目前,钢铁行业节能降碳的主要路径,是以清洁能源代替煤炭。氢气是最理想的清洁能源,受到了极大地关注[13~15]。氢基直接还原法炼铁可使钢铁行业低碳甚至零碳排放,最可能替代高炉炼铁[16~18]。氢基直接还原炼铁,其还原气氛有富氢和全氢。富氢直接还原炼铁是用天然气、焦炉煤气等还原铁矿石,但未能使二氧化碳的排放降到最低。而全氢直接还原炼铁,使用的气源是绿色氢气。在氢气还原赤铁矿的过程中还原产物为铁和水[19],可从源头上降低碳排放量。当前的氢基直接还原炼铁工艺,有流化床法和竖炉法。虽然流化床法无需对原料进行造块处理,但其成本及维护费用较高,且在生产中流化床反应器所需的气流量比理论上大得多,导致还原气体的利用率较低。因此,全氢竖炉直接还原炼铁,是钢铁行业实现低碳发展的未来技术选择。
全氢直接还原技术能节能减排,受到了广泛的关注。但是这种方法的还原气体利用率低和气固反应效率低。全氢还原具有较强的吸热效应,其还原反应效率受工艺参数和竖炉内气体流速等多种因素的影响。在低能耗的情况下提高氢气的还原效率是该工艺的难题,急需深入研究氢基直接还原炼铁动力学。
温度对提高赤铁矿在氢气中的还原效率有重要影响。Lu等[11]用热重分析(TGA)方法研究了不同浓度H2在600~900 ℃对磁铁矿粉末的等温还原过程,发现在一定的氢气浓度下还原速率与温度正相关,Fe3O4→FeO反应受形核或界面反应控制;FeO→Fe反应受形核和扩散混合控制。Bahgat和Khedr[20]研究了磁铁矿单晶在900~1100 ℃纯H2气氛中的还原动力学和微观结构演化,发现初期的还原速率最高,后期因致密铁层的形成而下降。同时还发现,随着温度的升高还原时间急剧减少。Man等[21]研究了球团矿的还原动力学,发现还原速率随温度的升高而提高,在900 ℃以下球团的还原由扩散控制,在1100 ℃转变为混合控制。
1 实验方法
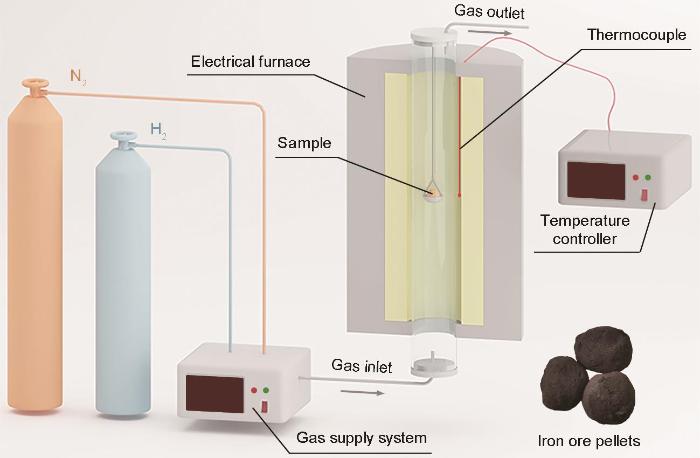
实验用材料是球径为8 mm的赤铁矿球团。实验前将球团矿样品称重。将球团矿在不同温度的纯氢气氛中恒温还原10 min,实验温度为600~900 ℃,实验流程图如图1所示。将球团矿置于炉内,抽真空后通入氮气,按预定程序将反应腔体加热到实验温度并保温20 min,随后通入氢气对球团矿进行还原,氢气的流量为0.5 L/min。还原10 min后关闭氢气阀门和加热开关,持续通入大流量氮气使球团矿冷却。实验结束后取出样品并称重,之后将样品保存在干燥皿中。
图1
用扫描电子显微镜(SEM)表征球团矿还原10 min后的截面微观形貌并使用配套的能谱分析仪(EDS)分析其元素分布。用EBSD表征在900 ℃还原10 min的球团矿的微观组织结构和晶体取向,并分析样品的界面特征和物相组成。
2 实验结果
2.1 温度对还原热力学和动力学的影响
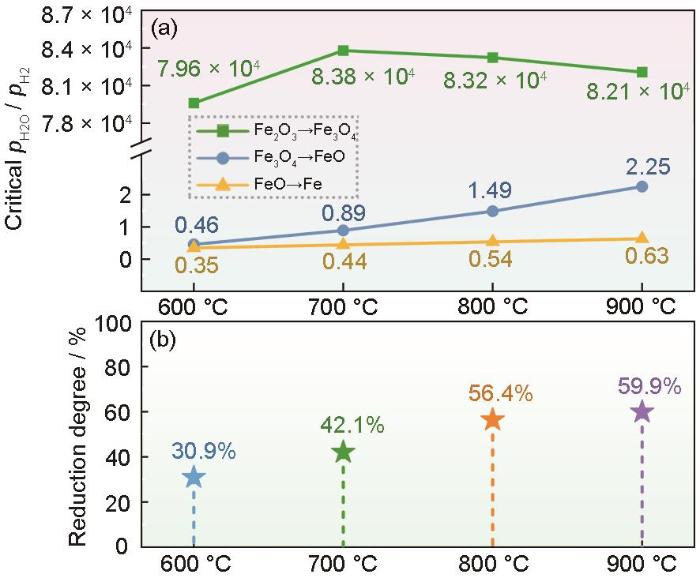
为了揭示温度对球团矿还原效率的影响,分别研究了在不同温度下赤铁矿(Fe2O3)还原的热力学和动力学。温度高于570 ℃时Fe2O3的还原反应为Fe2O3→Fe3O4→FeO→Fe[22]。根据不同温度下三种反应的平衡常数和对应的平衡氧分压,计算出反应平衡时的水分压与氢气分压的比值(pH2O/pH2)。从图2a可见,在相同的温度下Fe2O3→Fe3O4反应的临界pH2O/pH2比值远大于其余两个反应,前者平衡时的氢分压很低而水分压极高。这表明,在热力学上Fe2O3→Fe3O4的还原反应更易发生,球团矿在该反应中对还原剂H2的扩散迁至及对反应产物H2O的扩散迁离的需求远不及还原反应Fe3O4→FeO和FeO→Fe。因此,关于氢基直接还原的氢气逃逸,主要影响后两步反应,特别是所需临界pH2O/pH2比最低的FeO→Fe反应。
图2
图2
在不同温度下铁氧化物发生还原反应的临界水/氢分压比和在氢气中还原10 min球团矿的还原程度
Fig.2
Critical ratio of water partial pressure to hydrogen partial pressure for the reduction reaction of different iron oxides (a) and the reduction degree of iron ore pellet after 10 min reaction in hydrogen (b) at different temperatures
由于Fe3O4→FeO→Fe的还原反应驱动力远小于Fe2O3→Fe3O4,研究工艺参数对炼铁反应的影响时重点关注这两步反应。由图2a还可见,随着温度的升高,尽管Fe2O3→Fe3O4所需的临界pH2O/pH2比先增后降,但是Fe3O4→FeO和FeO→Fe所需的临界pH2O/pH2比都逐渐增大,表明升温可促进Fe3O4向FeO转变以及FeO向Fe的转变。需要注意的是,对于热力学上最难发生的FeO→Fe反应,其临界pH2O/pH2比随温度的变化并不明显。因此,在热力学上升温可提高球团矿的还原效率,但是对FeO→Fe这一难反应的加速作用较为有限。
图2b反映了球团矿在不同温度下还原10 min后的还原程度。可以看出,随着温度的升高球团矿的还原程度显著提高。在动力学上,升温可提高球团矿的还原效率。但是,当温度由800 ℃升到900 ℃时还原程度的提高变得较为缓慢,只提高了3.5%。其原因可能是,在900 ℃反应10 min后球团矿的大量区域落到了FeO→Fe这一热力学难反应阶段。另外,还有动力学上的阻滞因素,例如氧化物周围生成了致密的还原Fe层等。
2.2 温度对微观结构演化的影响
图3
图3
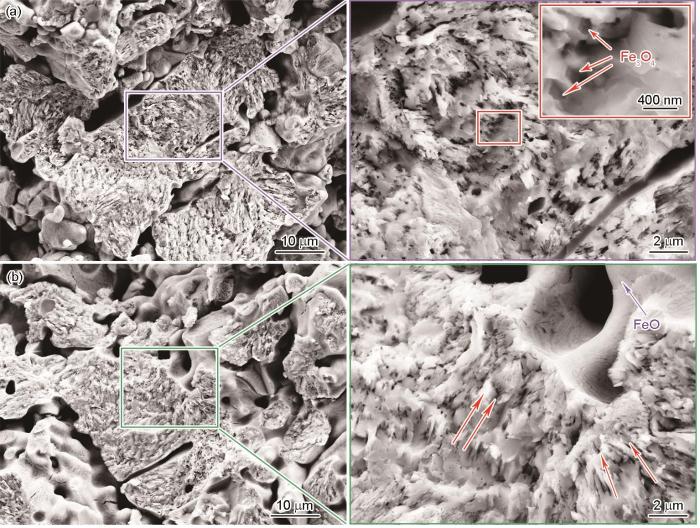
球团矿在600 ℃纯氢气氛中还原10 min的截面形貌
Fig.3
Cross-sectional morphologies of pellet ore reduced in hydrogen atmosphere at 600 oC for 10 min (a) center region, (b) edge region
图4
图4
球团矿在700 ℃纯氢气氛中还原10 min的截面形貌
Fig.4
Cross-sectional morphologies of pellet ore reduced in hydrogen atmosphere at 700 oC for 10 min (a) center region, (b) edge region
图5
图5
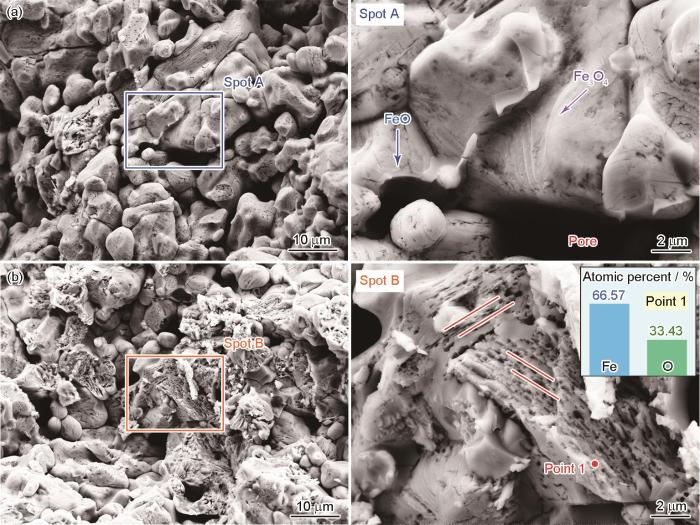
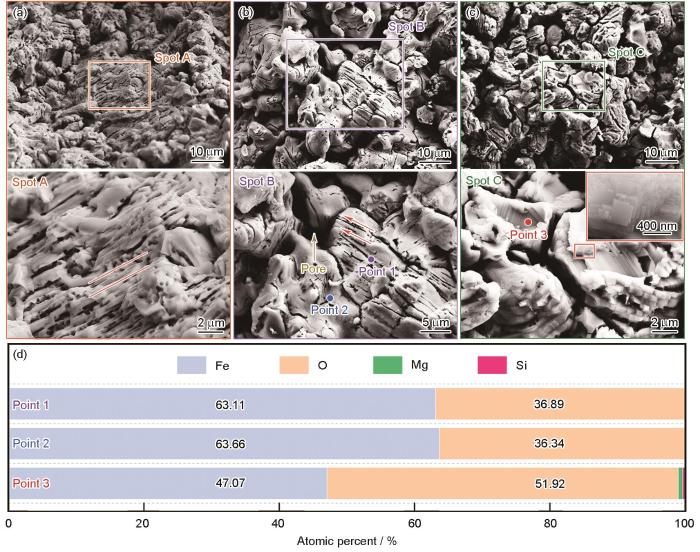
球团矿在800 ℃纯氢气氛中还原10 min的截面形貌和EDS结果
Fig.5
Cross-sectional morphologies and EDS results of pellet ore reduced in hydrogen atmosphere at 800 oC for 10 min (a) center region, (b-c) edge region, (d) EDS analytics
图6
图6
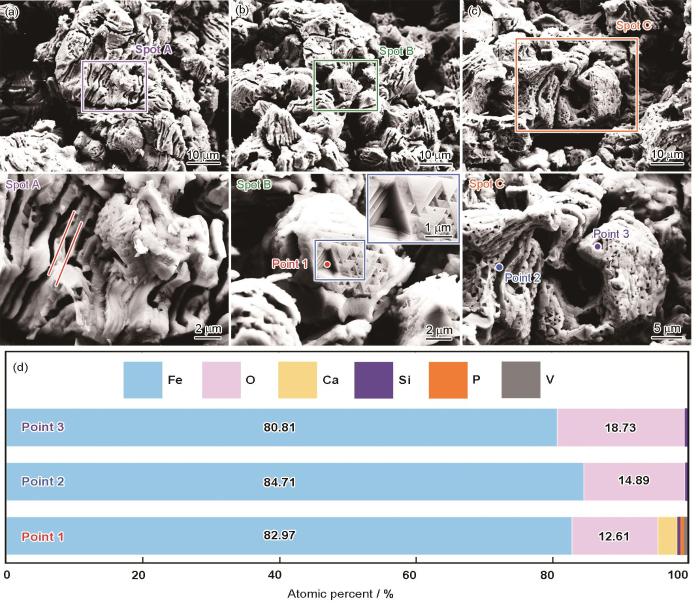
球团矿在900 ℃纯氢气氛中还原10 min的截面形貌和EDS结果
Fig.6
Cross-sectional morphologies and EDS results of pellet ore reduced in hydrogen atmosphere at 900 oC for 10 min (a) center region, (b-c) edge region, (d) EDS analytics
图7
图7
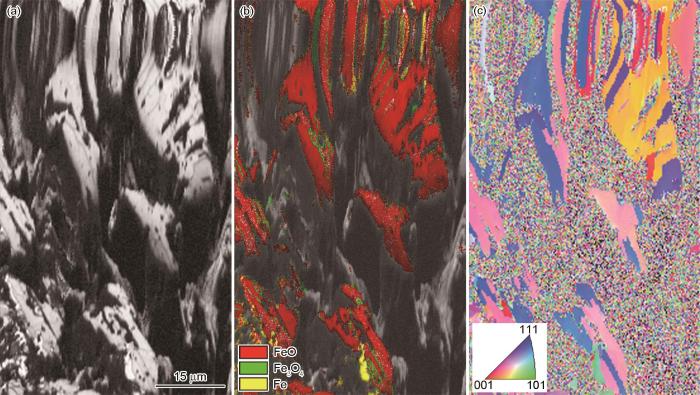
球团矿中心区域在900 ℃纯氢气氛中还原10 min的EBSD图
Fig.7
EBSD plot of pellet ore reduced in hydrogen atmosphere at 900 oC for 10 min (a) microscopic morphology, (b) physical phase distribution, (c) IPF map
3 讨论
根据热力学,在相同温度Fe2O3→Fe3O4反应对还原剂H2的扩散迁至以及反应产物H2O的扩散迁离的需求较低,因此在该阶段还原反应更容易发生。相比之下,Fe3O4→FeO和FeO→Fe两个还原对H2和H2O的扩散需求较高,因此为了提高赤铁矿还原效率,应更加关注后两步反应。随着温度的升高Fe3O4→FeO和FeO→Fe反应所需的临界pH2O/pH2比都逐渐增大,但是FeO→Fe反应的临界pH2O/pH2比的增幅较小。这表明,根据热力学,升温可增大Fe3O4→FeO和FeO→Fe两个反应的还原驱动力,但是温度对FeO→Fe这一反应的加速作用较为有限。
在还原反应过程中观察到球团矿中的细长孔隙有特定的生长取向,且随着温度的升高更加明显。这种孔隙结构的变化,与物质的扩散迁移相关。在微观上,在球团矿的还原过程中H2分子扩散到铁氧化物反应界面与O原子反应生成H2O,水分子迁移离开界面使界面O原子的浓度降低[28]。因此,在浓度梯度的作用下O原子继续向反应界面迁移,使还原反应持续进行。大孔洞附近的还原速率较高而需要补充大量O原子,O原子的大规模迁移使还原的铁氧化物颗粒内出现空穴。空穴逐渐生长为孔洞并受扩散的影响呈现生长取向[29]。因此,在球团矿部分区域可观察到细长孔隙。随着还原反应的进行和温度的提高,开放孔隙的数量增加且孔隙尺寸变大。大量的孔隙缩短了反应的扩散传质路径,加速了气固相的扩散传质,进而使还原速率提高。这也是大孔洞附近的氧化物优先还原的重要原因。
随着温度由800 ℃上升到900 ℃,球团矿孔隙率大幅度提高,但是其还原速率并没有显著提高。在800~900 ℃球团矿的大部分区域处于FeO→Fe的还原反应阶段,虽然升温可促进FeO向Fe的转变,但是其作用较为有限。这种孔隙结构的变化可能与Fe的形核和生长相关。图6给出的结果表明,在900 ℃球团矿孔隙附近出现了Fe的形核和生长,其生长方式与800 ℃时截然不同。在还原反应的初始阶段,Fe核垂直于FeO表面(z方向)生长和沿其表面(x和y方向)生长[29]。球团矿孔隙附近的还原速率较高,Fe优先形核和生长[30]。在800 ℃球团矿中生成的Fe核呈四面体形状。随着还原反应的进行,Fe核沿着垂直方向以四面体形状生长。在平行方向也生成了大量Fe核,在球团矿内多个区域生成了四面体层状晶体(图5c)。Fe核相互碰撞而合并,形成的铁层覆盖在FeO表面[29]。在900 ℃,Fe颗粒的生长呈现出三角形状。随着还原的进行第二层颗粒在第一层颗粒上继续成核生长,其表现是从球团矿表层区域向中心区域不断堆叠生长,最终孔隙形状变化为三角形层状孔隙。三角形孔隙的出现加快了Fe的生成,外层生长较快的Fe形成的孔隙其尺寸小于内层,使三角形层状孔隙展现出阶梯型。同时,在900 ℃球团矿内可能生成了γ-Fe [31, 32]。FeO还原成Fe的过程,涉及到Fe-O键的断裂和Fe-Fe键的形成。α-Fe为体心立方结构,在较低温度下更稳定;γ-Fe为面心立方结构,在较高温度下更为稳定。面心立方结构比体心立方结构排列更密集和键合更为复杂,从而使FeO转变为γ-Fe需要克服更高的能垒。这种热力学上的阻滞因素,也影响了900 ℃时球团矿的还原反应速率。因此,由800 ℃升到900 ℃还原程度的提高变得较为缓慢。
4 结论
(1) 在热力学上Fe2O3→Fe3O4的还原反应更易发生,球团矿在该阶段对还原剂H2的扩散迁至及对反应产物H2O的扩散迁离的需求远不及Fe3O4→FeO和FeO→Fe的还原反应。随着温度的升高Fe2O3→Fe3O4的还原驱动力先增后降,而Fe3O4→FeO和FeO→Fe的还原驱动力逐渐增大,进而使球团矿的还原程度提高,但当温度由800 ℃升到900 ℃时还原程度的提高放缓。
(2) 在氢还原过程中,球团矿内沿着一定方向出现细长的孔隙,且随着温度的升高这种趋势更加明显,与O原子的扩散迁移相关;随着温度的升高球团矿内开放孔隙的数量增加且尺寸变大。大量的开孔使球团矿的还原速率提高。
(3) 在不同温度下Fe的形核生长呈现出不同的形态,使球团孔隙率和孔隙形状发生变化。在800 ℃ Fe是四面体层状晶体,在900 ℃ Fe的成核生长表现为三角形状,并由球团矿表层向中心区域堆叠生长,使球团矿内产生三角形层状孔隙。
参考文献
A comprehensive random pore model kinetic study of hematite to iron reduction by hydrogen
[J].
Iron and steel industry emissions: a global analysis of trends and drivers
[J].
Review of influencing factors on the reduction disintegration performance of iron ore oxidized pellets
[J].
铁矿氧化球团低温还原粉化性能的影响因素评述
[J].
Towards green steel-energy and CO2 assessment of low carbon steelmaking via hydrogen based shaft furnace direct reduction process
[J].
Toward a fossil free future with HYBRIT: development of iron and steelmaking technology in Sweden and Finland
[J].The Swedish and Finnish steel industry has a world-leading position in terms of efficient blast furnace operations with low CO2 emissions. This is a result of a successful development work carried out in the 1980s at LKAB (Luossavaara-Kiirunavaara Aktiebolag, mining company) and SSAB (steel company) followed by the closing of sinter plants and transition to 100% pellet operation at all of SSAB’s five blast furnaces. However, to further reduce CO2 emission in iron production, a new breakthrough technology is necessary. In 2016, SSAB teamed up with LKAB and Vattenfall AB (energy company) and launched a project aimed at investigating the feasibility of a hydrogen-based sponge iron production process with fossil-free electricity as the primary energy source: HYBRIT (Hydrogen Breakthrough Ironmaking Technology). A prefeasibility study was carried out in 2017, which concluded that the proposed process route is technically feasible and economically attractive for conditions in northern Sweden/Finland. A decision was made in February 2018 to build a pilot plant, and construction started in June 2018, with completion of the plant planned in summer 2020 followed by experimental campaigns the following years. Parallel with the pilot plant activities, a four-year research program was launched from the autumn of 2016 involving several research institutes and universities in Sweden to build knowledge and competence in several subject areas.
Hydrogen reduction of iron ore pellets: a surface study using ambient pressure X-ray photoelectron spectroscopy
[J].
Hydrogen direct reduction (H-DR) in steel industry—An overview of challenges and opportunities
[J].
Parametric study on hematite pellet direct reduction by hydrogen
[J].
Influence of hydrogen flow rate on multistep kinetics of hematite reduction
[J].
Greening steel industry by hydrogen: lessons learned for the developing world
[J].
Multistep kinetic study of magnetite reduction by hydrogen based on thermogravimetric analysis
[J].
Decarbonisation and hydrogen integration of steel industries: recent development, challenges and technoeconomic analysis
[J].
Kinetics of hydrogen reduction of magnetite ore fines
[J].
Research progress on hydrogen-assisted fatigue crack growth of pipeline steels in hydrogen-blended natural gas environment
[J].
掺氢天然气环境下管线钢氢致疲劳裂纹扩展研究进展
[J].天然气掺氢输送是实现氢能长距离、低成本、大规模输运的重要途经,但管线因疲劳载荷作用可能发生氢致疲劳损伤,严重威胁掺氢天然气管线的服役安全。因此,本文首先介绍了管线钢氢致疲劳裂纹扩展的机理和模型,重点论述了微观组织结构及焊接、载荷和服役环境等因素对掺氢天然气环境下管线钢氢致疲劳裂纹扩展的影响,最后对该领域的未来研究方向提出了展望。
Research progress on hydrogen damage mechanism of pipeline steel in contact with hydrogen environment
[J].
临氢环境下管线钢氢损伤机理研究进展
[J].
Particle-resolved computational modeling of hydrogen-based direct reduction of iron ore pellets in a fixed bed. Part I: methodology and validation
[J].
Hydrogen-based direct reduction of combusted iron powder: deep pre-oxidation, reduction kinetics and microstructural analysis
[J].
Reaction kinetics of direct reduction of mineral iron carbonate with hydrogen: determination of the kinetic triplet
[J].
Kinetics analysis of direct reduction of iron ore by hydrogen in fluidized bed based on response surface methodology
[J].
Reduction kinetics, magnetic behavior and morphological changes during reduction of magnetite single crystal
[J].
Influence of temperature and time on reduction behavior in iron ore–coal composite pellets
[J].
Kinetics of reduction of iron oxides by H2: Part I: low temperature reduction of hematite
[J].
Effect of basicity on the structure characteristics of chromium-nickel bearing iron ore pellets
[J].High MgO content and the dehydroxylation of laterite ore particles lead to poor compressive strength of fired chromium-nickel bearing iron ore pellets at natural basicity. For improving the compressive strength of the fired chromium-nickel bearing iron ore pellets, burnt lime was applied as the additive to improve the pellet structure. Results showed that with an increase in quaternary basicity, the compressive strength of fired pellets was enhanced obviously. The optimum basicity of fired chromium-nickel bearing iron ore pellets is suggested at 1.15. Under the optimal test conditions, the compressive strength of fired pellets with natural basicity is only 607 N/pellet, but the compressive strength of fired pellets reaches 1991 N/pellet at basicity of 1.15. The structure of fired chromium-nickel pellets at 1.15 basicity greatly depends on the liquid bonding phases among chromite, hematite and enstatite particles, which are composed of kirschsteinite, SFCA and glass, and amount to 1927% in fired pellets. The liquid bonding phases fill in the voids among mineral particles and interconnect them to form the intertwined structure, which increases the shrinkage and significantly improves the compression strength of fired pellets. (C) 2018 Elsevier B.V.
Effect of reducing atmosphere on the direct reduction of iron oxides pellets
[J].
Hydrogen direct reduction and reoxidation behaviour of high-grade pellets
[J].
Gas–solid reduction behavior of in-flight fine hematite ore particles by hydrogen
[J].
Experimental study and numerical simulation on porosity dependent direct reducibility of high-grade iron oxide pellets in hydrogen
[J].
Influence of microstructure and atomic-scale chemistry on the direct reduction of iron ore with hydrogen at 700 °C
[J].
Morphological changes during reduction of magnetite compacts
[J].
Investigation of iron oxide reduction by TEM
[J].
Influence of original structure on the kinetics and mechanisms of carbon monoxide reduction of hematite compacts
[J].