以减少CO2排放为目标的化学工业去化石化,受限于许多含碳基产品的使用。为此,可应用电化学甘油氧化反应(Glycerol oxidation reaction,GOR)等技术将生产生物柴油的(副产品)甘油转化[1]。甘油的理论能量密度为5.00 kWh/kg,与其他燃料如甲醇(6.10 kWh/kg)和乙醇(8.00 kWh/kg)的能量密度相当[2]。在温和条件下可使用电催化剂将甘油选择性氧化,但是缺少高活性、高选择性和高稳定性的电催化剂[1]。铂(Pt)是一种性能优异的GOR电催化剂,但是其资源稀缺特别是CO中毒使其很快失活。对于在中等温度运行的燃料电池,钯(Pd)或Pd基电催化剂具有优异的抗中毒性和较高的电流密度且成本较低[3]。在自组装过程中,Pd纳米粒子可充当构建块构建一维(1D)、二维(2D)和三维(3D)纳米结构。Pd纳米片(Palladium Nanosheets,PdNSs)的表面积较大,可为反应物提供更多的活性位点[1]。2D金属纳米片对于直接醇类燃料电池具有优异的电化学行为[4]。超薄纳米片PdNSs具有更高的甘油电催化氧化活性。Liu等[5]用简单的湿化学方法制备了厚度约为1.20 nm的磷掺杂超薄和弯曲的钯-钼纳米片(P-PdMo),在碱性介质中对甘油的电氧化表现出CO抗中毒性能和优异的电氧化性能,质量活性高达4.06 A/mg。Wang等[6]在CO辅助下合成出分层多孔的PdRuC纳米片,具有较大的电化学活性表面积、丰富的PdRuCu活性位点和稳定的纳米片结构,对GOR的质量活度和比表面活度分别达到1083 mA/mg和38.80 A/m2。
用缺陷工程可调整纳米材料的电子及表面性质,暴露更多的催化剂活性位点,调整催化剂的表面结构和提高其催化活性[7~11]。因此,根据设计制备策略和后处理产生表面缺陷,可制备具有较高的活性、稳定性和电化学性能的富缺陷PdNSs。模板合成法的产率高、成本低和工艺简便,可制备2D纳米材料[12]。十六烷基三甲基溴化铵(CTAB)是一种阳离子型表面活性剂,具有良好的表面活性、稳定性及乳化作用,可与纳米粒子表面紧密结合形成表面活性剂双层胶束结构,为纳米粒子的生长提供模板。同时,CTAB分子可稳定胶体溶液,限制其暴露于大气中氧化;CTAB分子还能增大纳米粒子之间的静电斥力使金属纳米粒子分散,使其稳定性提高和防止其聚集以增大其表面积和平滑度[13]。本文制备富缺陷超薄PdNSs,研究GOR并揭示PdNSs的生成机理。
1 实验方法
1.1 PdNSs电催化剂的制备
将0.70 × 10-2 g的Pd(NO3)2·2H2O、1.80 × 10-2 g的抗坏血酸(AA,AR)和2.80 g的CTAB (AR)溶于水/乙醇(体积比4∶1)体系中并用功率为500 W的超声波细胞粉碎机搅拌,在(14 ± 1) ℃和氮气(N2)保护下反应3 h,得到黑色悬浮液。将黑色悬浮液依次用去离子水和乙醇(C2H5OH,AR)充分离心洗涤(各3次),将得到的产物在38 ℃真空干燥后得到PdNSs电催化剂。
1.2 性能表征
将1.37 g的CTAB,配制成浓度为1.50 × 10-2 mol/L的CTAB母液,用水/乙醇(体积比4∶1)体系将母液稀释成不同浓度的CTAB溶液。在25 ℃测试不同浓度CTAB溶液的紫外吸收光谱。用紫外可见分光光度计(UV-vis,T9)测量样品在200~500 nm内的吸光度(A);用场发射扫描电镜(FESEM,Sirion200)观察样品的形貌;采用透射电子显微镜(TEM,JEOL-2010)和高分辨率透射电镜(HRTEM,JEM-2100f)分析微观形貌;采用X射线衍射仪(XRD,XD-3)测定样品的XRD谱,以Cu Kα为放射源,2θ范围为30°~90°。用CHI760E电化学工作站检测PdNSs对甘油的电催化氧化。三电极体系中,Pt丝为对电极,饱和甘汞电极(SCE)为参比电极,纳米Pd修饰的玻碳电极(GCE)为工作电极。将Pd的乙醇悬浮液6 μL滴在干净的纳米Pd修饰的GCE表面,在室温下干燥后再滴6 μL的Nafion溶液(0.10%,质量分数),得到纳米材料修饰电极。实验中通N2保护。
2 结果和讨论
2.1 水/乙醇体系中CTAB的转变
为了制备2D纳米片须控制模板的用量。水溶液中CTAB达到第一临界胶束浓度(Critical micelle concentration, CMC)时,单分子形成球形胶束;CTAB达到第二CMC时,球形胶束转变为棒状胶束[14]。棒状胶束与金属纳米粒子表面紧密结合成纳米粒子生长的模板,引导纳米粒子定向生长。但是,乙醇的介电常数比水的介电常数低,使加入乙醇的体系中CTAB阳离子之间的排斥力增大,从而使CMC增大[15]。为此,测试水/乙醇(体积比4∶1)体系中不同浓度的CTAB紫外吸收光谱(图1a)。谱中最大吸收波长和吸光度随着CTAB浓度的提高而增大,说明CTAB具有增色效应,不用探针即可检测CTAB的CMC值[16]。根据不同浓度的CTAB紫外吸收光谱,画出最大吸收波长处的A与浓度(c)的双对数曲线(图1b)。双对数曲线有两个拐点,分别对应CTAB的第一CMC (2.17 × 10-3 mol/L)和第二CMC (8.72 × 10-3 mol/L)。CTAB的浓度达到2.17 × 10-3 mol/L时,溶液中的CTAB从单体转变到球形胶束;CTAB的浓度达到8.72 × 10-3 mol/L时,CTAB从球形胶束转变为棒状胶束[16]。
图1
图1
CTAB的紫外可见光谱以及第一和第二CMC
Fig.1
UV-vis spectra (a), and the first and second CMCs (b) of CTAB
2.2 纳米片的形貌和物相
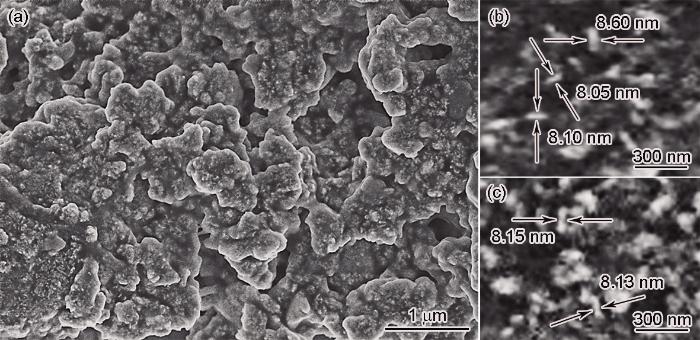
依据得到的CMC,设计浓度高于CMC浓度的CTAB为模板制备PdNSs纳米材料,FESEM如图2所示。可以看出,PdNSs的厚度约为8.10 nm (图2b和c),纳米片垂直生长并互相交织,可为电化学反应提供更多的活性位点[17]。在低倍TEM照片(图3a)中0.75 μm × 0.76 μm视野内可观察到呈堆叠状的超薄PdNSs,纵向尺寸达到几百纳米。在高倍TEM照片(图3b)中的暗区是竖直的PdNSs,可提供更多的吸附点和活性位点[4]。图3c给出了纳米片的XRD谱。谱中衍射角为40.02°、46.67°、68.07°、82.03°和86.67°处出现5个衍射峰,对应面心立方Pd衍射晶面的(111)、(200)、(220)、(311)和(222)的特征峰(PDF卡片#87-0643)。
图2
图2
PdNSs的FESEM图和厚度分布
Fig.2
FESEM image (a) and thickness distribution images (b, c) of PdNSs
图3
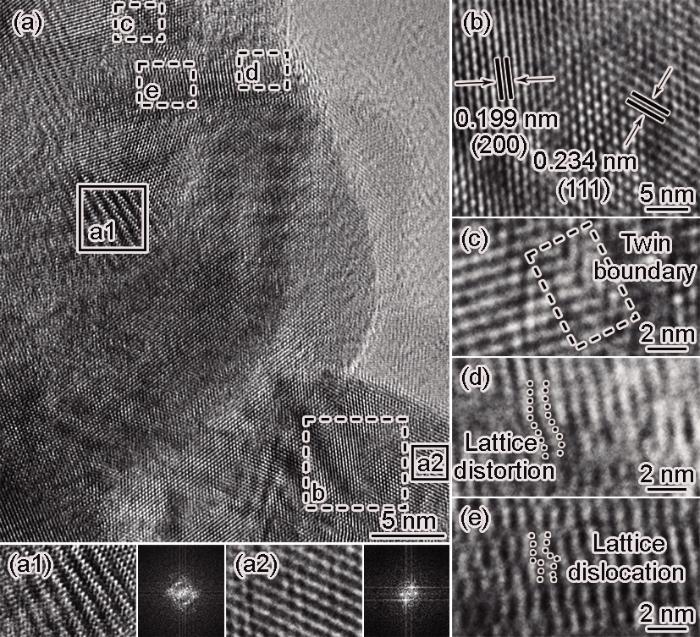
进一步进行HRTEM分析,图4a实线框中区域(图4a1和a2)的傅里叶变换(FFT)显示出两组不同的衍射斑点。这两个区域不同的晶体取向是晶体与其相邻晶体之间的原子不匹配所致。图4b是图4a中虚线框的放大图像,可见两组晶面间距分别为0.199和0.234 nm,分别为面心立方Pd的(200)和(111)晶面。与PDF卡片#87-0643(111)的0.226 nm相比明显增大。其主要原因是,Pd纳米粒子的晶格扩张使PdNSs具有良好的催化活性和优异的耐久性[18]。由图4a中的放大区域(图4c~e)可见孪晶界、晶格畸变和晶格位错缺陷。这些缺陷为电催化提供了额外的活性位点[8]。这些活性位点具有更高的能量和更丰富的电子结构,促进甘油分子在催化过程中的吸附和活化,从而加速甘油的氧化反应。材料中的应力分布与晶格缺陷密度密切相关。纳米晶体Pd中的晶格缺陷使应变增大和衍射峰分裂,导致图3c中主要衍射峰(如(200)、(220)和(311))分裂[19]。
图4
图4
PdNSs的HRTEM图、实线框的FFTs和虚线框放大图
Fig.4
HRTEM image of PdNSs (a) and FFTs from the solid boxes (a1, a2), magnification HRTEM images from the dashed boxes (b-e)
2.3 纳米片的生成机理
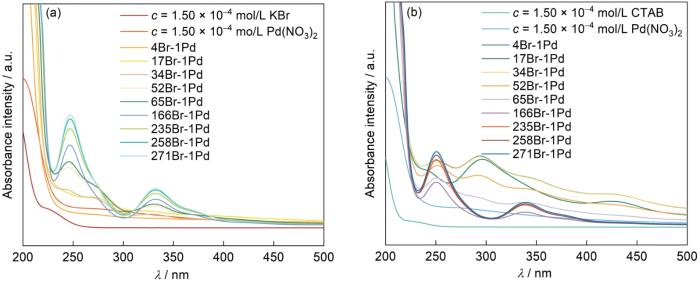
图5
图5
在Pd(NO3)2溶液逐步加入KBr和CTAB的紫外吸收光谱
Fig.5
UV-vis spectra of Pd(NO3)2 solution upon the addition of KBr (a) and CTAB (b)
Br-浓度的增大使紫外吸收峰的强度提高,表明Br-与Pd2+的络合程度提高。CTAB和Pd(NO3)2混合溶液的紫外光谱,如图5b所示。可以看出,n[Br-]/n[Pd2+]为4、17、34、52时,光谱中除了在250、345 nm处有紫外吸收峰外,在295 nm处还出现了紫外吸收谱宽带。这个宽谱带形成的原因,可能是CTAB溶解在水相中生成C16TA+和Br-,C16TA+和[PdBr4]2-之间存在着相互作用。CTAB浓度高于一定值(即n[Br-]/n[Pd2+]为166、235、258、271)时,在光谱中295 nm处的紫外吸收宽谱带逐渐消失,250 nm处的吸收峰逐渐增强,345 nm处的紫外吸收峰逐渐蓝移至338 nm处。这表明,[PdBr
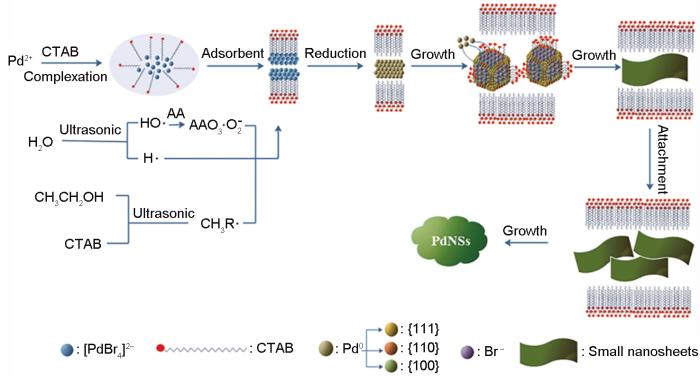
体系中抗坏血酸的还原速率较低,在反应的初始阶段Pd2+缓慢地还原为Pd0生成了少量小尺寸纳米颗粒,并被大量的表面活性剂覆盖。其他Pd纳米颗粒受到CTAB强疏水作用经历传统的Ostwald熟化过程附着到这些小尺寸纳米颗粒上,这些小尺寸纳米颗粒作为种子生成了中等尺寸的PdNSs。PdNSs侧壁上的低表面电荷,使PdNSs持续生长。另一方面,按照几何选择理论[25],不同晶面的生长速率不同。生长速率高的晶面最终变成点,而缓慢生长的晶面生长成宏观保留面。Pd的晶体结构为面心立方(FCC),有(111)、(100)、(110)等晶面。在CTAB胶束模板的作用下,CTAB和游离的Br-吸附在Pd纳米粒子的(100)晶面,限制Pd纳米晶体沿(100)晶面生长,而其它晶面晶粒的生长速率较高,使Pd纳米晶体在CTAB形成的层状胶束下生长成PdNSs[25],其机理如图6所示。
图6
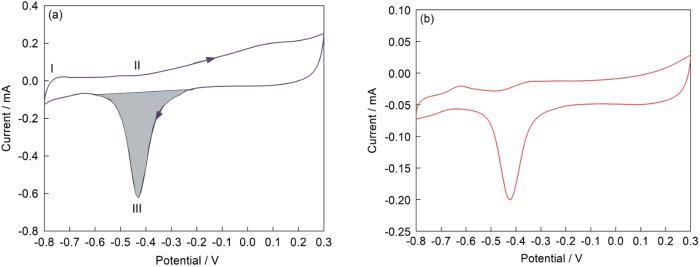
2.4 电催化性能
图7
图7
PdNSs和商业Pd/C的循环伏安曲线 (溶液:1 mol/L KOH;扫描速率:50 mV/s)
Fig.7
CV curves of PdNSs (a) and commercial Pd/C (b) (solution: 1 mol/L KOH; scan rate: 50 mV/s)
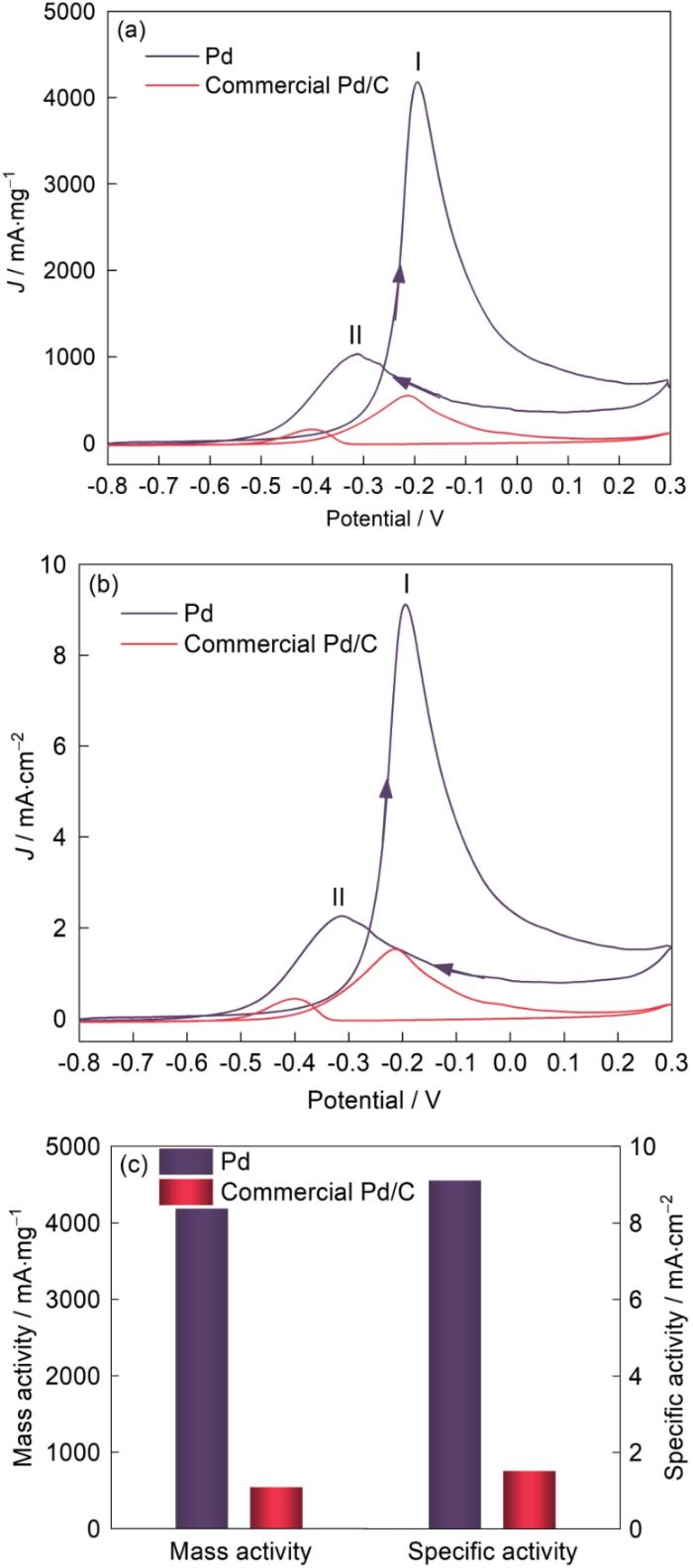
图8a给出了PdNSs和商业Pd/C在1 mol/L KOH + 1 mol/L C3H8O3的CV曲线。以Pd负载质量将氧化电流归一化,以比较两个催化剂的质量活度[30]。可以看出,PdNSs的氧化峰Ⅰ (4179.82 mA/mg)是商业Pd/C (562.77 mA/mg)催化剂的7.43倍。图8b给出了将电流以ECSA归一化后的比表面活度曲线,可见PdNSs的氧化峰Ⅰ比表面活度(9.12 mA/cm2)是商业Pd/C (1.57 mA/cm2)的5.81倍(图8c),即PdNSs氧化甘油的催化活性较高。这个结果,可归因于PdNSs晶界的缺陷结构。在PdNSs中的晶界产生的晶格应变改变了对O/OH的结合亲和力,从而改变了整个路径的总势垒[31]。同时,PdNSs用于GOR的氧化电流在大约-0.64 V出现,大于-0.16 V时显著增大,即Pd-OHads开始形成(图8a)。GOR电流密度的提高主要是双功能效应引起的,即Pd原子表面解离吸附的甘油分子与在相邻的Pd原子生成的OHads耦联[32]。同时,GOR的电流密度正向扫描峰值电位约为-0.19 V,而在反向扫描中氧化电流在约-0.01 V开始产生。其原因是,在正向扫描中,随着Pd-OHads/PdO覆盖率的提高用于GOR的新Pd的表面减少,一些吸附性强且难以去除的碳中间体致使出现了氧化峰Ⅱ[26];而在反向扫描中,PdO的还原恢复了Pd表面,残留的中毒中间体再次氧化去除,导致氧化电流的再次出现[33]。将在正向扫描(if)和反向扫描(ib)中峰电流密度的比值作为表征催化剂表面清洁度或者对中毒物质的耐受性的标准。与商业Pd/C催化剂(0.29)相比,PdNSs的ib/if值为0.25。ib/if的大小与电催化剂的抗CO中毒能力相关,即ib/if的值越小则该电催化剂的抗中毒性能越高[26]。
图8
图8
PdNSs和商业Pd/C的Pd质量归一化CV曲线、ECSA归一化CV曲线、催化剂相应的质量活度和比表面活度(溶液:1 mol/L C3H8O3 + 1 mol/L KOH;扫描速率:50 mV/s)
Fig.8
Pd-mass (a) and ECSA (b) normalized CVs of the PdNSs and commercial Pd/C, and corres-ponding mass activities and specific activities of catalysts (c) (solution: 1 mol/L C3H8O3 + 1 mol/L KOH; scan rate: 50 mV/s)
GOR在碱性介质中Pd电催化剂上的反应可分为[34]
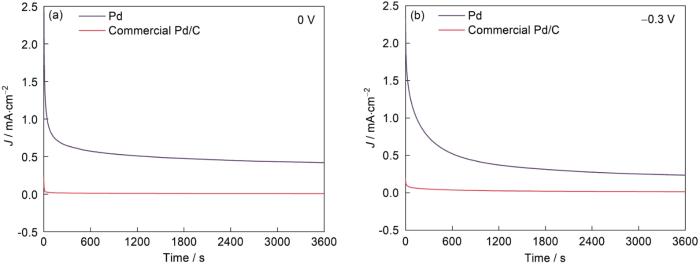
图9给出了对PdNSs和商业Pd/C的500次耐久性测试结果。图9c给出了质量活度的变化趋势。可以看出,经过500次循环后PdNSs的GOR质量活度为3272.69 mA/mg,损失了21.70%。与商业Pd/C(307.62 mA/mg,44.03%)和近年获得的Pd基电催化剂(表1)相比,其较高的电流密度和较低质量活性损失表明,PdNSs在KOH溶液中对GOR的耐久性良好。图10给出了在1 mol/L KOH + 1 mol/L C3H8O3溶液中PdNSs和商业Pd/C在0和-0.3 V电压下氧化电流密度随时间的变化。在固定电位下,醇的氧化产生中间产物(如CO)。这些中间产物可能吸附并积聚在催化剂修饰的电极表面。CO的吸附使催化剂失活和氧化电流密度降低。氧化电流密度的降低,与催化剂性能的衰减密切相关。以在60 min与5 min时的氧化电流密度之比(i60/i5)作为催化剂衰减的指标,PdNSs在0 V时的i60/i5比率(0.65)高于-0.3 V时的比率(0.32)。这表明,在0 V时抑制了催化剂的衰减。双功能效应强烈抑制了活性点位因吸附的碳基中间体中毒,降低了氧化电流密度下降的幅度[26]。值得注意的是,PdNSs在所有阶段其电流密度都高于商业Pd/C,即PdNSs对比商业Pd/C的电化学稳定性更好。
图9
图9
500次循环加速耐久性实验前后PdNSs和商业Pd/C的CV曲线、基于500次循环起始峰值电流密度PdNSs和商业Pd/C的耐久性以及500次循环后的标准化质量活性(溶液:1 mol/L C3H8O3 + 1 mol/L KOH;扫描速率:50 mV/s)
Fig.9
CVs obtained before and after accelerated durability tests for PdNSs (a) and commercial Pd/C (b) for 500 cycles, durability properties of PdNSs and commercial Pd/C based on the onset peak current density for 500 cycles (c), normalized mass activity of catalysts after 500 cycles (d) (solution: 1 mol/L C3H8O3 + 1 mol/L KOH; scan rate: 50 mV/s)
表1 近年来关于Pd基催化剂对GOR行为的研究成果[35~40]
Table 1
| Catalysts | Electrolyte | Scan rate / mV·s-1 | ECSA / m2·g-1 | Specific activity / mA·cm-2 | Mass activity / mA·mg | ib / if | Ref. |
|---|---|---|---|---|---|---|---|
| PdNSs | 1 mol/L KOH + 1 mol/L glycerol | 50 | 46.31 | 9.12 | 4179.82 | 0.25 | This work |
| Pd NPs/C110 | 0.5 mol/L KOH + 1 mol/L glycerol | 50 | 22.69 | - | 1026 | 0.34 | [35] |
| PdFe/C | 0.5 mol/L KOH + 1 mol/L glycerol | 50 | 38.99 | 1.10 | - | 0.64 | [36] |
| PdNi/C | 1 mol/L KOH + 0.5 mol/L glycerol | 50 | - | 2.2 | 211 | - | [37] |
| Pd/C | 1 mol/L KOH + 1 mol/L glycerol | 50 | 43.30 | - | 1150 | - | [38] |
| AgPd (1∶1)/C | 2 mol/L KOH + 1.5 mol/L glycerol | 20 | - | - | 220.27 | - | [39] |
| Pd3Pb | 1 mol/L KOH + 1 mol/L glycerol | 50 | - | - | 916 | - | [40] |
图10
图10
PdNSs和商业Pd/C分别在0和-0.3 V电压下的计时电流曲线(溶液:1 mol/L C3H8O3 + 1 mol/L KOH;扫描速率:50 mV/s)
Fig.10
Chronoamperometric curves of PdNSs and commercial Pd/C at a fixed potential of 0 V (a) and -0.3 V (b) (solution: 1 mol/L C3H8O3 + 1 mol/L KOH; scan rate: 50 mV/s)
3 结论
以Pd(NO3)2·2H2O为前驱体,可在水/乙醇(体积比4∶1)体系中构建CTAB模板制备富缺陷PdNSs。CTAB中的Br-与Pd2+相互作用生成[PdBr4]2-配合物,[PdBr4]2-在静电力作用下嵌入CTAB的棒状胶束使AA还原Pd2+的速率降低,为制备2D Pd纳米材料提供动力学条件。
参考文献
From waste to value-glycerol electrooxidation for energy conversion and chemical production
[J].
Electrocatalytic oxidation of crude glycerol from the biodiesel production on Pd-M (M = Ir, Ru or Pt) sub-10 nm nanomaterials
[J].
Glycerol electro-oxidation in alkaline media using Pt and Pd catalysts electrodeposited on three-dimensional porous carbon electrodes
[J].
Two-dimensional engineering of Pd nanosheets as advanced electrocatalysts toward formic acid oxidation
[J].
Phosphorus doped PdMo bimetallene as a superior bifunctional fuel cell electrocatalyst
[J].
One-step CO assisted synthesis of hierarchical porous PdRuCu nanosheets as advanced bifunctional catalysts for hydrogen evolution and glycerol oxidation
[J].
Defect rich structure activated 3D palladium catalyst for methanol oxidation reaction
[J].
Defect chemistry of nonprecious-metal electrocatalysts for oxygen reactions
[J].
Vacancy-induced ferromagnetism of MoS2 nanosheets
[J].
Engineering surface atomic structure of single-crystal cobalt (II) oxide nanorods for superior electrocatalysis
[J].
Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel
[J].
Ultrathin Pd-based nano-sheets: syntheses, properties and applications
[J].Two-dimensional (2D) noble metal-based nanosheets (NSs) have received considerable interest in recent years due to their unique properties and widespread applications. Pd-based NSs, as a typical member of 2D noble metal-based NSs, have been most extensively studied. In this review, we first summarize the research progress on the synthesis of Pd-based NSs, including pure Pd NSs, Pd-based alloy NSs, Pd-based core-shell NSs and Pd-based hybrid NSs. The synthetic strategy and growth mechanism are systematically discussed. Then their properties and applications in catalysis, biotherapy, gas sensing and so on are introduced in detail. Finally, the challenges and opportunities towards the rational design and controlled synthesis of Pd-based NSs are proposed.
Synthesis and characterisation of Nickel oxide nanoparticles using CTAB as capping agent
[J].
Determination of the first and second CMCs of surfactants by adsorptive voltammetry
[J].
Micellization of cationic surfactants in alcohol—water mixed solvent media
[J].
Determination of the second critical micelle concentration of CTAB by UV spectra without probe
[J].
无探针紫外光谱法测定CTAB的第二临界胶束浓度
[J].应用无探针的紫外吸收分光光谱法(UV)测定了十六烷基三甲基溴化铵(CTAB)溶液的第一、第二临界胶束浓度(CMC), 并用<sup>1</sup>H NMR谱和动态光散射的实验方法检测到了两个浓度时溶液中聚集体的转变, 从而验证了无探针紫外光谱法测定CTAB溶液第二临界胶束浓度的可行性. 此外, 我们还利用紫外光谱法研究了CTAB/KBr体系, 证实KBr可诱导CTAB形成蠕虫状胶束.
Flexible non-enzymatic glucose biosensor based on CoNi2S4 nanosheets grown on nitrogen-doped carbon foam substrate
[J].
Palladium nanobelts with expanded lattice spacing for electrochemical oxygen reduction in alkaline media
[J].
Correlating deformation mechanisms with X-ray diffraction phenomena in nanocrystalline metals using atomistic simulations
[J].
Mixed ligand complexes of palladium (II) with chloride and bromide
[J].
Seed-mediated synthesis of pd nanocrystals: factors influencing a kinetic- or thermodynamic-controlled growth regime
[J].
Sonolysis of water-methanol mixtures
[J].
Hydroxyl radical scavenging activity and kinetics of Vitamin C
[J].<p>For humanbeings, radicals scavenging plays an important role for maintaining the fundamental physiological and anti-ageing need. Comparison for competing scavenging ability of Vitamin C(V<sub>C</sub>) with salicylic acid gives optimal scavenging condition for V<sub>C</sub>. By fitting the Alberty equation, the maximum 46.23% clearance of ·OH by V<sub>C</sub> reached when V<sub>C</sub> concentration is 4.54 mmol/L at 47 ℃ for 90 min, and the maximum reaction rate is 0.11 mmol/(L·min). The dissociation constant for salicylic acid and V<sub>C</sub> is 30.2 mmol/L and 1.2×10<sup>-3</sup> mmol/L, respectively. The experimental data are well-fitted with relative error of 1.66%~5.40%. This is helpful for the effective and scientific usage of V<sub>C</sub>.</p>
Fenton反应考察抗坏血酸清除羟基自由基能力及动力学
[J].
Ab initio study of the structural properties of ascorbic acid (vitamin C)
[J].
Cetyltrimethylammonium bromide assisted preparation and characterization of Pd nanoparticles with spherical, worm-like, and network-like morphologies
[J].
十六烷基三甲基溴化铵辅助作用下球形、蠕虫状和网状Pd纳米粒子的制备与表征
[J].以十六烷基三甲基溴化铵 (CTAB) 为表面活性剂, NaBH4 为还原剂, 通过调节水相体系中 CTAB 和 NaBH4 浓度, 合成了一系列不同形貌的 Pd 纳米粒子, 合成过程简便易行且环境友好. 结果表明, 随着 CTAB 浓度的增加, Pd 粒子形貌由纳米微球逐渐向纳米线网络形态转变. CTAB 浓度和 NaBH4 浓度是决定 Pd 粒子形貌的两个重要因素.
Preparation of PdAg and PdAu nanoparticle-loaded carbon black catalysts and their electrocatalytic activity for the glycerol oxidation reaction in alkaline medium
[J].
Electro-catalytic activity of composite films of Pd-doped bacterial cellulose Nano-fibers for ethanol oxidation
[J].Bacterial cellulose nanofibers (BCFs) were synthesized by fermentation process and then with the prepared BCFs as carrier material, composite films of Pd-doped bacterial cellulose nanofibers (Pd/BCF) were prepared by depositing nano-particles of Pd (Pd-NPs) on the carrier via chemical reduction process. The prepared Pd-doped BCFs and composite films Pd/BCF were characterized by X-ray diffraction (XRD), scanning electron microscope (SEM), transmission electron microscopy (TEM), cyclic voltammetry (CV), electrochemical impedance spectra (EIS), chronoamperometry (CA), and chronopotentiometry (CP). The results show that Pd-NPs were well dispersed on BCFs and especially in the mesoporous of BCFs. The composite films contain c.a. 19% (mass fraction) of Pd-NPs with a mean particle size c.a. 10 nm. The composite of Pd/BCF had better catalytic activity in contrast to the traditional carbon carrier material and Pt-catalyst. Besides, the composite of Pd/BCF exhibited relatively high poison tolerance during the ethanol oxidation process.
载钯细菌纤维素纳米纤维复合膜用于乙醇的电催化氧化
[J].以发酵合成的细菌纤维素(BC)为载体支架,用一步化学还原法在BC上直接生长钯纳米颗粒(Pd NPs),制备出载钯细菌纤维素纳米纤维复合膜(Pd/BCF)。X射线衍射(XRD)、扫描电子显微镜(SEM)及透射电子显微镜(TEM)等测试结果表明,Pd NPs比较均匀地分散在纤维表面及介孔中,粒径约为10 nm,载量约为19.0%。电化学测试如循环伏安(CV)、电化学阻抗谱(EIS)、计时电流(CA)、计时电位曲线(CP)等的测试结果表明,与传统的碳材料载体和Pt催化剂相比,Pd/BCF对碱性介质中乙醇电催化氧化的活性显著提高,且在反应中的抗中毒能力较强。
Poly (pyrrole-co-aniline) hollow nanosphere supported Pd nanoflowers as high-performance catalyst for methanol electrooxidation in alkaline media
[J].
Electrooxidation of sodium borohydride at Pd, Au, and Pd x Au1- x carbon-supported nanocatalysts
[J].
Highly-effective palladium nanoclusters supported on para-sulfonated calix[8]arene-functionalized carbon nanohorns for ethylene glycol and glycerol oxidation reactions
[J].
Interlaced Pd–Ag nanowires rich in grain boundary defects for boosting oxygen reduction electrocatalysis
[J].
Enhancement effect of Ag for Pd/C towards the ethanol electro-oxidation in alkaline media
[J].
Promotion of palladium catalysis by silver for ethanol electro-oxidation in alkaline electrolyte
[J].
Glycerol electrooxidation on Pd, Pt and Au nanoparticles supported on carbon ceramic electrode in alkaline media
[J].
Glycerol oxidation at Pd nanocatalysts obtained through spontaneous metal deposition on carbon substrates
[J].
Microwave-induced defective PdFe/C nano-electrocatalyst for highly efficient alkaline glycerol oxidation reactions
[J].
Synergistic bimetallic PdNi nanoparticles: enhancing glycerol electrooxidation while preserving C3 product selectivity
[J].
Single atom iron carbons supported Pd-Ni-P nanoalloy as a multifunctional electrocatalyst for alcohol oxidation
[J].
AgPd nanoparticles as a potential electrocatalyst for enhanced performance in direct glycerol fuel cells
[J].
Raspberry-like Pd3Pb alloy nanoparticles: superior electrocatalytic activity for ethylene glycol and glycerol oxidation
[J].