常见的QSE聚合物基质,有聚氧化乙烯(PEO)[12]、聚偏氟乙烯(PVDF)[13]、聚丙烯腈(PAN)[14]等。这些QSE聚合物基质的电解液不易泄漏,电化学性能也较为优异。聚偏氟乙烯-六氟丙烯(PVDF-HFP)具有优异的离子传导性和较低的结晶度,是QSE的理想聚合物基质[15]。提高QSE性能最简单的方法,是在其中加入无机填料以提高其离子电导率、机械强度和界面稳定性[16~18]。石墨相氮化碳(g-C3N4)具有类似石墨的层状结构和较高的稳定性,还富含氮元素。g-C3N4的富电子特性使其具有Lewis碱性质,有助于吸附Li+并提供额外的活性位点。同时,g-C3N4三嗪环结构中的空位作为Li+迁移的潜在通道可提高离子电导率[19~21]。g-C3N4材料还具有良好的化学稳定性和热稳定性,能抵抗电解质腐蚀和高温等恶劣环境影响。将g-C3N4引入聚合物电解质,可显著降低其结晶度和提高综合性能[22]。Song等将石墨相氮化碳(g-C3N4)作为电解质添加剂,用于提高锂离子电池石墨负极的充电性能和循环稳定性[23]。Han等制备了g-C3N4-LiI-PEO SPE,g-C3N4和LiI使其离子电导率提高和改善与Li电极的界面相容性[24]。Huang等发现,将g-C3N4引入Li金属中可将Li金属/石榴石型SSE界面从点接触转变为紧密接触,还能抑制锂枝晶的生成[25]。Hu等验证了g-C3N4与Li盐中阴离子之间的强相互作用。这种强相互作用使其tLi+迁移数较高[26]。但是,g-C3N4的机械强度和界面稳定性较低[27]。PVDF-HFP准固态电解质具有良好机械性能和离子传导性,但是其结晶度还有待优化[2]。引入无机填料虽然能降低结晶度,但是难以构建连续离子传输通道和不能储存液态电解质[28]。g-C3N4的层状结构和丰富的活性位点,能为离子传输提供更多的通道且增强其相互作用使离子传导效率显著提高。同时,其较高的化学稳定性可提高电解质抗恶劣环境能力和延长其使用寿命[21]。这表明,将g-C3N4与PVDF-HFP结合,既能提高PVDF-HFP的结晶度又能克服g-C3N4的不足,可用于构建高性能锂离子电池电解质体系。
本文用纳米片自组装制备介孔球状g-C3N4多孔中空微球,将其与PVDF-HFP复合构建三维互穿网络结构[29]。将不同比例的介孔球状g-C3N4掺入PVDF-HFP基质制备准固体电解质PHCN-X系列,研究其性能和组装的锂电池的性能。
1 实验方法
1.1 介孔球状g-C3N4 的合成
将三聚氰胺1.9977 g,溶于80 mL的二甲基亚砜(DMSO)中,将相等摩尔数的三聚氰酸(2.0446 g)溶于40 mL的二甲基亚砜中。将这两种溶液在60 ℃搅拌使其均匀溶解后充分混合,再在60 ℃搅拌10 min得到混合液,然后静置30 min冷却至室温。将混合液离心分离后将产物用乙醇充分洗涤,然后置于50 ℃的真空干燥箱中干燥10 h得到白色粉末。将白色粉末放入已置于管式炉的陶瓷坩埚中,在高纯氩气氛中以2.3 ℃/min的速率升温至550 ℃,烧结4 h后得到棕黄色的粉末样品,即介孔球状g-C3N4。
1.2 PHCN准固态电解质的制备
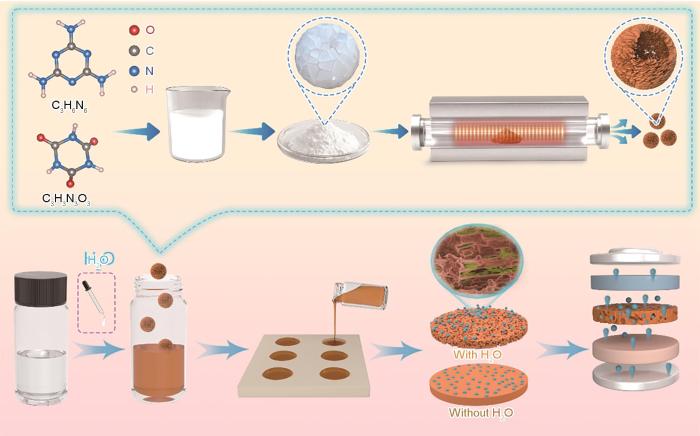
用溶液浇铸法制备PVDF-HFP基QSE,如图1所示。在室温下向5 mL丙酮溶剂中加入500 mg的PVDF-HFP,在50 ℃搅拌2 h;在得到的溶液中加入250 μL去离子水(电阻率为18.2 MΩ·cm),继续搅拌1 h;在PVDF-HFP溶液中加入不同质量分数(1%、2%、3%、4%)的介孔球状g-C3N4颗粒制成电解质膜PHCN-X,分别为“PHCN-1、PHCN-2、PHCN-3、PHCN-4”。随后在50 ℃连续磁力搅拌8 h形成均匀溶液,冷却至室温后将其倒入75 mm直径的玻璃培养皿中,在通风橱中自然蒸发6 h得到厚度约为200 μm的固态聚合物膜。将电解质膜转移到100 ℃真空干燥箱中干燥6 h。将膜切割成直径为16 mm的圆片,将其在手套箱中用1 mol/L LiPF6电解液浸泡2 h得到PHCN QSE。将样品保存在手套箱中。
图1
图1
PHCN QSE的制造工艺示意图
Fig.1
Schematic diagram of the fabrication process for PHCN QSE
1.3 电极的制备和电池的组装
以LiFePO4为正极、PHCN QSE为电解质、锂金属为负极,组装准固态硬币半电池。将LiFePO4正极,LiFePO4、PVDF和Super P以8∶1∶1的质量比分散在NMP溶剂中。充分研磨后将浆料均匀地涂覆到铝箔上,然后在120 ℃真空干燥12 h,制备出质量密度约为1.5 mg·cm-2的正极片。将正极片切割,得到直径为12 mm的电极。
1.4 性能表征
利用场发射扫描电子显微镜(FE-SEM)分析材料的表面形貌和纳米结构。结合能量色散光谱(EDS)进行元素分析。使用氮吸附分析仪进行氮吸附/解吸测试,以分析g-C3N4的比表面积和孔径分布。X射线衍射仪(XRD)用于测试g-C3N4的XRD谱,2θ的范围为5°~80°。使用Fourier变换红外光谱(FTIR)分析50~2000 cm-1范围内的分子结构和化学键信息。用同步热分析仪(TG-DSC)评估PHCN电解质膜的热稳定性,温度范围为30~600 ℃,加热速率为10 ℃·min-1。
使用CHI760E电化学工作站测量PHCN-X的离子电导率(σ)、电化学稳定性窗口和Li+迁移数(tLi+)。用电化学阻抗光谱法(EIS)在0.1~106 Hz频率范围内测定基于SS/PHCN-X-LiPF6/SS阻塞电池(SS为不锈钢片)的离子电导率(S·cm-1)
其中
根据
计算ZSPH QSE的激活能Ea。其中Ea代表热活化能,单位是eV;A:指前因子,单位同电导率,反映离子传导最大可能速率相关系数。T代表绝对温度(K);R:理想气体常数,值为8.314 J/(mol·K)。
采用线性扫描伏安法(LSV)测定了PHCN-X在不对称电池(Li/PHCN-X-LiPF6/SS)中对金属锂的电化学稳定性,扫描速率为10 mV·s-1,扫描频率范围为0~5.5 V。PHCN-X的tLi+值
是使用对称电池(Li/PHCN-X-LiPF6/Li)结合直流极化和交流阻抗测量的。式中I0为初始电流,ISS为稳态电流,R0为极化前的界面阻抗,RSS为极化后的界面阻抗,
其中W1为PHCN-X渗透到电解质膜中之前的膜质量,W2为渗透之后的质量。使用称量纸从薄膜表面清除任何多余的电解质。使用LANHE CT2001A充放电系统,在室温下在各种电流密度(0.2 mA·cm-2)下进行2 h锂对称电池的充放电循环,评价PHCN-X对锂金属的循环稳定性。以LiFePO4为正极材料,以金属锂为负极材料,使用LANHE CT2001A充放电系统测试以PHM9为电解液的锂金属全电池的循环性能和比容量特性。测试离子电导率、离子迁移数、电化学阻抗图和电池性能,测试三组,选择一组误差不大于± 5%数据。
2 结果和讨论
先制备三聚氰胺-三聚氰酸超分子聚集体,然后将其热缩聚合成介孔纳米球状g-C3N4,再引入多级通道PVDF-HFP 中。图1表明,在高纯氩气氛中将先已生成的三聚氰胺-三聚氰酸超分子聚集体热聚合。热缩聚时,三聚氰胺和三聚氰酸前驱体以二维的方式结合组装成纳米片,再组装成三维球状结构的g-C3N4多孔微球。以PVDF-HFP为基体,以介孔球状g-C3N4为填料,制备出QSE复合材料。将不同浓度(1%~4%)的g-C3N4多孔微球掺入PVDF-HFP的多级通道中,再将其油浴搅拌使溶剂蒸发后真空干燥,得到PHCN-X。
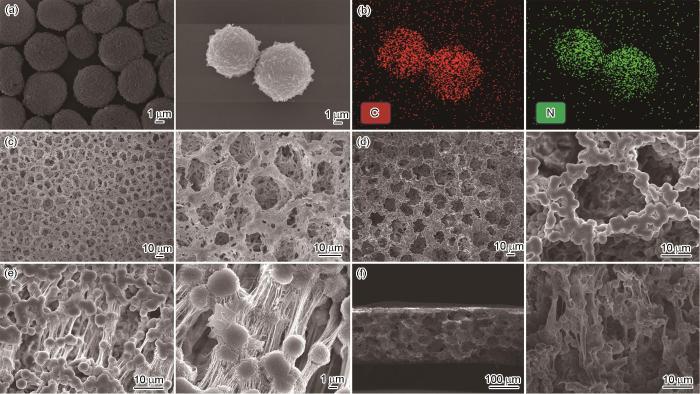
图2
图2
介孔球状g-C3N4的SEM图像和EDS能谱、多通道PVDF-HFP的SEM图像、PHCN-3的SEM图像、拉丝状结构以及横截面SEM图像
Fig.2
SEM images (a) and EDS spectrum (b) of mesoporous spherical g-C3N4, SEM image of multi-channel PVDF-HFP (c), SEM images of PHCN-3 (d) showing the fibrillated structure (e) and cross-sectional view (f)
将所得混合凝胶浇铸到玻璃培养皿中,干燥后形成厚度约为200 μm的淡黄色、平坦光滑的膜。图2d给出了PHCN-3的微观结构。可以看出,引入介孔球状g-C3N4后PHCN-3保持了原有的蜂窝状多孔结构,介孔中的球状g-C3N4微米颗粒均匀分布在PHCN膜的表面和孔洞结构内。介孔球状g-C3N4微米颗粒的引入破坏了聚合物的结晶,从而提高了PHCN QSE的离子电导率[30]。独特的球状结构和丰富的活性位点,为锂离子传输提供通道和使其更好的相互作用,从而显著提高离子传导效率。从图2e可见,PHCN中还有“拉丝”结构。形成这种“拉丝”结构的原因,可能是在混合过程中介孔球状g-C3N4与PVDF-HFP发生了特殊的相互作用。将介孔球状g-C3N4微米颗粒引入PVDF-HFP时,g-C3N4 表面的活性位点与PVDF-HFP的聚合物链段产生了物理吸附或者化学结合。在干燥过程中,随着溶剂的挥发这种相互作用使聚合物链的局部发生拉伸和取向而形成了类似“拉丝”的结构。同时,介孔球状g-C3N4 的引入破坏了PVDF-HFP原来的结晶结构,使聚合物链的排列更加无序。在干燥收缩过程中无序的链段相互纠缠和拉伸而形成了“拉丝”结构的。这种“拉丝”结构增大了电解质内部的表面积,为锂离子提供了更多的吸附位点,有助于锂离子在电解质中的存储和传输。其次,这些“拉丝”也是一种特殊的离子传输通道,其相互交织形成一种连续的网络,促进了离子在电解质中的扩散而使离子电导率提高。同时,这种结构在一定程度上还提高了电解质的机械性能,使电解质在电池充放电过程中能更好地承受电极体积变化产生的应力,提高了结构的稳定性和减少了电极与电解质之间的界面副反应。从图2f中PHCN-3的横截面SEM图像可见,PHCN-3内部依然是多孔和“拉丝”结构,内部的介孔球状g-C3N4 类似一串串“冰糖葫芦”。这些g-C3N4 球体的高比表面积和介孔结构提供了丰富的离子活性位点,并将锂离子迁移路径从长程扩散转变为g-C3N4球体表面的短程跳跃传输。
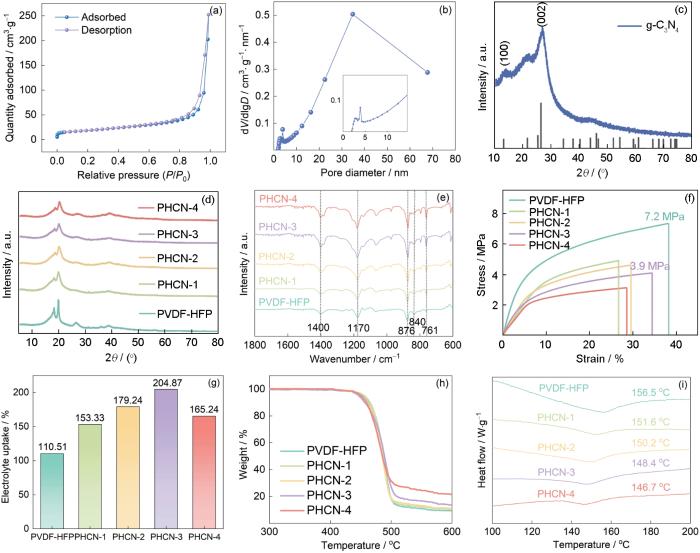
图3
图3
介孔球状g-C3N4的N2吸附脱附曲线和孔径分布、介孔球状g-C3N4的XRD谱、PHCN-X和PVDF-HFP的XRD谱、FTIR光谱、应力-应变曲线、吸液率、热重分析以及DSC分析
Fig.3
N2 adsorption-desorption isotherm (a) and pore size distribution (b) of mesoporous spherical g-C3N4, XRD pattern of mesoporous spherical g-C3N4 (c), XRD patterns (d), FTIR spectra (e), stress-strain curves (f), liquid electrolyte uptake (g), TG analysis (h), and DSC analysis (i) of PHCN-X and PVDF-HFP
图3d给出了PHCN-X和PVDF-HFP的XRD谱,谱中18.5°、20.2°、26.6°和38.8°处出现了PVDF-HFP的衍射峰,对应PVDF-HFP的(020)、(110)、(022)和(041)晶面。对比PHCN-1和PVDF-HFP的XRD谱表明,引入介孔球状g-C3N4后PHCN QSE的强衍射峰强度降低,弱衍射峰消失。这表明,介孔球状g-C3N4的引入使PHCN QSE的结晶度降低,非晶区增大。结晶度的降低促进了聚合物链段的迁移,并在一定程度上增强了Li+的传输[29]。随着介孔球状g-C3N4含量的提高,PHCN QSE中介孔球状g-C3N4 的衍射峰强度逐渐提高。根据FTIR对介孔球状g-C3N4与PVDF-HFP之间的特征官能团谱带及其相互作用进行了深入研究。图3e中位于761 cm-1和1402 cm-1处的特征峰可归属于PVDF-HFP的α相,位于876 cm-1和840 cm-1处的峰则表明在PVDF-HFP中存在β相。随着介孔球状g-C3N4含量的提高PHCN-X的FTIR光谱特征峰没有显著的变化,β相的特征峰逐渐加强,PVDF-HFP中β相的存在加速了锂盐的解离并调节了Li+ 通量的分布[34]。
图3f表明,随着介孔球状g-C3N4颗粒的引入PVDF-HFP的应力降低,表明PVDF-HFP的结晶度降低。同时,PHCN-3的应力为3.9 MPa,适当的应力变化范围确保其为准固态电解质提供了机械支撑能力。PHCN-3的机械坚固性可阻止锂枝晶的形成和生长,能更好地适应电镀/剥离过程中的体积变化和减轻内部短路引起安全问题。
电解质膜的表面润湿性决定于化学组成与微观结构的协同作用。PVDF-HFP含高电负性氟官能团,具有疏水性和低表面能特性。复合介孔球状g-C3N4构建的PHCN准固态电解质,其多孔结构与g-C3N4中空结构协同提高了表面粗糙度和增加了有效接触面积,使表面润湿性和表面能显著提高。多孔纳米填料固定了部分液态电解质,增强了复合聚合物电解质与电解液界面的接触。同时,纳米多孔填料促进Lewis酸-阴离子相互作用显著强化了Li+传输[35]。将PHCN电解质膜浸渍于LiPF6电解液2 h,根据其质量变化计算吸液率。如图3g所示,引入g-C3N4使吸液率提高到110.51%~204.87%。其中PHCN-3的吸液率达到峰值,而PHCN-4的吸液率因填料过量堵塞离子传输通道而降低。
如图3h所示,PHCN准固态电解质在100~450 ℃温度区间其热稳定性较高,几乎没有质量损失。直至450~500 ℃温度区间才出现显著的质量损失,表明PVDF-HFP有机聚合物发生了降解。PHCN中介孔球状g-C3N4比例的提高使剩余质量增加,而纯 PVDF-HFP的质量损失较高。这一结果表明,介孔球状g-C3N4的热稳定性良好。玻璃化转变温度(Tg),表征聚合物的链段运动[36]。在聚合物电解质体系中Li+的传输靠非晶相链段之间的运动。非晶相链段的占比越高,Tg则越低,有利于Li+的高效输运。如图3i所示,随着介孔球状g-C3N4含量的提高Tg值降低。与PVDF-HFP相比,PHCN-3的Tg值更低,只有148.4 ℃。结晶度较低的电解质,其Tg也较低。这表明,介孔球状g-C3N4的引入有利于降低PHCN准固态电解质的结晶度。同时,Tg的降低能提高Li+的室温迁移率,有利于提高离子电导率。PHCN-3的热稳定性优异和Tg值较低。
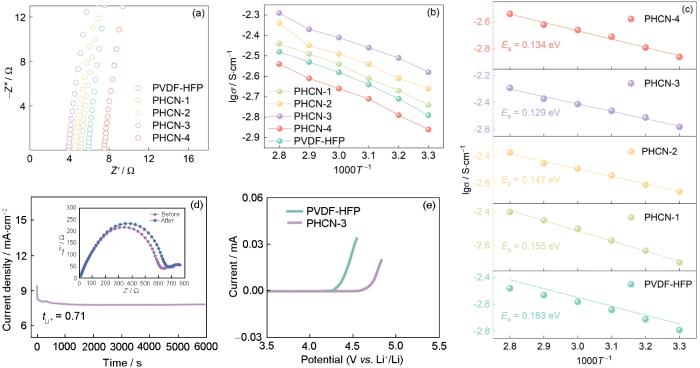
图4
图4
温度为30 ℃时Nyquist图、阿伦尼乌斯曲线、活化能曲线、PHCN-3在30 ℃极化前后的电池极化曲线和阻抗图、PHCN-3和PVDF-HFP准固态电解质在30 ℃、扫描速率为10 mV·s-1的LSV曲线
Fig.4
Nyquist plots at 30 oC (a), Arrhenius plots (b), activation energy plots (c), chromoamperometry curve and corresponding Nyquist plots before and after polarization for PHCN-3 at 30 oC (d), LSV curves of PHCN-3 and PVDF-HFP quasi-solid electrolytes at 30 oC with a scan rate of 10 mV·s-1 (e)
较高的tLi+值有利于使Li+高速且均匀的沉积,进而抑制锂枝晶的生成[38]。根据
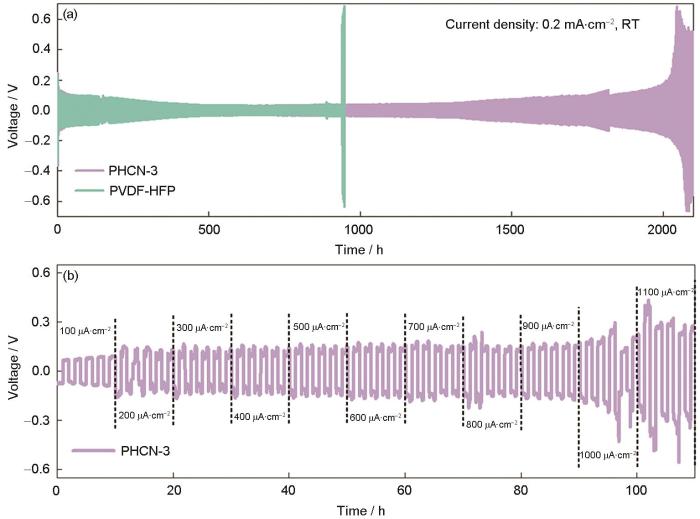
图5a给出了温度为30 ℃、电流密度为0.2 mA·cm-2条件下Li/PVDF-HFP-LiPF6/Li和Li/PHCN-3-LiPF6/Li对称电池的循环性能。可以看出,Li/PHCN-3-LiPF6/Li对称电池在极低的极化电压(约35 mV)下超过2000 h的优异循环稳定性,在整个电镀/剥离循环过程中过电位没有显著升高。与其相比,基于PVDF-HFP的锂对称电池在约940 h后极化电压突然升高,因为锂电极电场不均使锂枝晶生长。锂枝晶刺穿隔膜使局部短路或电阻增大导致恒流条件下电压升高。
图5
图5
用PVDF-HFP和PHCN-3组装的锂对称电池的恒电流循环曲线以及PHCN-3的临界电流密度曲线
Fig.5
Galvanostatic cycling profiles of Li symmetric cells assembled with PVDF-HFP and PHCN-3 (a), and critical current density (CCD) curve for PHCN-3 (b)
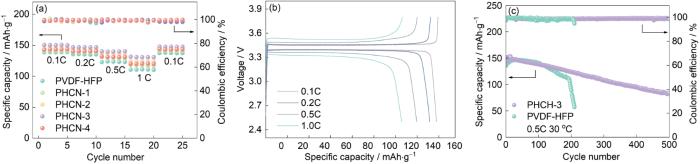
使用PHCN基准固态电解质构建的LiFePO4/Li金属电池(LiFePO4/PHCN-LiPF6/Li)在2.5~3.8 V电压窗口其电化学性能优异。恒流充放电测试结果表明,引入介孔球状g-C3N4填料使电解质体系的倍率性能显著提高。图6a表明,PVDF-HFP基电解质在0.1 C倍率下放电比容量为140.4 mAh·g-1,但是在1C倍率下极化效应加剧使其容量衰减到110.5 mAh·g-1(容量保持率78.6%)。与其相比,优化后的PHCN-3电解质的性能更优异:在0.1C倍率和初始电流密度下放电容量达150.1 mAh·g-1。随着倍率提高到0.2 C、0.5 C和1 C,其容量依次保持为146.9、139.8和130.8 mAh·g-1。特别是经历多级倍率循环后恢复到0.1C的倍率仍维持147.5 mAh·g-1的容量,对应的容量保持率为98.2% (图6a,b)。
图6
图6
LiFePO4/PHCN-3-LiPF6/Li电池的倍率性能和在不同循环速率下的首次充放电曲线、PHCN-3和PVDF-HFP在0.5C和30 ℃下的循环稳定性
Fig.6
Rate performance (a) and initial charge-discharge curves at various C-rates (b) for the LiFePO4/PHCN-3-LiPF6/Li cell, Cycling stability of PHCN-3 and PVDF-HFP at 0.5C and 30 oC (c)
在PHCN QSE体系中,循环稳定性和Coulomb效率是评价锂金属电池(LMB)的重要指标[40,41]。在30 ℃、电压窗口范围为2.5~3.8 V和倍率为0.5C的条件下测试使用PHCN-3电解质的LiFePO4/Li电池的循环稳定性,结果在图6c中给出。可以看出,其首圈放电容量为155.9 mAh·g-1 (Coulomb效率为98.22%),200次循环后Coulomb效率为98.7%,容量保持率为88.33%。与其对比,PVDF-HFP基电池的初始容量为145 mAh·g-1,但是200次循环后容量急剧衰减。其原因是,PVDF-HFP体系中锂枝晶的持续生长和堆积使电极空间和活性物质的面积减少。PHCN-3优异的电化学性能,可归因于其多孔拉丝结构。PVDF-HFP和g-C3N4材料组分和结构之间的协同效应构建了多级“驿站”式离子传输通道,保障了Li+的高速迁移,还使锂金属均匀沉积而抑制了电解质界面的副反应。
图7给出了对LiFePO4/PHCN-3-LiPF6/Li电池循环500圈后锂片表面的SEM照片。可以看出,PHCN-3准固态电解质能抑制锂枝晶生长。在锂片表面没有尖锐、粗壮且无序生长的锂枝晶。
图7
图7
LiFePO4/PHCN-3-LiPF6/Li电池循环500圈后锂片的表面形貌
Fig.7
Surface morphologies of the lithium metal anode after 500 cycles in the LiFePO4/PHCN-3-LiPF6/Li cell
3 结论
(1) PHCN QSE电解质中掺入介孔球状g-C3N4,可形成“驿站”式离子传输通道。这种独特的结构可为组装的电池供给充足的电荷载体,用以搭建的高效Li+传输路径可实现锂离子的高效传输(tLi+ = 0.71)。
(2) PVDF-HFP基质使PHCN QSE具有优异的机械强度和储存性能。介孔球状g-C3N4填料的引入大幅度提高了QSE的电化学稳定性,将其电化学窗口拓宽至约4.6 V。这种填料可提高离子电导率(30 ℃时达2.62 × 10-3 S·cm-1)和抑制锂枝晶的生长。在电流密度为0.2 mA·cm-2和约35 mV的低极化电压条件下,PHCN-3 QSE锂对称电池具有优异的稳定性。LiFePO4/PHCN-3-LiPF6/Li电池还具有较高的容量保持率。
参考文献
Advancements and challenges in organic-inorganic composite solid electrolytes for all-solid-state lithium batteries
[J].To address the limitations of contemporary lithium-ion batteries, particularly their low energy density and safety concerns, all-solid-state lithium batteries equipped with solid-state electrolytes have been identified as an up-and-coming alternative. Among the various SEs, organic–inorganic composite solid electrolytes (OICSEs) that combine the advantages of both polymer and inorganic materials demonstrate promising potential for large-scale applications. However, OICSEs still face many challenges in practical applications, such as low ionic conductivity and poor interfacial stability, which severely limit their applications. This review provides a comprehensive overview of recent research advancements in OICSEs. Specifically, the influence of inorganic fillers on the main functional parameters of OICSEs, including ionic conductivity, Li+ transfer number, mechanical strength, electrochemical stability, electronic conductivity, and thermal stability are systematically discussed. The lithium-ion conduction mechanism of OICSE is thoroughly analyzed and concluded from the microscopic perspective. Besides, the classic inorganic filler types, including both inert and active fillers, are categorized with special emphasis on the relationship between inorganic filler structure design and the electrochemical performance of OICSEs. Finally, the advanced characterization techniques relevant to OICSEs are summarized, and the challenges and perspectives on the future development of OICSEs are also highlighted for constructing superior ASSLBs.
Challenges in speeding up solid-state battery development
[J].
Enabling high-performance multivalent metal-ion batteries: current advances and future prospects
[J].This review discusses challenges and innovations in multivalent metal-ion batteries, focusing on materials, electrolytes, separators, advanced characterization as well as role of machine learning for high performance, stability, and scalability.
Lithium battery chemistries enabled by solid-state electrolytes
[J].
Polyfluorinated crosslinker-based solid polymer electrolytes for long-cycling 4.5 V lithium metal batteries
[J].\n Solid polymer electrolytes (SPEs), which are favorable to form intimate interfacial contacts with electrodes, are promising electrolyte of choice for long-cycling lithium metal batteries (LMBs). However, typical SPEs with easily oxidized oxygen-bearing polar groups exhibit narrow electrochemical stability window (ESW), making it impractical to increase specific capacity and energy density of SPE based LMBs with charging cut-off voltage of 4.5 V or higher. Here, we apply a polyfluorinated crosslinker to enhance oxidation resistance of SPEs. The crosslinked network facilitates transmission of the inductive electron-withdrawing effect of polyfluorinated segments. As a result, polyfluorinated crosslinked SPE exhibits a wide ESW, and the Li|SPE|LiNi\n 0.5\n Co\n 0.2\n Mn\n 0.3\n O\n 2\n cell with a cutoff voltage of 4.5 V delivers a high discharge specific capacity of ~164.19 mAh g\n −1\n at 0.5 C and capacity retention of ~90% after 200 cycles. This work opens a direction in developing SPEs for long-cycling high-voltage LMBs by using polyfluorinated crosslinking strategy.\n
Dendritic solid polymer electrolytes: a new paradigm for high-performance lithium-based batteries
[J].
Designing solid-state electrolytes for safe, energy-dense batteries
[J].
A review of composite solid-state electrolytes for lithium batteries: fundamentals, key materials and advanced structures
[J].All-solid-state lithium ion batteries (ASSLBs) are considered next-generation devices for energy storage due to their advantages in safety and potentially high energy density. As the key component in ASSLBs, solid-state electrolytes (SSEs) with non-flammability and good adaptability to lithium metal anodes have attracted extensive attention in recent years. Among the current SSEs, composite solid-state electrolytes (CSSEs) with multiple phases have greater flexibility to customize and combine the advantages of single-phase electrolytes, which have been widely investigated recently and regarded as promising candidates for commercial ASSLBs. Based on existing investigations, herein, we present a comprehensive overview of the recent developments in CSSEs. Initially, we introduce the historical development from solid-state ionic conductors to CSSEs, and then summarize the fundamentals including mechanisms of lithium ion transport, key evaluation parameters, design principles, and key materials. Four main types of advanced structures for CSSEs are classified and highlighted according to the recent progress. Moreover, advanced characterization and computational simulation techniques including machine learning are reviewed for the first time, and the main challenges and perspectives of CSSEs are also provided for their future development.
Current status and future directions of multivalent metal-ion batteries
[J].
Application of advanced Wide-Temperature range and flame retardant "Leaf-Vein" Structured functionality composite Quasi-Solid-State electrolyte
[J].
Opportunities of flexible and portable electrochemical devices for energy storage: expanding the spotlight onto semi-solid/solid electrolytes
[J].The ever-increasing demand for flexible and portable electronics has stimulated research and development in building advanced electrochemical energy devices which are lightweight, ultrathin, small in size, bendable, foldable, knittable, wearable, and/or stretchable. In such flexible and portable devices, semi-solid/solid electrolytes besides anodes and cathodes are the necessary components determining the energy/power performances. By serving as the ion transport channels, such semi-solid/solid electrolytes may be beneficial to resolving the issues of leakage, electrode corrosion, and metal electrode dendrite growth. In this paper, the fundamentals of semi-solid/solid electrolytes (e.g., chemical composition, ionic conductivity, electrochemical window, mechanical strength, thermal stability, and other attractive features), the electrode-electrolyte interfacial properties, and their relationships with the performance of various energy devices (e.g., supercapacitors, secondary ion batteries, metal-sulfur batteries, and metal-air batteries) are comprehensively reviewed in terms of materials synthesis and/or characterization, functional mechanisms, and device assembling for performance validation. The most recent advancements in improving the performance of electrochemical energy devices are summarized with focuses on analyzing the existing technical challenges (e.g., solid electrolyte interphase formation, metal electrode dendrite growth, polysulfide shuttle issue, electrolyte instability in half-open battery structure) and the strategies for overcoming these challenges through modification of semi-solid/solid electrolyte materials. Several possible directions for future research and development are proposed for going beyond existing technological bottlenecks and achieving desirable flexible and portable electrochemical energy devices to fulfill their practical applications. It is expected that this review may provide the readers with a comprehensive cross-technology understanding of the semi-solid/solid electrolytes for facilitating their current and future researches on the flexible and portable electrochemical energy devices.
Strong Lewis-acid coordinated PEO electrolyte achieves 4.8 V-class all-solid-state batteries over 580 Wh kg-1
[J].
Oxygenated carbon nitride-based high-energy-density lithium-metal batteries
[J].
In situ polymerization derived from PAN-based porous membrane realizing double-stabilized interface and high ionic conductivity for lithium-metal batteries
[J].
Filler-integrated composite polymer electrolyte for solid-state lithium batteries
[J].
Tailoring practically accessible polymer/inorganic composite electrolytes for all-solid-state lithium metal batteries: a review
[J].
Composite solid electrolytes for all-solid-state lithium batteries
[J].
Recent progress of composite solid polymer electrolytes for all-solid-state lithium metal batteries
[J].
Preparation and electrochemical study of PVDF-HFP/LATP/g-C3N4 composite polymer electrolyte membrane
[J].
g-C3N4 nanosheets enhanced solid polymer electrolytes with excellent electrochemical performance, mechanical properties, and thermal stability
[J].
2-Dimensional g-C3N4 nanosheets modified LATP-based “Polymer-in-Ceramic” electrolyte for solid-state lithium batteries
[J].
Emerging chemical functionalization of g-C3N4: covalent/noncovalent modifications and applications
[J].
Graphitic carbon nitride (g-C3N4) as an electrolyte additive boosts fast-charging and stable cycling of graphite anodes for Li-ion batteries
[J].
Exploiting Iodine redox chemistry for achieving high-capacity and durable PEO-based all-solid-state LiFePO4/Li batteries
[J].
Graphitic carbon nitride (g-C3N4): an interface enabler for solid-state lithium metal batteries
[J].
Unlocking solid-state conversion batteries reinforced by hierarchical microsphere stacked polymer electrolyte
[J].Pursuing all-solid-state lithium metal batteries with dual upgrading of safety and energy density is of great significance. However, searching compatible solid electrolyte and reversible conversion cathode is still a big challenge. The phase transformation at cathode and Li deformation at anode would usually deactivate the electrode-electrolyte interfaces. Herein, we propose an all-solid-state Li-FeF conversion battery reinforced by hierarchical microsphere stacked polymer electrolyte for the first time. This g-CN stuffed polyethylene oxide (PEO)-based electrolyte is lightweight due to the absence of metal element doping, and it enables the spatial confinement and dissolution suppression of conversion products at soft cathode-polymer interface, as well as Li dendrite inhibition at filler-reinforced anode-polymer interface. Two-dimensional (2D)-nanosheet-built porous g-CN as three-dimensional (3D) textured filler can strongly cross-link with PEO matrix and LiTFSI (TFSI: bistrifluoromethanesulfonimide) anion, leading to a more conductive and salt-dissociated interface and therefore improved conductivity (2.5 × 10 S/cm at 60 °C) and Li transference number (0.69). The compact stacking of highly regular robust microspheres in polymer electrolyte enables a successful stabilization and smoothening of Li metal with ultra-long plating/striping cycling for at least 10,000 h. The corresponding Li/LiFePO solid cells can endure an extremely high rate of 12 C. All-solid-state Li/FeF cells show highly stabilized capacity as high as 300 mAh/g even after 200 cycles and of ~200 mAh/g at extremely high rate of 5 C, as well as ultra-long cycling for at least 1200 cycles at 1 C. High pseudocapacitance contribution (>55%) and diffusion coefficient (as high as 10 cm/s) are responsible for this high-rate fluoride conversion. This result provides a promising solution to conversion-type Li metal batteries of high energy and safety beyond Li-S batteries, which are difficult to realize true "all-solid-state" due to the indispensable step of polysulfide solid-liquid conversion.Copyright © 2020 Science China Press. Published by Elsevier B.V. All rights reserved.
Graphitic carbon nitride as a catalyst support in fuel cells and electrolyzers
[J].
Extraordinary ionic conductivity excited by hierarchical ion-transport pathways in MOF-based quasi-solid electrolytes
[J].
Recent progress on metal-organic framework/polymer composite electrolytes for solid-state lithium metal batteries: ion transport regulation and interface engineering
[J].This review provides an overview of different strategies to improve the ion transport of MOF/polymer composite electrolytes and stabilize the electrode/electrolyte interface.
The critical role of fillers in composite polymer electrolytes for lithium battery
[J].With excellent energy densities and highly safe performance, solid-state lithium batteries (SSLBs) have been hailed as promising energy storage devices. Solid-state electrolyte is the core component of SSLBs and plays an essential role in the safety and electrochemical performance of the cells. Composite polymer electrolytes (CPEs) are considered as one of the most promising candidates among all solid-state electrolytes due to their excellent comprehensive performance. In this review, we briefly introduce the components of CPEs, such as the polymer matrix and the species of fillers, as well as the integration of fillers in the polymers. In particular, we focus on the two major obstacles that affect the development of CPEs: the low ionic conductivity of the electrolyte and high interfacial impedance. We provide insight into the factors influencing ionic conductivity, in terms of macroscopic and microscopic aspects, including the aggregated structure of the polymer, ion migration rate and carrier concentration. In addition, we also discuss the electrode-electrolyte interface and summarize methods for improving this interface. It is expected that this review will provide feasible solutions for modifying CPEs through further understanding of the ion conduction mechanism in CPEs and for improving the compatibility of the electrode-electrolyte interface.© 2023. The Author(s).
Single-ion Li+, Na+, and Mg2+ solid electrolytes supported by a mesoporous anionic Cu-azolate metal-organic framework
[J].
Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: from photochemistry to multipurpose catalysis to sustainable chemistry
[J].
From melamine-cyanuric acid supramolecular aggregates to carbon nitride hollow spheres
[J].
High-performance dual-salt plastic crystal electrolyte enabled by succinonitrile-regulated porous polymer host
[J].
Upgrading traditional organic electrolytes toward future lithium metal batteries: a hierarchical nano-sio2-supported gel polymer electrolyte
[J].
Recent advances in solid polymer electrolytes for lithium batteries
[J].
Solid polymer electrolytes with high conductivity and transference number of Li ions for Li-based rechargeable batteries
[J].
Artificial Interphases for highly stable lithium metal anode
[J].
Critical interphase overpotential as a lithium dendrite-suppression criterion for all-solid-state lithium battery design
[J].
Pathways for practical high-energy long-cycling lithium metal batteries
[J].State-of-the-art lithium (Li)-ion batteries are approaching their specific energy limits yet are challenged by the ever-increasing demand of today's energy storage and power applications, especially for electric vehicles. Li metal is considered an ultimate anode material for future high-energy rechargeable batteries when combined with existing or emerging high-capacity cathode materials. However, much current research focuses on the battery materials level, and there have been very few accounts of cell design principles. Here we discuss crucial conditions needed to achieve a specific energy higher than 350 Wh kg(-1), up to 500 Wh kg(-1), for rechargeable Li metal batteries using high-nickel-content lithium nickel manganese cobalt oxides as cathode materials. We also provide an analysis of key factors such as cathode loading, electrolyte amount and Li foil thickness that impact the cell-level cycle life. Furthermore, we identify several important strategies to reduce electrolyte-Li reaction, protect Li surfaces and stabilize anode architectures for long-cycling high-specific-energy cells.