油页岩是一种高灰分有机沉积岩,由有机质、矿物质和少量沥青组成[1]。油页岩中有机质的含量约为15%,无机矿物质的含量较高且种类较多[2]。用油页岩制取页岩油时,产生大量的灰渣[3]。长期堆放的灰渣占用土地资源还污染环境,因此综合利用油页岩灰渣是当务之急[4]。油页岩灰渣的主要成分,有SiO2、Al2O3以及少量的Fe2O3、CaO、Na2O和K2O。油页岩灰渣的矿物组成和层状结构,与制备分子筛的粘土矿物非常相似。用油页岩灰渣制备分子筛,可大大降低成本。Shawabkeh等[5]以约旦油页岩灰为原料,采用碱熔水热法合成了P型分子筛;Bao等[6]以大庆油页岩灰渣为原料,用碱熔水热法制备了X型分子筛。
印染废水污染环境和损害人类的健康。处理印染废水的方法,有物理、化学和生物法等[7]。吸附法的操作简单、见效快、二次污染风险较小,可用于处理印染废水[8]。分子筛是一种具有三维空间结构的硅酸铝盐晶体矿物质,具有良好的离子交换能力、特殊的孔隙结构和高比表面积[9]。但是,用粉末状分子筛处理印染废水时难以与废水快速分离。将强吸附剂(如分子筛、活性炭等)与磁性物质结合,用磁场可实现吸附剂的快速分离。Cheng等[10]在NaP1型分子筛上负载磁性SrFe12O19,外加磁场实现了分子筛与MB溶液的快速分离。Liu等[11]在NaA型分子筛上负载Fe3O4制备NaA型分子筛,具有良好的磁化和磁稳定性。Fe3O4负载量为4.7%的磁性沸石,对Cu2+、Pb2+的吸附率仍高于95%。鉴于此,本文以北票油页岩灰为原料制备NaX型分子筛和磁性分子筛,研究其对亚甲基蓝(MB)的吸附性能并揭示其机理。
1 实验方法
1.1 实验用材料
北票油页岩、NaOH(96%)、FeCl3·6H2O(99%)、乙二醇(99%)、乙酸铵(98%)、亚甲基蓝(MB)、KCl(99%)、Na2CO3(99%)、CaCl2(99%)、Na2SO4(99%)和去离子水,电阻为18.25 MΩ。
1.2 实验用仪器
MiniFlex600型X射线衍射仪(XRD),Zetium波长型X射线荧光光谱仪(XRF),LakeShore7404型振动样品磁强计(VSM),ZEISS Sigma 300型扫描电子显微镜(SEM),Jem-2100F Jeol型透射电子显微镜(TEM),ASAP 2020型比表面积及孔径分析仪(BET),Escalab250型X射线光电子能谱仪(XPS),Nicolet-is5型傅里叶变换红外光谱仪(FTIR),以及ZS90型电位分析仪(Zeta)。
1.3 磁性分子筛的制备
将油页岩过100目筛子后在950 ℃马弗炉中焙烧6 h,得到油页岩灰(BPA,主晶相是SiO2)。将BPA与NaOH按照1∶2的质量比混合后研磨均匀,在温度为600 ℃的马弗炉中熔融60 min。将适量熔融后得到的固体粉末与去离子水按照1 g∶6 mL的比例混合,在常温下搅拌3 h后装入聚四氟乙烯水热反应釜中。将反应釜置于温度为80 ℃的恒温干燥箱中反应12 h;反应结束后,过滤反应物并用去离子水将滤渣洗至中性。将滤渣置于110 ℃恒温干燥箱中,干燥5 h后得到分子筛[12]。
将1 g分子筛和适量的Fe3O4颗粒[13]放入30 mL水中搅拌3 h,然后将其转移到聚四氟乙烯水热反应釜中,将反应釜置于120 ℃的恒温干燥箱中反应7 h。反应结束后,过滤反应物并用去离子水将滤渣洗至中性。将滤渣置于60 ℃恒温干燥箱中,干燥6 h后得到磁性分子筛。
1.4 磁性分子筛性能的表征
用X射线衍仪表征BPA、NaX和Fe3O4@NaX型分子筛的物相组成,测试条件为40 kV和20 mA,扫描范围为5°~90°,扫描间隔为0.02°。用荧光光谱仪分析BPA的化学组成。用振动样品磁强计测试Fe3O4@NaX型分子筛的磁性。用扫描电子显微镜观察NaX和Fe3O4@NaX型分子筛的表面形貌。用透射电子显微镜观察Fe3O4@NaX型分子筛的形貌并分析选定区域的元素分布。用比表面积及孔径分析仪测试Fe3O4@NaX型分子筛的N2吸附-脱附等温线和孔径分布曲线。用傅里叶变换红外光谱仪分析吸附前后Fe3O4@NaX型分子筛的结构变化,扫描范围为4000~400 cm-1,分辨率为2 cm-1。用X射线光电子能谱仪分析吸附前后Fe3O4@NaX型分子筛的元素组成。用电位分析仪分析NaX和Fe3O4@NaX型分子筛的表面电位。用紫外可见分光光度计(Shimadzu UV2450)测分子筛吸附和再生后分子筛吸附MB后上清液的吸光度。
将磁性分子筛(0.5~2.5 g/L)与一定体积和用量的MB溶液(初始浓度为10~50 mg/L)装入锥形烧瓶中,然后置于不同温度(25~65 ℃)的恒温水浴中,吸附平衡一定时间(0~120 min)后离心分离得到上清液。用紫外可见分光光度计测上清液的吸光度。用0.5 mol/L的氯化钠溶液对吸附后的磁性分子筛进行脱附再生[14],用再生后的分子筛对MB溶液进行重复吸附。磁性分子筛对MB的吸附率(R)和平衡吸附量(Q)分别为
式中ce和c0分别为MB的平衡浓度和初始浓度(mg/L,由与其吸光度之间关系的标准曲线方程求出),V为MB溶液的体积(L),m为分子筛的用量(g)。
2 结果和讨论
2.1 Fe3O4@NaX型分子筛的物相组成和形貌
表1 BPA的化学组成
Table 1
| SiO2 | Al2O3 | CaO | Fe2O3 | K2O | MgO | SO3 | Bal. |
|---|---|---|---|---|---|---|---|
| 60.08 | 14.38 | 9.13 | 5.08 | 3.66 | 3.22 | 2.09 | 2.35 |
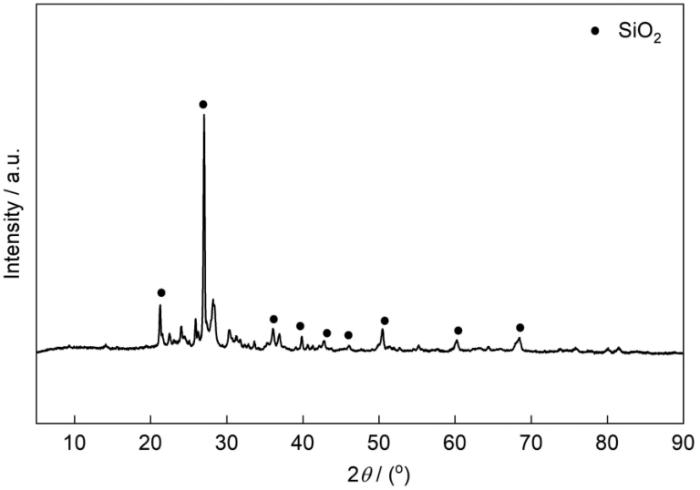
图1
图2
图2
NaX分子筛的XRD谱和FTIR谱
Fig.2
XRD pattern (a) and FTIR spectrum (b) of NaX zeolite
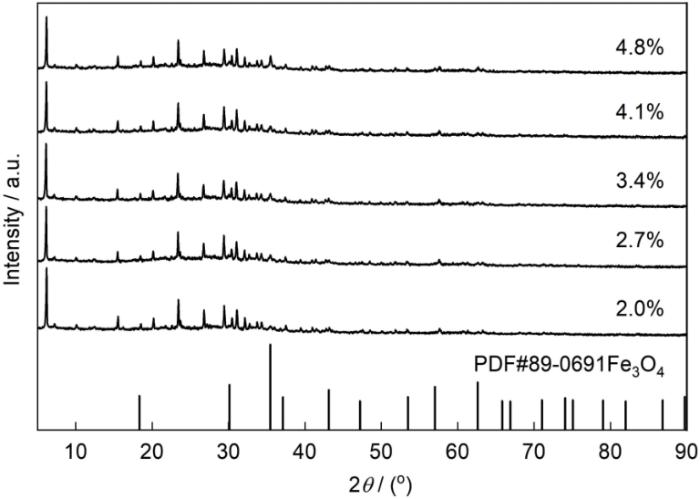
图3
图3
不同Fe3O4负载量的Fe3O4@NaX型分子筛的XRD谱
Fig.3
XRD patterns of Fe3O4@NaX zeolites with different contents of Fe3O4 (mass fraction)
图4
图4
NaX和Fe3O4@NaX型分子筛的SEM照片
Fig.4
SEM images of (a, b) NaX and (c, d) Fe3O4@NaX zeolites
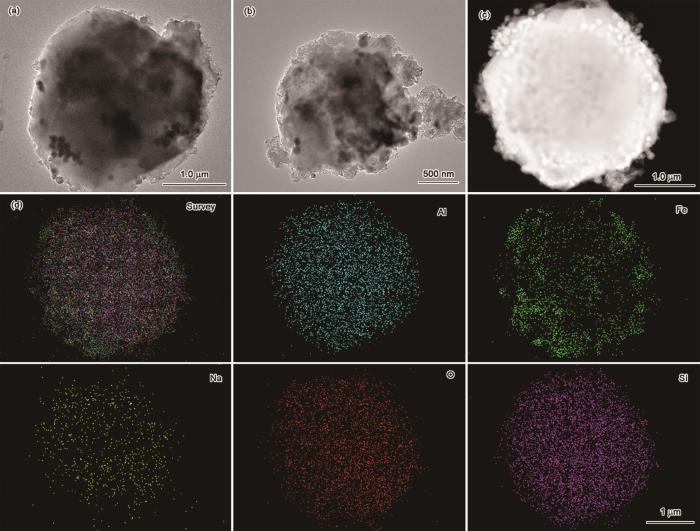
图5
图5
Fe3O4@NaX型分子筛的TEM照片和选定区域的元素分布
Fig.5
TEM analysis results of Fe3O4@NaX zeolite. (a, b) TEM images; (c, d) selected area and corresponding element distributions
2.2 Fe3O4@NaX型分子筛对MB的吸附率以及与MB溶液的分离
图6给出了不同Fe3O4负载量Fe3O4@NaX型分子筛对MB的吸附性能。可以看出,负载Fe3O4后分子筛对MB的吸附率没有较大的变化,而吸附速率有较大的提高。在吸附过程中,分子筛表面所带负电荷与MB所带正电荷之间的静电引力是吸附过程的主要动力。NaX和2.0% Fe3O4@NaX (质量分数,下同)型分子筛的电位分别为-28.3和-24.5 mV,表明Fe3O4的加入降低了分子筛表面的电位。分子筛表面电位的降低增大了其与MB之间的静电引力,使分子筛对MB的吸附速率提高。随着Fe3O4负载量的增大,分子筛对MB的吸附率和吸附速率没有较大的变化,Fe3O4的最佳负载量为2.0%。
图6
图6
Fe3O4负载量不同的Fe3O4@NaX型分子筛对MB的吸附率
Fig.6
MB removal efficiency of Fe3O4@NaX zeolites with different contents of Fe3O4
图7
图7
2.0% Fe3O4@NaX型分子筛的磁滞回线
Fig.7
Magnetic hysteresis loop of 2.0% Fe3O4@NaX zeolite
2.3 Fe3O4@NaX型分子筛的N2 吸附-脱附等温线和孔径分布
图8
图8
Fe3O4@NaX型分子筛的N2吸附-脱附等温线和孔径分布
Fig.8
N2 adsorption-desorption isotherms and pore size distribution curve (inset) of the Fe3O4@NaX zeolite
2.4 吸附条件对分子筛吸附MB性能的影响
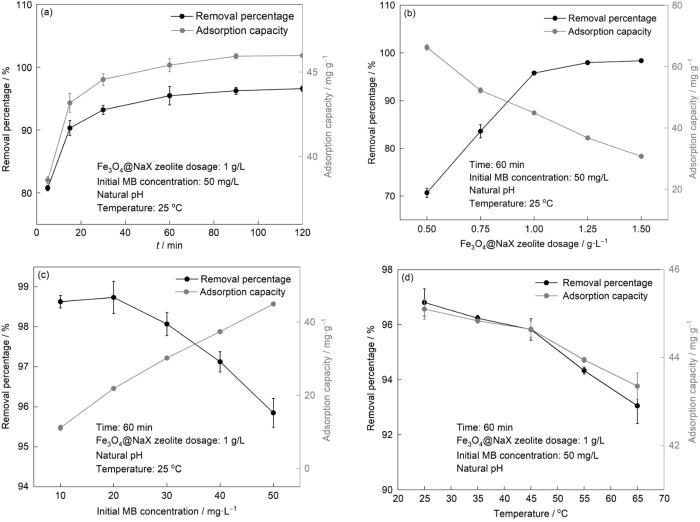
图9给出了在不同条件下分子筛对MB的吸附性能。从图9a可以看出,在吸附的前60 min内,分子筛对MB的吸附率和平衡吸附量从80.85%和38.65 mg/g提高到96.98%和45.19 mg/g,60和120 min的吸附率和平衡吸附量较为接近,因此选择分子筛对MB的吸附时间为60 min。从图9b可见,随着分子筛用量从0.5增加到1.0 g/L对MB的吸附率从69.61%提高到95.54%,因为分子吸附活性位点增多。但是,平衡吸附量从65.25降至30.76 mg/g,因为过多的吸附剂发生团聚,使其总表面积减小[21]。综合考虑分子筛对MB的吸附效率和吸附能力,选择1 g/L作为后续实验的分子筛用量。从图9c可见,随着MB初始浓度的提高分子筛对MB的吸附率和平衡吸附量分别从98.45%和11.48 mg/g变化到95.64%和44.72 mg/g。其原因是,随着MB初始浓度的提高,分子筛与MB分子之间的浓度梯度增大而使传质推动力增大[22]。吸附率降低的原因是,一定用量的分子筛提供的表面活性位点数量有限[23]。MB的初始浓度为50 mg/L时分子筛的吸附性能较高且吸附率高于95%。因此,选择MB的初始浓度为50 mg/L。从图9d可见,随着MB溶液温度的升高吸附率和平衡吸附量分别从96.98%和45.19 mg/g降到92.78%和43.23 mg/g。这表明,分子筛吸附MB的过程为放热过程[24],25 ℃是最佳吸附温度。
图9
图9
吸附条件对分子筛吸附性能的影响
Fig.9
Effects of adsorption conditions on the adsorption performance of the zeolite. (a) effect of adsorption time, (b) effect of zeolite dosage, (c) effect of the initial concentration of MB, (d) effect of MB solution temperature
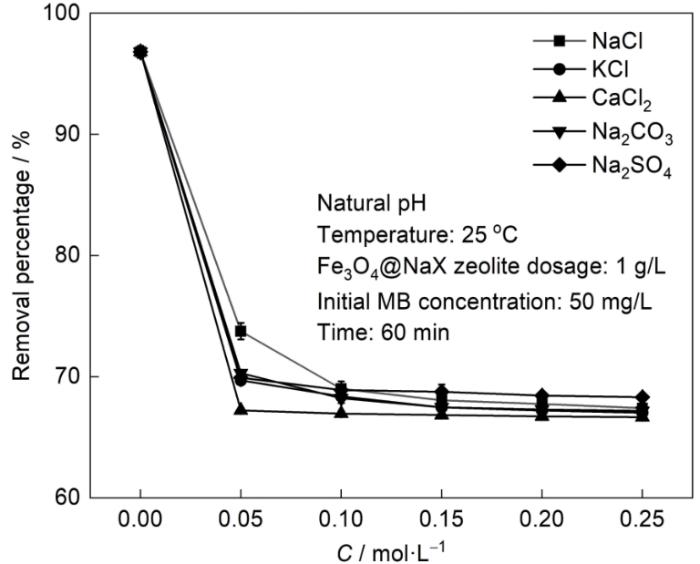
图10给出了NaCl、KCl、CaCl2、Na2CO3和Na2SO4 5种盐和盐度对分子筛吸附MB的影响。可以看出,溶液中的盐使分子筛对MB的吸附率降低。其原因是,存在盐离子的离子效应及其与MB之间的竞争吸附。当阳离子相同时,阴离子对MB吸附率没有显著的影响,因为静电引力是主要的吸附力;而阴离子相同时,Ca2+对MB吸附率的影响比K+和Na+的大,因为在浓度相同的条件下Ca2+带两个正电荷,Ca2+与MB之间对吸附位点较大的竞争使其对MB吸附率的影响更大。
图10
图10
几种盐和盐度对分子筛吸附MB的影响
Fig.10
Effects of different salts and salinities on the MB adsorption performance of the zeolite
表2列出了Fe3O4@NaX型分子筛对MB的吸附性能与其他吸附剂的比较。可以看出,本文合成的Fe3O4@NaX型分子筛对MB的吸附性能较好。
表2 不同吸附剂对MB的吸附性能
Table 2
| Adsorbents | Time / min | Dosage / g·L-1 | Initial concentration / mg·L-1 | pH value | Temperature / oC | Adsorption capacity / mg·g-1 | Ref. |
|---|---|---|---|---|---|---|---|
| Fe3O4@NaX | 60 | 1 | 50 | Natural | 25 | 45.19 | This work |
| WO3 | 3 | 0.05 | 15 | Natural | Natural | 1.64 | [25] |
| Zeolite HY-Fe3O4 from Zeolite HY and Fe3O4 | 40 | 1.186 | 10.23 | 9 | 53.9 | 3.24 | [26] |
| Natural zeolite from Sigmae-Aldrich | 120 | 0.6 | 20 | Natural | 25 | 9.19 | [27] |
| A mixture of zeolite A and P synthesized from electrolytic manganese residue | 200 | 6 | 200 | 6 | 40 | 33.57 | [28] |
| Zeo-FPT | 40 | 0.5 | 10 | 8 | 25 | 0.438 | [29] |
| Magnetic graphene oxide | 240 | 0.15 | 45 | 9 | 25 | 306.5 | [30] |
| Fe3O4@UIO-66-NH2 | 90 | 0.004 | 4 | 9 | Natural | 9.53 | [31] |
2.5 Fe3O4@NaX型分子筛的吸附动力学和吸附模型
将0.1 g的2.0% Fe3O4@NaX型分子筛与100 mL的浓度为50 mg/L的MB溶液装入锥形烧瓶中,然后置于不同温度(25、35、45、55和65 ℃)的恒温水浴进行吸附实验,得出不同吸附时间(5、15、30、60、90、120 min)下分子筛对MB的平衡吸附量,采用式(
表3 Fe3O4@NaX型分子筛吸附MB的动力学参数
Table 3
| Models | Temperature | 25 oC | 35 oC | 45 oC | 55 oC | 65 oC |
|---|---|---|---|---|---|---|
| Experiment | qe,exp / mg·g-1 | 47.80 | 45.07 | 52.38 | 52.38 | 50.94 |
| Pseudo-first-order | qe,cal / mg·g-1 | 5.64 | 4.68 | 4.02 | 3.80 | 4.41 |
| k1 / min-1 | 0.0115 | 0.0094 | 0.0085 | 0.0042 | 0.0041 | |
| R2 | 0.6108 | 0.4593 | 0.2464 | 0.3032 | 0.2157 | |
| Pseudo-second-order | qe,cal / mg·g-1 | 46.32 | 43.42 | 50.63 | 49.85 | 47.78 |
| k2 / g·mg-1·min-1 | 0.0237 | 0.0339 | 0.0599 | 0.1242 | 0.6337 | |
| R2 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9997 | |
| Intraparticle diffusion | c / mg·g-1 | 39.50 | 37.98 | 45.76 | 47.64 | 45.48 |
| kp / mg·g-1·min-1/2 | 0.0115 | 0.0094 | 0.0085 | 0.0042 | 0.0041 | |
| R2 | 0.6079 | 0.4797 | 0.4130 | 0.4876 | 0.4487 |
拟一级动力学模型为
拟二级动力学模型为
颗粒内扩散动力学模型为
式中,qt和qe(mg/g)分别为任意时间的吸附量和MB平衡吸附量,qe,exp和qe,cal(mg/g)分别为实验qe值和理论qe值,k1(min-1)为准一级吸附速率常数,k2(g·mg-1·min-1)为准二级吸附速率常数,kp(mg·g-1·min-1/2)为内扩散动力学吸附速率常数,c(mg/g)为反映边界层对吸附速率影响的常数。
表4列出了Fe3O4@NaX型分子筛吸附MB过程的Langmuir、Freundlich和Dubinin-Radushkevich吸附等温线参数。可以看出,Fe3O4@NaX型分子筛对MB的吸附遵循Langmuir吸附等温线模型,说明是单层吸附。
表4 Fe3O4@NaX型分子筛吸附MB的等温线参数
Table 4
| Langmuir | Freundlich | Dubinin-Radushkevich | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
qm / mg·g-1 | KL / L·mg-1 | R2 | KF / mg·g-1 | n | R2 | qm / mg·g-1 | KDR / mol2·J-2 | R2 | ||
| 57.3394 | 1.6998 | 0.9997 | 34.1461 | 1.9409 | 0.9495 | 42.6702 | 5.32×10-8 | 0.9734 | ||
Langmuir吸附等温线模型为
Freundlich吸附等温线模型为
Dubinin-Radushkevich吸附等温线模型为
式中,qm(mg/g)为最大理论的吸附量,KL(L/mg)为Langmuir常数,KF(mg/g)为Freundlich常数,KDR(mol2/J2)为吸附能相关参数,n为与温度有关大于1的常数,R(8.314 J·mol-1·K-1)为理想气体摩尔常数,
2.6 Fe3O4@NaX型分子筛的吸附热力学、吸附机理和重复使用性能
表5 Fe3O4@NaX型分子筛吸附MB的热力学参数
Table 5
| Temperature / K | Kc | ΔG / kJ·mol-1 | ΔH / kJ·mol-1 | ΔS / J·mol-1·K-1 |
|---|---|---|---|---|
| 298.15 | 32.1413 | -8.6019 | -16.3922 | -26.1432 |
| 308.15 | 26.4178 | -8.3880 | ||
| 318.15 | 24.4721 | -8.2136 | ||
| 328.15 | 16.9428 | -7.7205 | ||
| 338.15 | 12.8492 | -7.1782 |
Van't Hoff方程为
式中,Kc为吸附过程的平衡常数,ΔG(kJ/mol)为吸附过程的Gibbs自由能,ΔH(kJ/mol)为等温吸附过程的焓变,ΔS(kJ/(mol·K))为等温吸附过程的熵变。
图11
图11
Fe3O4@NaX型分子筛吸附前后的XPS谱
Fig.11
XPS spectra of Fe3O4@NaX zeolite before and after adsorption: (a) survey spectra, (b) Na 1s, (c) Si 2p, (d) C 1s
图12
图12
Fe3O4@NaX型分子筛吸附前后的FTIR谱
Fig.12
FTIR spectra of Fe3O4@NaX zeolite before and after adsorption
图13表明,这种分子筛的电位为-24.5 mV。基于以上分析提出一种Fe3O4@NaX型分子筛吸附MB的机理:MB分子所带的正电荷与分子筛表面的负电荷之间的静电吸引力,促进了分子筛对MB分子的吸附;分子筛表面的-OH基团与MB分子中的N原子形成氢键,进一步促进了吸附;分子筛具有丰富的孔结构,MB分子通过孔隙扩散或毛细管冷凝渗透到分子筛内并被吸附。
图13
图13
Fe3O4@NaX型分子筛吸附MB的机理
Fig.13
Mechanism of adsorption of MB by Fe3O4@NaX molecular sieve
图14
图14
Fe3O4@NaX型分子筛的可重复利用性能和洗脱前后的XRD谱
Fig.14
Reusability (a) and XRD patterns before and after elution (b) of Fe3O4@NaX zeolite
3 结论
以(北票)油页岩灰为原料可制备Fe3O4@NaX型磁性分子筛。Fe3O4@NaX型分子筛对MB的吸附遵循拟二级动力学和Langmuir等温线模型,是自发的、放热的熵减过程,吸附的推动力为静电引力、氢键和孔内扩散。经过5次循环再生后,分子筛对MB的吸附率不变且其结构没有明显的改变。加一外磁场可将这种磁性分子筛与MB溶液快速分离。这种分子筛的结构稳定,具有较好的可再生重复使用性能。
参考文献
Experimental study on the thermophysical properties of Jimsar oil shale
[J].
Modified oil shale ash and oil shale ash zeolite for the removal of Cd2+ ion from aqueous solutions
[J].
Alkali activation of oil shale ash based ceramics
[J].
Synthesis of mesoporous silica SBA-15 from geothermal sludge
[J].
Conversion of oil shale ash into zeolite for cadmium and lead removal from wastewater
[J].
Adsorption of heavy metal ions from aqueous solutions by zeolite based on oil shale ash: kinetic and equilibrium studies
[J].
Enhanced adsorption of dyes on microwave-assisted synthesized magnetic zeolite-hydroxyapatite nanocomposite
[J].
Adsorption and recovery of low concentration coal-bed methane by zeolite ZSM-5
[J].As a high-silica hydrophobic material, zeolite ZSM-5 was used for the adsorption and recovery of low concentration coal-bed methane. Theoretical and experimental studies were conducted on adsorption equilibrium, adsorption kinetics and vacuum pressure swing adsorption (VPSA). Single and binary competitive adsorption equilibrium experimental data of CH4/N2 on zeolite ZSM-5 were measured by gravimetric method and breakthrough-curve method. The multi-site Langmuir model was used to fit the experimental data. Microporous diffusion coefficients were calculated based on the experimental data of diluted breakthrough curves and simulated results of an isothermic mathematical bi-disperse model without momentum loss. Competitive breakthrough curves of CH4 and N2 on zeolite ZSM-5 were predicted by fixed bed adsorption models with mass, momentum and energy transfer. Effects of CH4 concentration in feed, feed flow rate, feed time and purge/feed flow rate ratio on separation performances of ZSM-5 packed single column four-step VPSA process were investigated. It showed that ZSM-5 zeolite exhibited a relatively good selectivity for CH4 and the diffusion in micropores was the rate determining step for both CH4 and N2 adsorption. By the process of VPSA, CH4 purity can be concentrated from 20% to 31%~41% and the recovery can be up to 93%~98%.
沸石ZSM-5吸附回收低浓度煤层气中CH4
[J].利用高硅疏水性沸石ZSM-5吸附回收低浓度煤层气中的甲烷,对其吸附平衡、吸附动力学以及真空变压吸附分离过程进行了理论和实验研究。通过重量法和穿透曲线法测定了CH<sub>4</sub>/N<sub>2</sub>单组分及双组分的竞争吸附平衡数据,并采用Multisite Langmuir吸附等温线模型对其进行拟合。结合CH<sub>4</sub>和N<sub>2</sub>稀释穿透曲线实验数据和等温无动量损失的双分散二级孔结构扩散模型,获得CH<sub>4</sub>和N<sub>2</sub>在沸石ZSM-5上的微孔扩散系数。建立并求解包含质量、动量及能量传递的固定床吸附分离模型方程,预测了CH<sub>4</sub>和N<sub>2</sub>在沸石ZSM-5上的竞争吸附穿透曲线。进一步采用ZSM-5吸附剂填充床单柱四步真空变压吸附实验考察了进料浓度、进料流速、进料时间以及吹扫比对分离效果的影响。结果发现沸石ZSM-5对CH<sub>4</sub>具有较好的选择性,沸石晶粒内的微孔扩散为吸附速率控制步骤,真空变压吸附工艺可将模拟煤层气中20%的CH<sub>4</sub>提纯至31%~41%,回收率为93%~98%。
Utilization of red mud waste into mesoporous ZSM-5 for methylene blue adsorption-desorption studies
[J].
Feasible low-cost conversion of red mud into magnetically separated and recycled hybrid SrFe12O19@NaP1 zeolite as a novel wastewater adsorbent
[J].
Magnetic zeolite NaA: synthesis, characterization based on metakaolin and its application for the removal of Cu2+, Pb2+
[J].The optimum parameters for synthesis of zeolite NaA based on metakaolin were investigated according to results of cation exchange capacity and static water adsorption of all synthesis products and selected X-ray diffraction (XRD). Magnetic zeolite NaA was synthesized by adding Fe3O4 in the precursor of zeolite. Zeolite NaA and magnetic zeolite NaA were characterized with scanning electron microscopy (SEM) and XRD. Magnetic zeolite NaA with different Fe3O4 loadings was prepared and used for removal of heavy metals (Cu(2+), Pb(2+)). The results show the optimum parameters for synthesis zeolite NaA are SiO2/Al2O3=2.3, Na2O/SiO2=1.4, H2O/Na2O=50, crystallization time 8h, crystallization temperature 95 °C. The addition of Fe3O4 makes the NaA zeolite with good magnetic susceptibility and good magnetic stability regardless of the Fe3O4 loading, confirming the considerable separation efficiency. Additionally, Fe3O4 loading had a little effect on removal of heavy metal by magnetic zeolite, however, the adsorption capacity still reaches 2.3 mmol g(-1) for Cu(2+), Pb(2+) with a removal efficiency of over 95% in spite of 4.7% Fe3O4 loading. This indicates magnetic zeolite can be used to remove metal heavy at least Cu(2+), Pb(2+) from water with metallic contaminants and can be separated easily after a magnetic process.Copyright © 2013 Elsevier Ltd. All rights reserved.
The methyl blue adsorption performance and mechanism of NaX zeolite synthesized from Huadian oil shale ash
[J].
Synthesis and characterization of magnetic core with two shells: mordenite zeolite and CuO to form Fe3O4@MOR@CuO core-shell: as a visible light driven photocatalyst
[J].
Synthesis, characterization, and use of an amine-functionalized mesoporous silica SBA-15 for the removal of Congo Red from aqueous media
[J].
Characterization and application of lamellar zeolite-X prepared from kaolin
[J].
Synthesis and characterization of zeolite X obtained from coal gangue for adsorption of Cu2+
[J].
Feasible synthesis of magnetic zeolite from red mud and coal gangue: preparation, transformation and application
[J].
Structural, magnetic and electromagnetic properties of SrFe12O19 ferrite with particles aligned in a magnetic field
[J].
Systematic review on applicability of magnetic iron oxides-integrated photocatalysts for degradation of organic pollutants in water
[J].
Synthesize and characterize of Fe3O4/zeolite 4A magnetic nanocomposite
[J].
Effective removal of ruthenium (III) ions from wastewater by amidoxime modified zeolite X
[J].
Properties of methylene blue in the presence of zeolite nanoparticles
[J].
Preparation of zeolite 4A for adsorptive removal of methylene blue: optimization, kinetics, isotherm, and mechanism study
[J].In this study, we have reported a facile preparation of zeolite 4A from Ethiopia kaolin for the adsorptive removal of methylene blue (MB) dye. The formation of highly crystalline and pure phase zeolite 4A with cubic morphology was confirmed by powder X-ray diffraction (XRD) and scanning electron microscope (SEM) analysis. The efficiency of zeolite 4A for abatement of MB was investigated at varying adsorption parameters. Response surface methodology coupled with Box Behnken Design (RSM-BBD) was employed to optimize adsorption parameters. High regression (R-2 = 0.9947) and the low probability (p value <0.0001) values signify the validity of the quadratic model to predict the removal (%) of MB. The maximum MB removal (%) was 99.37% at the optimum combination of 50 mL of 10 mg/L of MB, 39.05 mg zeolite 4A, and 179.82 contact time (min). The adjusted R-square (R-adj(2)) and standard deviations (SD) were validated the best fitting of pseudo-second-order kinetics and the Langmuir isotherm model for experimental values. The maximum adsorption capacity (Q(max)) of zeolite 4A towards MB was found to be 44.35 mg g(-1). Electrostatic interaction, hydrogen bond, and n to pi bond formation are predicted to be the plausible interaction mechanism of MB to the surface of zeolite 4A. The Fukui function and vibration analysis based on the density functional theory (DFT) further strengthened the proposed mechanism.
Adsorption of methylene blue and methyl red dyes from aqueous solutions onto modified zeolites
[J].Zeolite, hematite, modified zeolite and commercial activated charcoal were examined for their ability to remove methylene blue (MB) and methyl red (MR) from their aqueous solutions. Modified zeolite and hematite were produced according to the Schwertmann and Cornell method while zeolite and commercial activated charcoal were obtained from S&B and Fluka AG companies, respectively. Adsorption experiments were conducted at three different adsorbent-to-solution ratios, namely 8, 16 and 24 g/L under environmental conditions and continuous stirring. Equilibrium isotherms of MB and MR were studied at different initial concentrations (from 5 × 10(-4) to 5 × 10(-3) g/L). MB adsorption kinetics were also studied. The maximum adsorption of MB and MR from their aqueous solutions was achieved at 24 g/L (adsorbent-to-dye solution ratio) after 1 h and was equal to 100% (MB) on modified zeolite and 99% (MR) on commercial activated charcoal, respectively. All the other materials achieved intermediate values of dye adsorption. From the applied kinetic models, the pseudo-second-order equation best described the adsorption of MB and MR. Consequently, modified zeolite showed the highest adsorption capacity for MB, while commercial activated charcoal showed the highest adsorption capacity of MR. The studied adsorbents can be used as filters to remove dyes from wastewaters.
Photodecoration of tungsten oxide nanoparticles onto eggshell as an ultra-fast adsorbent for removal of MB dye pollutant
[J].Nowadays, the use of natural wastes and adsorbents along with their modification by simple and new methods based on metal oxides to remove dye pollutants has been the focus of many researchers. In this study, for the first time, simple and low-cost modification of eggshell (EGS) with tungsten oxide (WO) based on the photochemical modification method as a green, ultra-fast, cost-effective, and biodegradable adsorbent is reported to remove of methylene blue (MB) dye pollutant. The EGS modified by WO was investigated by EDX, EDX mapping, XRD, FE-SEM, and UV-Vis Diffuse Reflectance (DRS) analyses. The obtained results show that the modified EGS by WO has more than ten times (78.5%) the ability to remove MB dye pollutant within 3 min compared to bare EGS (11%). Various parameters including dye pollutant pH, dye concentration, adsorbent dosage, and reusability of the WO/EGS adsorbent for removal of MB dye pollutant were investigated and the result show that the adsorbent capacity of WO/EGS is 1.64 mg g. EGS adsorbent The synthesis of WO/EGS adsorbent with a novel photochemical method as a fast and very cheap adsorbent with excellent efficiency can be a promising alternative adsorbent for various purposes in removing dye pollutants from water environments.© 2024. The Author(s).
Modification of zeolite by magnetic nanoparticles for organic dye removal
[J].
Evaluation of physicochemical methods in enhancing the adsorption performance of natural zeolite as low-cost adsorbent of methylene blue dye from wastewater
[J].
Removal of basic dye (methylene blue) from aqueous solution using zeolite synthesized from electrolytic manganese residue
[J].
An investigation of a natural biosorbent for removing methylene blue dye from aqueous solution
[J].
Removal of mercury(II) and methylene blue from a wastewater environment with magnetic graphene oxide: adsorption kinetics, isotherms and mechanism
[J].
Fe3O4@UiO-66-NH2 core-shell nanohybrid as stable heterogeneous catalyst for Knoevenagel condensation
[J].
核壳结构Fe3O4@UiO-66-NH2磁性纳米复合材料的合成及其催化Knoevenagel缩合反应性能
[J].金属有机骨架(MOF)材料是由过渡金属离子与有机配体通过配位键连接构成的高度有序的超分子化合物.这类材料比表面积大,孔隙率高,热稳定性好,而且具有规整可调控的孔结构、易于功能化的骨架金属离子和有机配体,在多相催化领域具有潜在应用前景.将纳米尺寸的MOF材料等多孔材料作为催化剂,可以提高反应传质效率,从而提高催化反应活性,但纳米MOF催化剂的分离和回收困难.将磁性纳米粒子和MOF材料组装成核壳结构的磁性MOF材料,不仅可简化催化剂的分离回收,而且通过控制壳层材料的厚度可以实现催化剂的高活性和高选择性.我们曾将磁核Fe<sub>3</sub>O<sub>4</sub>纳米粒子交替放入含有一种MOF材料前体的DMF溶液中,采用层层组装法制备了磁性Fe<sub>3</sub>O<sub>4</sub>@UiO-66-NH<sub>2</sub>纳米复合材料.经过十步组装后的材料的透射电镜(TEM)结果证实为核壳结构.但未出现明显的UiO-66-NH<sub>2</sub>的X射线衍射(XRD)特征峰,说明壳层材料UiO-66-NH<sub>2</sub>的结晶度较低;同时由于其孔结构的破坏或堵塞,在反应中出现明显失活.本文进一步改进自组装方法制备了核壳结构的磁性Fe<sub>3</sub>O<sub>4</sub>@UiO-66-NH<sub>2</sub>纳米复合材料,用XRD、傅里叶变换红外光谱(FT-IR)、TEM、扫描电镜(SEM)和氮气吸附等方法对材料的组成和结构进行了表征,并考察了其在Knoevenagel缩合反应中的催化性能.结果表明,所制材料是以Fe<sub>3</sub>O<sub>4</sub>为核,以UiO-66-NH<sub>2</sub>为壳的核-壳结构材料.经三次组装后出现了一系列UiO-66-NH<sub>2</sub>的XRD特征峰,说明采用新方法制备的复合材料中壳层材料UiO-66-NH<sub>2</sub>结晶度高,晶体结构规整.N<sub>2</sub>吸附-脱附结果表明,材料具有较高的比表面积和孔容.该复合材料在Knoevenagel缩合反应中表现出与纳米UiO-66-NH<sub>2</sub>相当或更好的催化活性和选择性,而且因壳层材料的孔道限阈效应而对底物表现出尺寸选择性.由于材料结晶度和晶体结构规整度的提高,催化剂稳定性更好,通过简单磁性分离即可分离和回收催化剂,循环使用4次而未出现明显失活.相对于本课题组之前报道的Fe<sub>3</sub>O<sub>4</sub>@CuBTC-NH<sub>2</sub>,Fe<sub>3</sub>O<sub>4</sub>@IRMOF-3和Fe<sub>3</sub>O<sub>4</sub>@UiO-66-NH<sub>2</sub>材料,本文所制的Fe<sub>3</sub>O<sub>4</sub>@UiO-66-NH<sub>2</sub>是一类结构更加稳定的高效固体碱催化剂.
A new absorbent by modifying walnut shell for the removal of anionic dye: kinetic and thermodynamic studies
[J].
Comparison of photocatalytic efficiency of supported CuO onto micro and nano particles of zeolite X in photodecolorization of methylene blue and methyl orange aqueous mixture
[J].
Synthesis of Na-X zeolite from Longkou oil shale ash by alkaline fusion hydrothermal method
[J].