 S-scheme heterojunction photocatalyst for Congo Red photodegradation
[J].