金属-空气(Metal-air)电池的能量密度比较高、环保且成本较低,在储能领域备受关注[1, 2]。金属-空气电池包括:锂空气电池[3]、锌空气电池[4]、镁空气电池和铝空气电池[5, 6]。镁-空气(Mg-air)电池的质轻、成本低、理论能量密度高(6.8 kWh·kg-1)和电压高(3.1 V) [7~10],但是也有许多不足之处:(1)在放电过程中生成的Mg(OH)2沉积物妨碍阳极活性物质的利用[11];(2)阳极的析氢反应导致能量损失[12];(3)阳极的溶解不均匀 (大块效应)[13]。解决这些问题有两种方法:(1)将纯镁合金化。例如在Mg-Al-Zn系中加入少量的Gd[14,15]、La[16]、In[17]等稀土元素实现细化晶粒、均匀溶解和减少“大块效应”,从而提高放电效率;在Mg-Al-Pb系中加入少量的La[18]、In[19]、Ce和Y[20],进一步缩短阳极的激活时间[21~23];加入少量的Ca元素可提高合金的放电性能[24,25]。Yang等[26]的研究表明,块状不连续的(Mg,Al)2Ca相可作为牺牲阳极保护
1 实验方法
1.1 实验用合金的制备
实验用原料:纯Mg锭(99.99%,质量分数)、纯Al锭(99.99%,质量分数)、Mg-8%(质量分数)Mn中间合金和Mg-30%(质量分数)Ca中间合金。用高温电阻炉熔炼得到Mg-xAl-1Ca-0.7Mn (x = 0,1%,2%,3%,4%,质量分数)合金铸锭。熔炼时,将熔炉加热到720 ℃后将大块Mg锭放入熔炉内的坩埚中熔化,再依次放入其它材料。用CO2+SF6混合气体保护以防止Mg氧化甚至燃烧。原料全部融化并除渣后加入精炼剂(MgCl2)并搅拌,静置一段时间后将熔体浇铸在预先预热的模具中,熔体凝固后得到直径为90 mm、长度为300~320 mm的圆柱形铸锭。
将铸锭固溶处理(加热至400 ℃保温24 h取出后空冷)后在270 ℃挤压成厚度为4.96 mm的板材,挤压比和挤压速率分别为10∶1和0.1 mm/s。最后制成尺寸为10 mm × 10 mm × 10 mm的小块。将制得的挤压态Mg-xAl-1Ca-0.7Mn (x = 0,1%,2%,3%,4%,质量分数)合金(AMX合金)记为AMX011、AMX111、AMX211、AMX311、AMX411合金。
1.2 微观组织和性能表征
先将AMX合金试样的观察面用SiC砂纸打磨和机械抛光,直至没有划痕,用腐蚀液(挤压态:4%的硝酸酒精)腐蚀后依次用蒸馏水和酒精冲洗,吹干后用Zeiss 200 MAT显微镜观察其组织。用附带EDS的FEI Quanta 250扫描电子显微镜(SEM)观察挤压态AMX合金的微观组织形貌并进行微区成分分析。
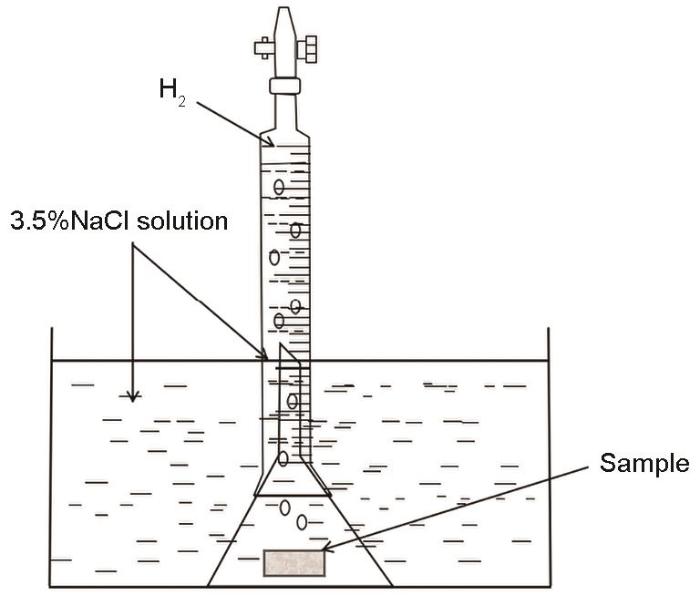
用排水法收集合金在室温条件下浸泡在3.5%(质量分数)NaCl溶液中产生的氢。析氢实验装置如图1所示。收集前,先用环氧树脂包裹待测合金试样只露出面积为10 mm × 10 mm的工作面。经析氢试样浸泡72 h,然后将带有腐蚀产物的试样置于200 g/L CrO3 + 10 g/L AgNO3溶液中超声振荡10 min,依次用无水乙醇和去离子水在超声波中清洗5 min,取出吹干后置于干燥皿中2 h后称重。为了观察合金腐蚀时的趋势,分别将试样浸泡3和24 h后用SEM观察实验样品去除腐蚀产物前后的腐蚀形貌。将腐蚀产物用200 g/L CrO3 + 10 g/L AgNO3溶液清洗。分别测量腐蚀实验前后实验的质量,腐蚀速率为[33]
式中ν为腐蚀速率(mg·cm-2·h-1);
图1
使用Zahner Zennium电化学工作站三电极体系进行室温电化学测试,工作电极(WE)为实验制备的挤压态AMX合金,辅助电极(CE)为铂电极,参比电极(RE)为饱和甘汞电极(SCE),电解液为3.5%(质量分数)NaCl溶液。用Nyquist图分析试样的腐蚀性能,使用ZsimpWin软件拟合实验数据。在非法拉第电位范围(-1.80 V~0.00 V vs. SCE)内以150 mV/s的扫描速率进行合金电极的循环伏安(CV)实验。在3.5%(质量分数)NaCl溶液中以4种不同的电流密度(5、10、20和30 mA·cm-2)恒流放电4 h。放电前称出待测工作电极的质量,放电后的样品在200 g/LCrO3 + 10 g/LAgNO3溶液中浸泡5 min以去除放电产物,取出后用去离子水冲洗后再用无水乙醇在超声波仪器中震荡5 min,取出吹干后称其质量。用放电前后的质量损失计算工作电极的阳极利用率[16,19,34]
式中
2 实验结果和讨论
2.1 AMX合金的微观结构
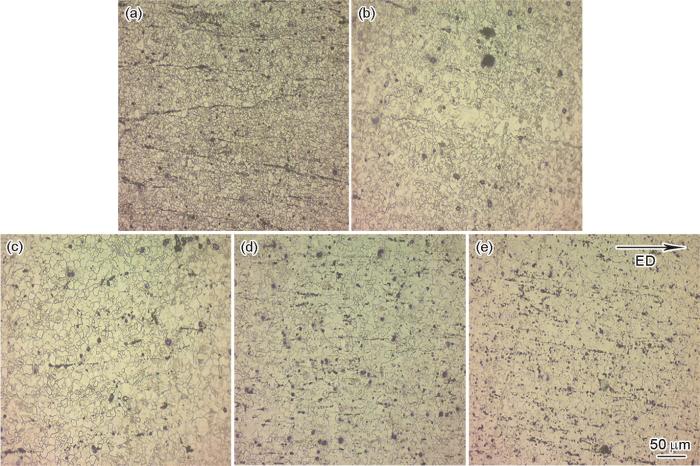
图2
图2
挤压态合金的OM图
Fig.2
OM diagram of extruded alloy (a) AMX011, (b) AMX111, (c) AMX211, (d) AMX311, (e) AMX411
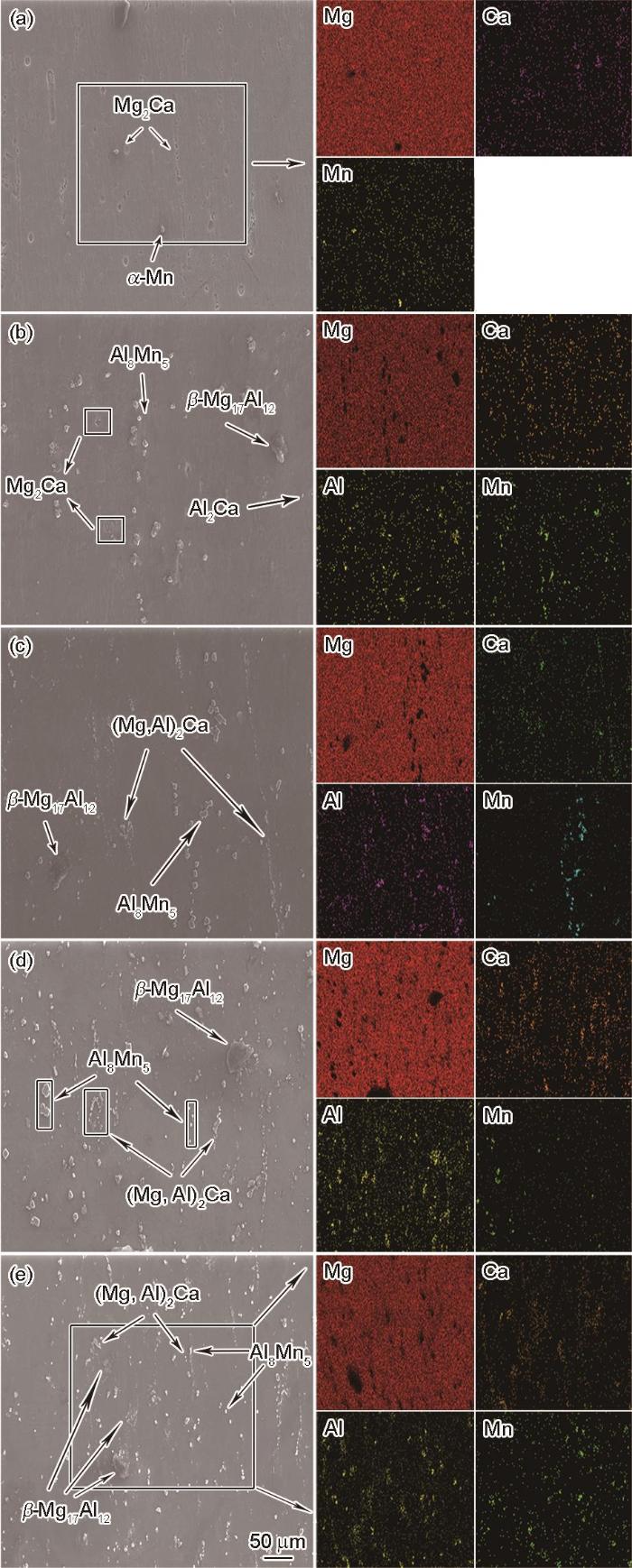
根据挤压态合金平行于挤压方向(ED)取样的SEM照片相关的面扫图(图3),深入分析合金的微观结构:在AMX011合金中,Mg2Ca相以零散颗粒的形式沿着挤压方向呈流线型排列在镁基体中。Mg2Ca相的电位低于镁基体,使其在微电偶腐蚀过程中易成为阳极先开始腐蚀[35]。因此AMX011合金中的Mg2Ca相先发生点蚀;AMX111合金中只有少量零散的Mg2Ca相,随着Al含量的提高,在晶界附近出现少量细小颗粒状Al2Ca相,其不均匀的分布影响电偶腐蚀的顺序和速率。Al含量提高到2%的合金(AMX211合金),开始出现了团聚状或沿挤压方向分布的半连续(Mg,Al)2Ca相和Al2Ca相。与AMX111合金相比,(Mg,Al)2Ca相和Al2Ca相分布较为均匀,而Mg2Ca颗粒几乎消失;与AMX111和AMX211合金相比,Al含量更高的AMX311合金中Al8Mn5、(Mg,Al)2Ca以及β-Mg17Al12相的尺寸更大。但是,AMX411合金的Al含量更高,其中更多的再结晶形核点使第二相的尺寸较小且分布更加均匀。含Al的第二相具有比镁基体更正的电极电位,是放电过程中的阴极。而合金中更小且均匀分布的第二相在电解过程中使放电产物均匀脱落,从而使其放电活性提高。
图3
图3
挤压态合金的SEM形貌
Fig.3
SEM diagram of extruded alloy (a) AMX011, (b) AMX111, (c) AMX211, (d) AMX311, (e) AMX411
2.2 挤压态合金的腐蚀行为
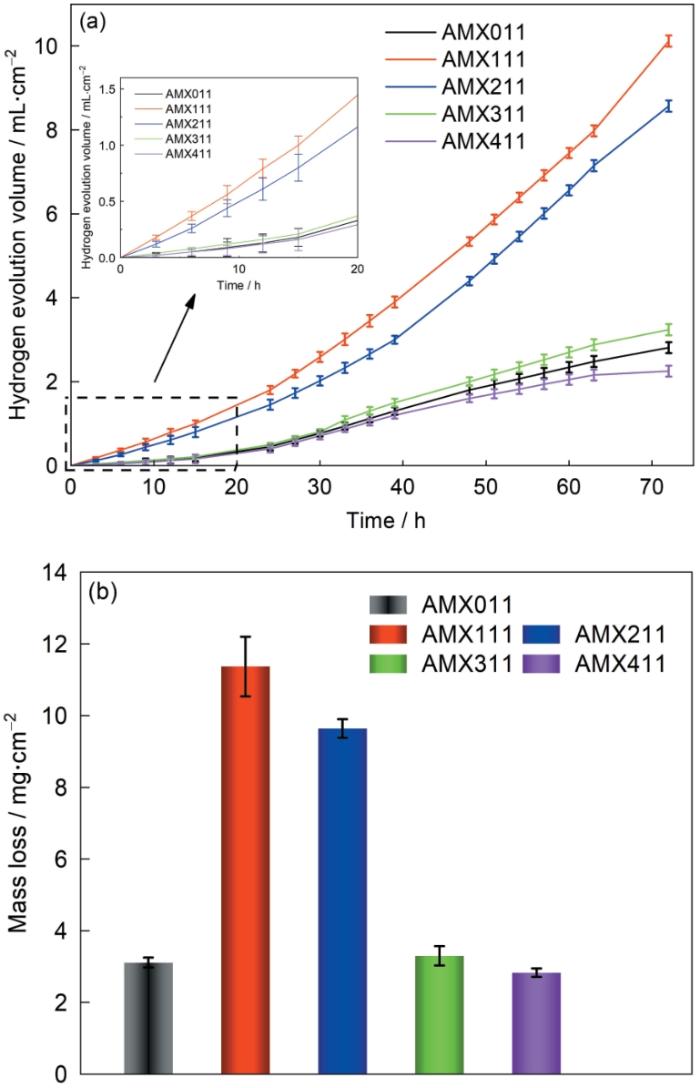
图4给出了挤压态合金在3.5%NaCl中的析氢曲线和质量损失柱状图,表明其腐蚀结果与极化曲线一致。在镁合金的浸泡初期(6 h),在表面生成的氧化膜的保护作用使其析氢速率较低。随着浸泡时间的延长,溶液中的Cl-对氧化膜的侵蚀使腐蚀深入合金的内部,从而使析氢速率提高。浸泡60 h后AMX111和AMX211合金的腐蚀速率进一步提高,而其它合金的腐蚀速率则不同程度的降低。可根据析氢评估镁合金的溶解速率和腐蚀速率,因为阴极的HER产生氢气。图4b给出了72 h的浸泡周期结束后各合金的质量损失率。可以看出,AMX011、AMX111、AMX211、AMX311和AMX411合金的质量损失率为3.11、11.37、9.64、3.30和2.83 mg·cm-2,由
图4
图4
AMX合金在3.5%NaCl中浸泡72 h后析氢量-时间曲线和质量损失
Fig.4
Hydrogen evolution volume (a) and total mass loss (b) of extruded alloys after immersion in 3.5%NaCl for 72 h
表1 挤压态合金动电位极化曲线拟合数据
Table 1
| Alloy | φcorr / V | Jcorr / µA·cm-2 |
|---|---|---|
| AMX011 | -1.591 | 80.5 ± 0.25 |
| AMX111 | -1.533 | 154.3 ± 0.64 |
| AMX211 | -1.540 | 133.5 ± 0.38 |
| AMX311 | -1.569 | 82.7 ± 0.21 |
| AMX411 | -1.267 | 67.9 ± 0.16 |
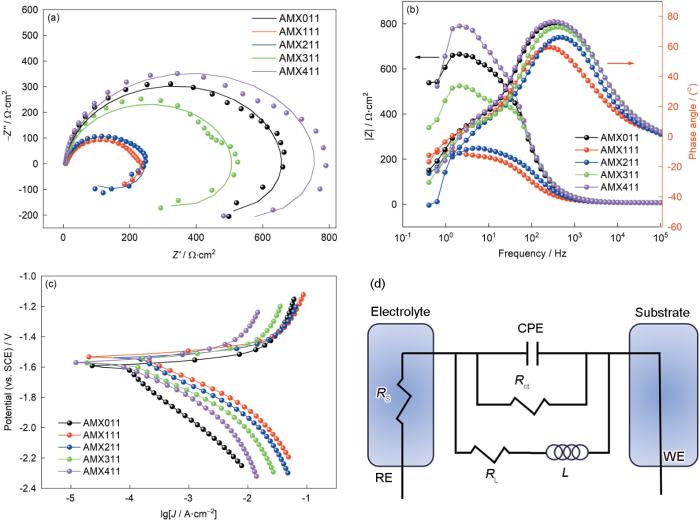
析氢质量损失和极化实验结果都表明,AMX411合金的耐腐蚀性能最好。图5a和b给出了AMX合金的Nyquist和Bode图。Nyquist图由高频容抗环和低频感抗环组成,其中的高频区容抗环与电极和电解液界面的电荷转移过程相关,环的大小表征电荷转移电阻的大小[37]。图5d给出了等效电路图,等效电阻RL与电感L串联再与电荷转移电阻Rct和表征双电层电容的常相位角元件CPEdl并联,最后与溶液电阻Rs串联,构成电极过程的等效电路。表2列出了所研究合金拟合的各电化学元件参数值,可见AMX411合金的RL电阻值(581.2 Ω·cm2)大于其电感L的阻抗值(368.4 H·cm2)。这表明,在开路电位下AMX411合金发生点蚀比活化溶解困难[38]。图5a中的AMX411合金的阻抗环最大,即对应其最大电荷转移电阻,表明其在腐蚀过程中的腐蚀屏蔽较好。所研究的镁合金电极在低频区有一个直径较小的感抗弧,与合金在低频区的Bode图中下凹的相位角峰对应。Nyquist图低频区中感抗弧,可能与电极表面局部腐蚀(如点蚀)的诱导期有关[39],而图6给出的各合金腐蚀初期确实以点蚀为主。AMX111和AMX211合金的电容环半径明显比其他各合金的小,表明Al添加比例低于2%~3%的合金其阻抗明显减小。Bode图中AMX411合金的模峰值和相位角峰值最大,也表明其耐蚀性最高。
图5
图5
AMX合金在3.5%NaCl溶液中的电化学阻抗谱、极化曲线和等效电路
Fig.5
Nyquist (a), Bode (b) plots, and polarization curves (c) of AMX alloy in 3.5%NaCl solution, and equivalent circuit diagrams (d)
表2 拟合AMX合金的EIS参数
Table 2
| Alloy | Rs / Ω·cm2 | CPEdl / µF·cm-2 | Rct / Ω·cm2 | L / H·cm2 | RL / Ω·cm2 | |
|---|---|---|---|---|---|---|
| Cdl | n | |||||
| AMX011 | 6.854 | 8.562 × 10-3 | 0.9489 | 656.1 | 537.6 | 463.1 |
| AMX111 | 7.956 | 3.543 × 10-2 | 0.556 | 226.4 | 291.1 | 57 |
| AMX211 | 6.825 | 1.458 × 10-2 | 0.9223 | 241.1 | 110.3 | 56.85 |
| AMX311 | 7.551 | 7.881 × 10-3 | 0.9454 | 503.8 | 263.4 | 258.1 |
| AMX411 | 7.041 | 8.052 × 10-3 | 0.9517 | 760.2 | 368.4 | 581.2 |
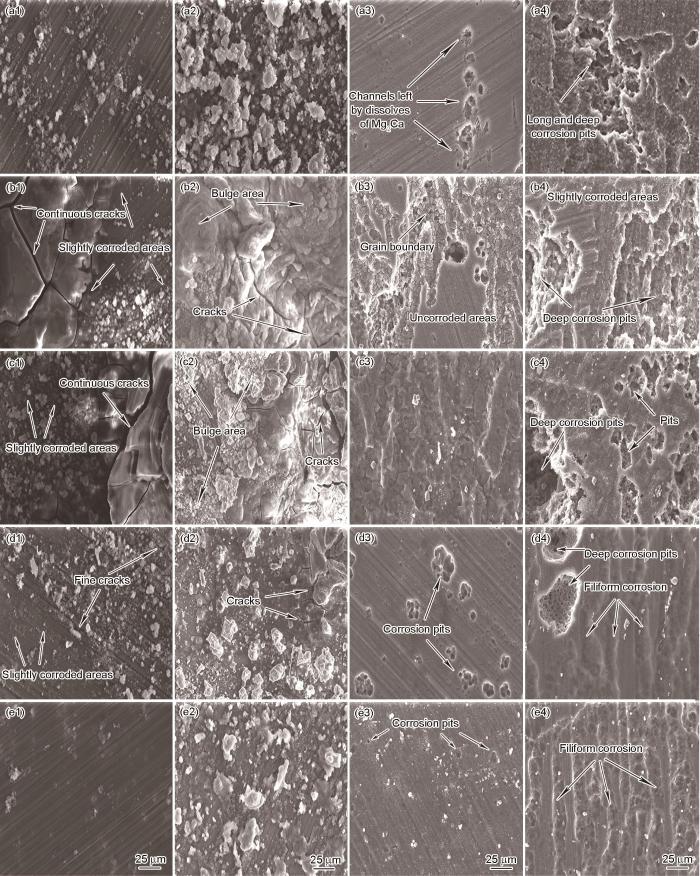
图6
图6
挤压态合金分别浸泡3和24 h后以及去除腐蚀产物后的形貌
Fig.6
Corrosion morphologies of alloys in extruded state with (a1~e1, a2~e2) and without corrosion (a3~e3, a4~e4) products after 3 h (a1~e1, a3~e3) and 24 h (a2~e2, a4~e4) immersion
图6a1~e1给出了浸泡3 h后带有腐蚀产物试样的照片,可见AMX011、AMX311以及AMX411合金的腐蚀表面比AMX111和AMX211更为平滑,表明其耐腐蚀性能较好。在5种合金中,AMX411合金的腐蚀产物最少,表明其腐蚀程度轻微。与此相反,AMX111和AMX211合金出现明显的局部自腐蚀,其中AMX111合金的腐蚀表面出现较长且较深的裂纹,表明其析氢倾向较强,是其自腐蚀严重的原因之一。在析氢较少的AMX311合金腐蚀表面出现了比AMX011合金更多的裂纹,表明其析氢量较大。在浸泡24 h后带有腐蚀产物使用的照片中,可见浸泡3 h的腐蚀趋势。AMX111和AMX211合金的腐蚀产物堆积最为严重,且腐蚀产物的表面布满了长而深的裂纹。这些裂纹,进一步加速了腐蚀向材料内部扩展。相反,AMX411合金的腐蚀产物最少且其表面平整光滑,没有出现明显的缺陷,再次印证其耐腐蚀性能优异。
图6a3~e3和a4~e4给出了去除腐蚀产物后AMX合金表面的微观结构,与a1~e1和a2~e2结合进一步分析其腐蚀机理:由于AMX011合金中Mg2Ca相的腐蚀电位低于镁基体,在浸泡3 h的电解过程中Mg2Ca相充当阳极保护阴极镁基体,表现为在除去腐蚀产物的图中沿挤压方向分布的小空洞,与图3中Mg2Ca相的分布相符。AMX111合金的腐蚀起始于晶界并向内部发展,表明其具有晶间腐蚀的特点。由于Al元素析出不均匀而在晶界附近出现贫Al区和富Al区,使Al富集区域的电位降低成为阴极,使贫Al区优先发生严重局部腐蚀,少量的颗粒状Mg2Ca相也使AMX111合金发生点蚀[40]。虽然AMX211合金也发生点蚀和晶间腐蚀,但是其Al元素含量的提高降低了晶界间的电势差并使第二相分布均匀,故其耐蚀性能优于AMX111合金。AMX311和AMX411合金的点蚀起始部位主要位于Al8Mn5、(Mg,Al)2Ca和β-Mg17Al12相。在腐蚀初期富含Al的氧化膜的保护作用使AMX411合金的腐蚀程度低于AMX311合金。同时,AMX411合金中第二相颗粒更小且分布更均匀,降低了整体电势差和微电偶腐蚀效应,从而展现出最佳的耐蚀性能[41]。
随着浸泡时间延长至24 h,合金的腐蚀加剧。在AMX011合金中,腐蚀从小孔逐渐向内发展并出现部分晶粒脱落而形成较大的深坑。在AMX111和AMX211合金中,腐蚀从贫Al区晶界向内扩展,使晶粒间的结合力降低而使大量未反应的基体材料脱落,形成大尺寸深坑。AMX311和AMX411合金的腐蚀从点状演变为丝状,可能与第二相在ED方向上的分布有关。AMX311合金中较大的颗粒状Al2Ca相因其电位较正而作为阴极,在周围镁基体完全腐蚀时大片第二相脱落而形成大且深的腐蚀坑。相反,AMX411合金中的第二相尺寸更小、分布更均匀且电位差较小,因此其腐蚀形貌为丝状浅坑。
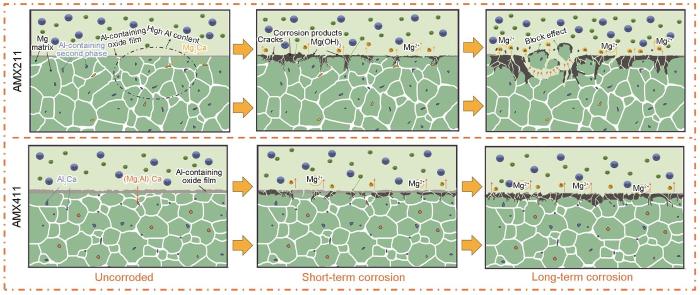
根据AMX合金的成分分布、微观结构和腐蚀行为,本文研究的合金其腐蚀机理(图7)可归结为两类:AMX111和AMX211合金的腐蚀类型相似,部分Mg2Ca作为腐蚀阳极先溶解形成初期的点蚀。含Al第二相的电位比镁基体的更正,成为腐蚀阴极。AMX111和AMX211合金沿晶界不均匀分布的含Al第二相产生区域的电位差异,导致区域的微电偶腐蚀而形成区域性的“大块效应”,严重影响其放电效率;AMX311和AMX411合金的腐蚀情况类似,生成的氧化膜比其他两种合金的更厚,延缓了腐蚀启动时间而使其耐腐蚀性能提高。AMX411合金中的第二相更小且分布得更均匀使其能均匀溶解,在长时间放电的情况下表现出优异的耐腐蚀性能和高利用效率。Qiu等[42]在本系列固溶态合金中发现,半连续的(Mg,Al)2Ca相具有一定的腐蚀屏蔽作用,使其耐腐蚀性能有所提高。在挤压后的AMX311和AMX411合金中出现了半连续的(Mg,Al)2Ca相,是其耐腐蚀性能高的原因之一。
图7
图7
AMX合金浸泡在3.5%NaCl溶液中不同时间后的腐蚀机理示意图
Fig.7
Schematic corrosion mechanism of AMX alloy in 3.5%NaCl solution for different time
2.3 挤压态合金的放电行为
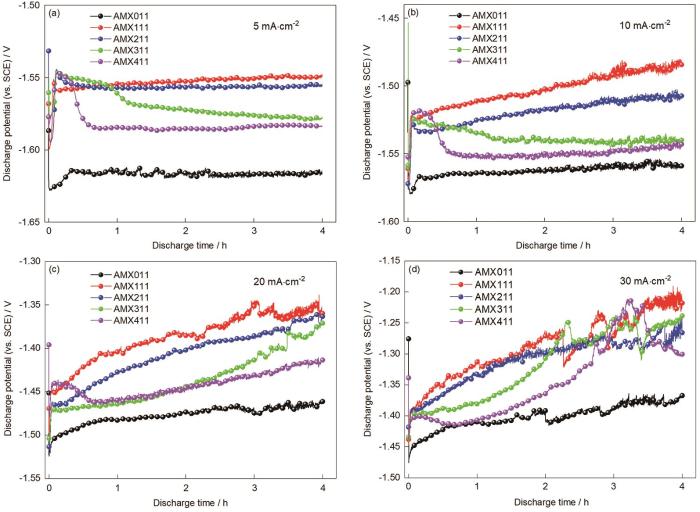
图8给出了挤压态合金在3.5%NaCl中放电4 h的恒电流放电曲线,表3列出了平均放电电压和阳极利用率等特性。可以看出,在电流密度为5和10 mA·cm-2的条件下,5种合金的放电过程较为稳定,表明合金的放电产物的产生和脱落保持动态平衡。AMX011合金的放电电位(-1.617 V)最低和激活时间较短,可归因于合金中的Mg2Ca相较多。Mg2Ca相提供了更多的电子而加速表面钝化膜的破裂,从而使其更快地进入有效放电阶段。AMX111和AMX211合金严重的析氢副反应使大量电子并未参与放电过程,是其平均放电电压比AMX111和AMX211合金正的原因之一[43]。而电化学活性较低的AMX311和AMX411合金内的第二相分布更为均匀,有助于在放电过程中减少析氢副反应,从而使其放电效率提高。
图8
图8
挤压态合金浸泡在3.5%NaCl中不同电流密度放电4 h的恒电流放电曲线
Fig.8
Constant current discharge curve of extruded alloy discharged in 3.5%NaCl for 4 h under the current density of 5 mA·cm-2 (a), 10 mA·cm-2 (b), 20 mA·cm-2 (c), and 30 mA·cm-2 (d)
表3 挤压态合金在不同电流密度下放电4 h的平均放电电压和阳极利用率
Table 3
| Parameters | Discharge current density / mA·cm-2 | AMX011 | AMX111 | AMX211 | AMX311 | AMX411 | |
|---|---|---|---|---|---|---|---|
| Average discharge potential / V | 5 | -1.617 | -1.553 | -1.557 | -1.567 | -1.581 | |
| 10 | -1.563 | -1.503 | -1.519 | -1.538 | -1.547 | ||
| 20 | -1.477 | -1.387 | -1.408 | -1.437 | -1.442 | ||
| 30 | -1.402 | -1.289 | -1.310 | -1.319 | -1.345 | ||
| Anode utilization / % | 5 | 43.32 | 30.36 | 33.49 | 43.27 | 44.39 | |
| 10 | 50.64 | 35.03 | 38.14 | 47.23 | 52.25 | ||
| 20 | 59.74 | 41.83 | 43.64 | 54.56 | 61.36 | ||
| 30 | 63.82 | 44.79 | 46.15 | 58.98 | 65.35 | ||
在电流密度为10 mA·cm-2的条件下,AMX011和AMX411合金之间的平均放电电压差异极小,分别为-1.563和-1.547 V。Al含量的提高使AMX411合金产生致密的放电产物,从而使其平均放电电位的正移,但是AMX411合金中细小、分布均匀的第二相有利于其均匀溶解而使其放电能力提高[44]。电流密度提高到20 mA·cm-2时AMX011和AMX411合金依然保持稳定的放电电位,而其他合金的放电电位开始正向偏移。其原因可能是高电流密度使其他合金的副反应加剧或界面阻抗增大[33]。进一步提高电流密度到30 mA·cm-2,破坏了此系列合金的溶解和放电产物的动态平衡而使放电产物迅速生成。但是放电产物从阳极表面剥离的速度跟不上产物生成的速度,产物的积累使放电电位正移。但是,大量产物的不均匀累积和随后的大块剥落使电位发生波动。需要指出的是,在此高电流密度下AMX011合金电位波动最小。这意味着,AMX011合金产生的放电产物极易剥离,从而使电化学反应较为稳定。
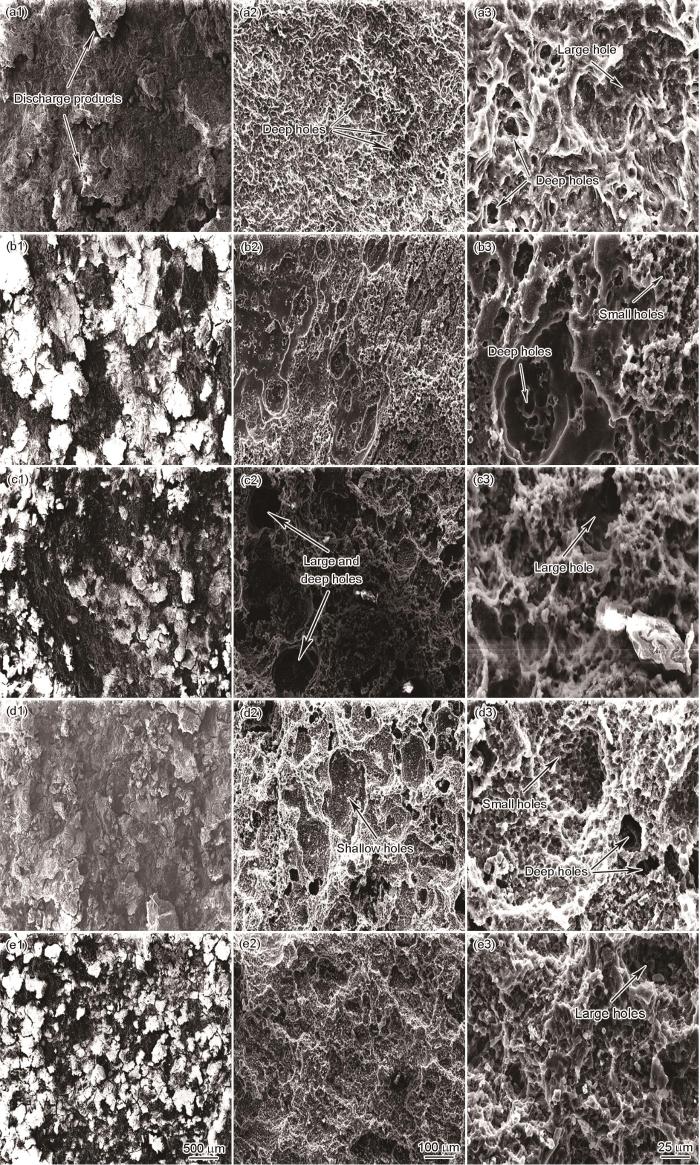
图9给出了放电后合金的表面形貌。可以看出,堆积在AMX011合金表面的放电产物最少,有利于电解液充分渗透并与阳极材料接触,从而维持较高的放电活性和负向的放电电位。去除放电产物后表面密集的小孔结构,表明Mg2Ca相发生了腐蚀。这些小孔增大了有效接触面积,使放电反应的效率提高; AMX111和AMX211合金的表面产生放电产物表明发生了严重的析氢副反应,导致的电子大量自腐蚀流失而使其放电活性降低。去除AMX111合金表面的放电产物后,可见表面的大且光滑的空洞(可能源于晶粒的脱落)和较小的放电孔。而AMX211合金表面不规则的凹坑和小孔,反映出阳极溶解的不均匀性和放电过程的不稳定。AMX311合金表面堆积较多放电产物且裂纹较少,表明出现了一定程度的放电屏蔽,降低了放电活性。这一点,表现为放电曲线上电位的正移。去除放电产物后,AMX311合金表面可见较大的放电孔和密集的小孔。出现较大的放电孔的原因是较大尺寸第二相的脱落,密集小孔是析氢引起的。这些孔洞,都比AMX111和AMX211合金的略浅。AMX411合金的放电产物剥落较少而使其放电电位比AMX011合金的稍正,但是放电产物表面的裂纹有助于电解液与阳极材料良好接触而使合金能在放电过程中保持相对平稳的电位。去除放电产物后AMX411合金的表面相对平整,只有轻微腐蚀形成的浅坑,表明其放电过程较为稳定。轻微的析氢反应减少了不必要的电子损失,提高了放电活性,并且均匀的放电特性使阳极材料的利用率提高。
图9
图9
挤压态合金在电流密度为20 mA·cm-2条件下放电4 h后以及去除放电产物的表面形貌
Fig.9
Surface morphologies of extruded alloy without removal (a1~e1) and with removal of discharge products (a2~e2, a3~e3) after 4 h discharge at 20 mA·cm-2 (a) AMX011, (b) AMX111, (c) AMX211, (d) AMX311, (e) AMX411
3 结论
(1) 在AMX合金中添加适量的Al可提高其综合性能。
(2) AMX111和AMX211合金的自腐蚀行为严重的原因,是其中含Al相的区域性分布;而在AMX411合金中含Al第二相细小且均匀分布,使其在电解液中均匀溶解。
(3) AMX合金Al含量的提高可降低其电化学活性和使其耐腐蚀性能最佳。
参考文献
Research advances of magnesium and magnesium alloys worldwide in 2022
[J].
Research advances of magnesium and magnesium alloys globally in 2023
[J].
A comprehensive review of carbon-based air cathode materials for advanced non-aqueous lithium-air batteries
[J].
A rechargeable zinc-air battery based on zinc peroxide chemistry
[J].Rechargeable alkaline zinc-air batteries promise high energy density and safety but suffer from the sluggish 4 electron (e)/oxygen (O) chemistry that requires participation of water and from the electrochemical irreversibility originating from parasitic reactions caused by caustic electrolytes and atmospheric carbon dioxide. Here, we report a zinc-O/zinc peroxide (ZnO) chemistry that proceeds through a 2e/O process in nonalkaline aqueous electrolytes, which enables highly reversible redox reactions in zinc-air batteries. This ZnO chemistry was made possible by a water-poor and zinc ion (Zn)-rich inner Helmholtz layer on the air cathode caused by the hydrophobic trifluoromethanesulfonate anions. The nonalkaline zinc-air battery thus constructed not only tolerates stable operations in ambient air but also exhibits substantially better reversibility than its alkaline counterpart.Copyright © 2021, American Association for the Advancement of Science.
Clarifying the roles of grain boundary and grain orientation on the corrosion and discharge processes of α-Mg based Mg-Li alloys for primary Mg-air batteries
[J].
Aluminum-air batteries: A review of alloys, electrolytes and design
[J].
Electrochemical performance of melt-spinning Al-Mg-Sn based anode alloys
[J].
The effect of small addition of tin and indium on the corrosion behavior of aluminium in chloride solution
[J].
Microstructure and properties of As-cast Mg-8Zn-4Al-0.5Cu-0.5Mn-xLi alloys with high modulus
[J].
Mg-8Zn-4Al-0.5Cu-0.5Mn-xLi高模量铸造镁合金的组织和性能
[J].设计和制备Mg-8Zn-4Al-0.5Cu-0.5Mn-xLi (x = 0,3,5,7)(%,质量分数)系列高比模量的镁合金,研究了它的组织和性能。Li含量为3.14%和5.37%的两种镁合金,其基体为单相α-Mg,在晶界析出骨骼状Mg<sub>32</sub>(Al, Zn)<sub>49</sub>相,在晶粒内有颗粒状Mg<sub>5</sub>Al<sub>2</sub>Zn<sub>2</sub>相和Al<sub>2</sub>Mn相;Li含量为7.57%的合金,其基体主要为双相组织α-Mg + β-Li。添加Li元素后在晶界附近生成的共晶组织由α-Mg相和层片状AlLi相组成,并随着Li含量的提高层片状AlLi相的含量随之提高。随着Li含量的提高,合金的屈服强度呈逐渐提高的趋势,而抗拉强度基本上不变,合金的弹性模量呈现先提高后降低的趋势。铸态合金ZA84-5Li的弹性模量可达51.89 GPa,比纯Mg的弹性模量高7 GPa,但是其力学性能基本不变,屈服强度为141 MPa,抗拉强度为189 MPa,密度为1.71 g/cm<sup>3</sup>。
Microstructure and mechanical properties of extruded Mg-Alloy Mg-Al-Ca-Mn-Zn
[J].The microstructure and mechanical properties of extruded Mg-alloy of Mg-1Al-0.4Ca-0.5Mn-0.2Zn (mass fraction, %) were systematically investigated. As indicated by the results, the incomplete dynamic recrystallization occurred for the alloys extruded at 260℃ (denoted as AXMZ1000-260) and 290°C (AXMZ1000-290) with recrystallized grain sizes of 0.75 μm and 1.2 μm, respectively. The two alloys have high-density G.P. regions and spherical nano-phases, which can effectively inhibit the dislocation motion and provide abundant nucleation sites for dynamic recrystallization. Moreover, the nano-phases precipitated along grain boundaries can restrain the migration of grain boundary and restrict the growth of DRXed grains, which results in the ultrafine grains with a size of 0.75 μm in AXMZ1000-260 alloy. The strength of the alloy decreases with the increase of extrusion temperature, and the change of elongation is not obvious. The yield strength and elongation of alloys extruded at 260℃ and 290℃ are approximately 322 MPa and 343 MPa, as well as 13.4% and 13%, respectively. The dynamic precipitation and recovery process are promoted by the increasing extrusion temperature, and a high-density G.P. zones and spherical nano-phases are accumulated in the alloy. At the same time, many dislocations are transformed into LAGBs by dynamic recovery, and the unDRXed areas are subdivided into dense lamellar subgrains. The nano-phases and LAGBs can effectively hinder the newly generated dislocation motion, which is the major reason that the alloy extruded at 290℃ still have a high yield strength and the change of ductility is not obvious. Furthermore, TEM observations show that the pinning effect of G.P. zones can impede the dynamic recovery to certain extent, resulting in a high number of residual dislocations in the alloy, which is conducive to the improvement of the yield strength.
Mg-Al-Ca-Mn-Zn变形镁合金的组织和力学性能
[J].
A novel rechargeable Magnesium-Air battery using “All in one” Mg anode with high reversibility
[J].
Development and application of plastic processing technologies of magnesium alloys
[J].China has the most abundant magnesium resources in the world. Magnesium and its alloys have the advantages of low density, high specific strength, good damping property, and exceptional electromagnetic-shielding and energy-storage characteristics. They are one of the most promising lightweight materials. The enhanced applications of magnesium alloys can save energy and reduce emissions and are significant to the new Chinese energy strategy. However, magnesium alloys have a hexagonal close-packed structure and exhibit relatively low ductility. A bottleneck in expanding the application of magnesium alloys is improving the ductility of magnesium alloys. For more than ten years, efforts have been made to improve the ductility and plastic deformation ability. Progress has been made in plastic-processing technologies of magnesium alloys. The novel alloy design theory “solid solution strengthening and ductilizing” and advanced preparation technologies such as “melt self-purification through varying temperature” have been established. Series of new magnesium alloys with good ductility and corresponding alloy grades have been developed, where the impurity content of iron can be reduced to below 10 × 10-6; the elongation was more than 60% for ultrahigh plasticity magnesium alloys and is above 10% for the ultrahigh-strength magnesium alloys (UTS > 550 MPa). New plastic-processing technologies, such as asymmetric extrusion, asymmetric rolling, asymmetric modification, cyclical multipass upsetting and squeezing, expansion control large ratio forging, and extrusion and forging composite forming, have been developed. These newly developed magnesium alloys and processing technologies weaken the basal texture in wrought magnesium alloys, improving the formability of sheets, tubes, profiles, and forgings and their product quality and reducing their product cost. These technologies have been successfully applied in the processing of magnesium sheets, pipe profiles, and forgings.
镁合金塑性加工技术发展及应用
[J].我国是世界上镁资源最为丰富的国家,镁及镁合金具有质轻、比强度高、阻尼减振、电磁屏蔽性能优良、储能特性好等优点,是最有潜力的轻量化材料之一,其推广应用对节能减排和能源转型战略具有重要意义。但镁合金具有密排六方晶体结构,塑性变形能力较差。如何改善镁合金的塑性变形能力是扩大镁合金应用的瓶颈问题之一。本文综述了10多年来,世界各国在改善镁合金塑性、提升镁合金塑性变形能力等方面所做的大量工作,及在镁合金塑性加工技术等方面取得的重要进展。发展了“固溶强化增塑”新型镁合金设计理论和“熔体变温自纯化”等关键制备技术,形成了一批高塑性变形镁合金材料和牌号,其中杂质Fe含量可降到10 × 10<sup>-6</sup>以下,超高塑性镁合金延伸率可达到60%以上,超高强度(抗拉强度大于550 MPa)镁合金延伸率可以达到10%以上;开发了非对称挤压、非对称轧制、非对称改性、往复循环多道次镦挤开坯技术、扩收控制大比率锻造技术、挤锻复合成形技术等一批镁合金新型塑性加工技术。这些合金和技术使变形镁合金基面织构显著弱化,明显提高了变形镁合金板材、管型材和锻件的塑性成形能力和制品质量,产品成本大幅度降低,在板材、管型材和锻件制备加工中实现了成功应用。
Metallic anodes for next generation secondary batteries
[J].Li-air(O2) and Li-S batteries have gained much attention recently and most relevant research has aimed to improve the electrochemical performance of air(O2) or sulfur cathode materials. However, many technical problems associated with the Li metal anode have yet to be overcome. This review mainly focuses on the electrochemical behaviors and technical issues related to metallic Li anode materials as well as other metallic anode materials such as alkali (Na) and alkaline earth (Mg) metals, including Zn and Al when these metal anodes were employed for various types of secondary batteries.
AZ61 and AZ61-La alloys as anodes for Mg-air battery
[J].AZ61 and AZ61-0.5La (wt.%) alloys are prepared by the conventional casting method and then, AZ61 and AZ61-0.5La alloy sheets are obtained with multi-pass hot rolling. Microstructures and electrochemical properties of four alloys are investigated, and discharge properties are tested by an assembled Mg-air battery using alloy anode sheets with a dimension of 70x100x2.5mm. Results show that Al11La3 phases are formed in as-cast AZ61-0.5La alloy and promote the grain refinement and the formation of uniform microstructure in as-rolled AZ61-0.5La alloy. As-rolled AZ61-0.5La alloy exhibits a weak self-corrosion tendency but a strong discharge activity. After discharge test for 5h, the Mg-air battery based on the as-rolled AZ61-0.5La sheet can provide the maximum power output of 1.625W, and as-rolled AZ61-0.5La anode can supply the maximum energy density of 1417mWhg(-1). In addition, the relationship between microstructures and discharge properties is sufficiently described and discussed in this paper.
Discharge properties and electrochemical behaviors of AZ80-La-Gd magnesium anode for Mg-air battery
[J].
Performance of Mg-9Al-1In alloy as anodes for Mg-air batteries in 3.5 wt% NaCl solutions
[J].
Synergistic effect of second phase and grain size on electrochemical discharge performance of extruded Mg-9Al-xIn alloys as anodes for Mg-air battery
[J].
Discharge performance of Mg-Al-Pb-La anode for Mg-air battery
[J].
Discharge behaviour of Mg-Al-Pb and Mg-Al-Pb-In alloys as anodes for Mg-air battery
[J].
Electrochemical discharge performance of the Mg-Al-Pb-Ce-Y alloy as the anode for Mg-air batteries
[J].
Effect of rolling-induced microstructure on corrosion behaviour of an as-extruded Mg-5Li-1Al alloy sheet
[J].
Discharge performance of the magnesium anodes with different phase constitutions for Mg-air batteries
[J].
Composition and microstructure dependent corrosion behaviour of Mg-Li alloys
[J].
The influence of heat treatment on discharge and electrochemical properties of Mg-Gd-Zn magnesium anode with long period stacking ordered structure for Mg-air battery
[J].
Effect of microalloyed Ca on the microstructure and corrosion behavior of extruded Mg alloy AZ31
[J].
Effect of Ca addition on the corrosion behavior of Mg-Al-Mn alloy
[J].
Effect of extrusion on corrosion properties of Mg-2Ca-χAl (χ = 0, 2, 3, 5) alloys
[J].
Effect of Mn addition and refining process on Fe reduction of Mg-Mn alloys made from magnesium scrap
[J].
Mechanism of Mn on inhibiting Fe-caused magnesium corrosion
[J].In the present study, to understand the mechanism of Mn on inhibiting Fe-caused Mg corrosion, the corrosion behaviour of commercial pure Mg and Mg-6Mn alloy in 0.6 M NaCl solution is investigated. It is found that in Mg-6Mn alloy, Fe impurity is incorporated into Mn to form Mn (Fe) phase with Fe as solid solute. The initial galvanic corrosion cannot be reduced through converting Fe-rich phase to Mn (Fe) phase, since Mn (Fe) phase also has relatively strong cathodic activity and has much larger volume fraction than Fe-rich phase. However, the cathodic activation behaviour of pure Mg is inhibited. The cathodic activity even decreases for Mg-Mn alloy with increased exposure time, due to the reduced cathodic HER at the Mn (Fe) particles. Mn can be oxidized at the OCP of Mg-6Mn alloy, resulting in relatively dense Mn-rich corrosion film on particle surface, which separates the particle from the electrolyte and, consequently, inhibits HER. (C) 2020 Chongqing University. Publishing services provided by Elsevier B.V. on behalf of KeAi Communications Co. Ltd.
Effects of microstructure on the electrochemical discharge behavior of Mg-6wt%Al-1wt%Sn alloy as anode for Mg-air primary battery
[J].
Effect of heat treatment on microstructure and Al-water reactivity of Al-Mg-Ga-In-Sn alloys
[J].Al-Mg-Ga-In-Sn alloys with different Mg-contents were prepared and then subjected to solution and aging treatment. The microstructure and corrosion morphology after immersion in water of alloys was characterized by means of XRD and SEM with EDX. The Volta potential differences (ΔVPD) of interfacial phases with respect to Al matrix were measured using AFM/SKPFM. The Al-water reactivity of alloys in waters at different temperature were measured by using drainage method. The heat treatment influences the phase type, morphology of interfacial phases, and the content of Mg and Ga inside Al grains. As the Mg content is below 4% the heat-treated alloys contain interfacial phases of Mg2Sn, MgGa, MgGa2 andMgIn. Mg5Ga2 and Mg2Ga phases occurs as the Mg content of alloy is c.a. 5%. MgGa phase precipitates within Al grains of the aged alloys. The heat-treated alloys exhibit higher the Volta potential differences (ΔVPD) of interfacial phases with respect to Al in comparison with the cast ones. The generation rate and yield amount of hydrogen correlate with Mg contents of the heat-treated alloys. The reasons that the heat treatment affects the microstructures of alloys and the Volta potential differences (ΔVPD) of interfacial phases with respect to Al were analyzed, and the effect of heat treatment on the Al-water reactivity of alloys was also discussed.
热处理对Al-Mg-Ga-In-Sn合金微观结构和铝水反应的影响
[J].制备不同镁含量的Al-Mg-Ga-In-Sn合金并对其进行固溶和时效热处理,用XRD和SEM分析和观察了显微结构和腐蚀表面,用AFM/SKPFM测量了合金不同晶界相与铝晶粒间的电势差,用排水法测量了在不同水温下合金的铝水反应。结果表明,热处理改变了合金低熔点界面相的种类、形态以及合金晶粒内Mg和Ga含量。热处理态Mg含量低于4%的合金,其中有Mg<sub>2</sub>Sn、MgGa、MgGa<sub>2</sub>、MgIn界面相;在Mg含量为5%的热处理态合金中出现了Mg<sub>5</sub>Ga<sub>2</sub>、Mg<sub>2</sub>Ga相。在时效态合金晶粒内有MgGa相析出。与相同成分的铸态合金相比,时效态合金中各晶界相与铝基体间的电位差较大。热处理态合金的产氢速率和产氢率,与合金的Mg含量有关。分析了热处理使合金显微结构和晶界相与铝基体间电位差变化的原因,并讨论了热处理对合金铝水反应的影响。
Microstructures and mechanical properties of Mg-13Gd-1Zn alloy
[J].A ternary alloy with composition of Mg-13Gd-1Zn (%, mass fraction) was prepared by conventional smelting and casting technique. The microstructure and mechanical properties of the as-cast, as-annealed, as-extruded and as-aged (T5) alloy were investigated. The results show that the microstructure of the as-cast alloy consists of α-Mg matrix, (Mg, Zn)3Gd eutectic and a 14H long period staking ordered (14H-LPSO) phase. The significant increase of 14H-LPSO phase after annealing and ageing (T5) treatment in the alloy microstructure indicates that the precipitation of the 14H-LPSO phase occurs in a wide temperature range (200~510oC). The β' and β1 precipitates have also been observed in the alloy after ageing (T5) treatment. Under the combined action of precipitation strengthening and LPSO strengthening, the tensile strength, yield strength and elongation of the alloy are 397 MPa, 197 MPa and 2.56%, respectively. The creep properties of the Mg-13Gd-1Zn alloy are higher than those of the WE54 alloy in the two experimental conditions of 200oC/80 MPa and 200oC/120 MPa.
Mg-13Gd-1Zn合金的组织与力学性能
[J].
Effects of Al and Sn on microstructure, corrosion behavior and electrochemical performance of Mg-Al-based anodes for magnesium-air batteries
[J].
Hypoeutectic Mg-Zn binary alloys as anode materials for magnesium-air batteries
[J].
Approaches to construct high-performance Mg-air batteries: from mechanism to materials design
[J].
Electrochemical properties and discharge performance of Mg-3Sn-xCa alloy as a novel anode for Mg-air battery
[J].
Discharge properties and electrochemical behaviors of Mg-Zn-xSr magnesium anodes for Mg-Air batteries
[J].
Effect of crystallographic orientation on the discharge and corrosion behaviour of AP65 magnesium alloy anodes
[J].
Influence of chloride ion concentration on the electrochemical corrosion behaviour of plasma electrolytic oxidation coated AM50 magnesium alloy
[J].
Microstructure and corrosion behavior of as-extruded Mg-xLi-3Al-2Zn-0.2Zr alloys (x = 5, 8, 11 wt.%)
[J].
Improved corrosion behavior of AZ31 alloy through ECAP processing
[J].
The semi-continuous (Mg,Al)2Ca second phase on Mg-Al-Ca-Mn alloys as an efficient anti-corrosion anode for Mg-air batteries
[J].
Magnesium-air batteries: from principle to application
[J].
Advanced Mg-based materials for energy storage: fundamental, progresses, challenges and perspectives
[J].