本文用溶剂热法制备一种Z型异质结结构NH2-UiO-66/BiOBr复合催化剂,研究其在可见光照射下对OFX的光催化降解性能、可重复使用性能和稳定性,并揭示其光催化机理。
1 实验方法
1.1 实验用试剂
氯化锆、2-氨基对苯二甲酸、溴化钾、五水合硝酸铋、N,N-二甲基甲酰胺、乙二醇、异丙醇、L-组氨酸、碘化钾、对苯醌、氢氧化钠、盐酸、氯化钠、无水硫酸钠、磷酸二氢钾、硝酸钠以及无水乙醇,均为分析纯。
1.2 光催化剂的制备
用溶剂热法[23]合成NH2-UiO-66。将1.0 mmol的四氯化锆(ZrCl4)和1.0 mmol的2-氨基对苯二甲酸(BDC)分散到20 mL N,N-二甲基甲酰胺(DMF)中,磁力搅拌30 min使其充分溶解。然后将混合物倒入50 mL特氟龙内衬的不锈钢高压反应釜中,在120 ℃反应24 h。将所得沉淀物自然冷却并就那些离心分离,用DMF和乙醇将产物充分交替洗涤(3次)。最后,将所得产物在70 ℃真空干燥8 h,得到淡黄色NH2-UiO-66粉末样品。
将1.0 mmol的 Bi(NO3)3·5H2O溶于10 mL的乙二醇中,再加入不同质量(0.1524、0.3048、0.54572、0.6096 g)的NH2-UiO-66,将其磁力搅拌10 min完全溶解后得到混合溶液。将1.0 mmol的KBr溶解在5 mL的超纯水中,然后将其滴加到上述混合溶液中并持续剧烈搅拌,滴加结束后继续磁力搅拌30 min,然后将溶液转移至50 mL反应釜中在120 ℃高温反应5 h。自然冷却至室温后用去离子水和无水乙醇交替充分洗涤(3次)。离心分离后收集沉淀,将其在60 ℃真空干燥8 h,得到BiOBr白色粉末。将复合材料BiOBr白色粉末记为NUB-x,其中x (x = 0.5、1、1.5、2)表示NH2-UiO-66与BiOBr的质量比。单一BiOBr的制备方法与此类似[24,25],区别是滴加KBr前不加NH2-UiO-66。
1.3 催化剂的表征
分别用JSM-6710F型扫描电子显微镜(SEM)、FEI Tecnai G2 F20型透射电子显微镜(TEM)和能谱分析(EDS)表征催化剂的微观形貌和元素组成。使用Bruker D8型X射线衍射仪(XRD)测定催化剂的XRD谱。用ASAP 2020型N2吸附/解吸测定仪测定样品的比表面积、孔容和孔径分布。用EscaLab 250Xi型X射线光电子能谱仪(XPS)表征催化剂表面的化学组成和元素价态。测定Irprestige-21型傅里叶变换红外光谱(FT-IR)检测材料的官能团。使用配备积分球的UV-vis漫反射分光光度计(UV-Vis-DRS)表征催化剂的光吸收。使用CHI760E型电化学工作站测定催化剂样品的瞬态光电流响应和电化学阻抗谱(EIS),用F-7100荧光分光光度计测量样品的PL光谱。极限电子自旋共振(ESR)测出活性物种信号。
根据OFX的降解率评价催化剂的光催化性能。模拟太阳光的光源是500 W氙灯,将其置于圆形反应器中心对OFX进行光催化降解。实验时,将0.12 g催化剂和0.2 L的OFX水溶液(20 mg/L)加入石英玻璃管中,然后将其放置在距离氙灯约5 cm处。先在黑暗中磁力搅拌30 min实现吸附-解吸平衡。然后在已预热10 min的氙灯照射下进行光催化反应,每间隔20 min从其中取出约5 mL悬浮液,离心后吸取4 mL上清液,用去离子水将其稀释2.5倍,一紫外-可见分光光度计在293 nm处测定OFX的吸光度。
图1给出了OFX溶液的标准曲线,线性回归后得到曲线方程为y = 0.06674x + 0.001742,根据标准曲线可计算出OFX的浓度。用抽滤法收集反应后的催化剂,用DMF和无水乙醇交替充分洗涤后在真空条件下烘干,然后进行下一轮循环实验,循环使用实验共进行5次。
图1
初始OFX浓度、OFX浓度和降解率分别为
式中A0为初始氧氟沙星吸光度,C0为初始氧氟沙星浓度(mg/L),At为反应后氧氟沙星吸光度,Ct为反应后氧氟沙星浓度(mg/L),η为降解率,k为降解反应过程中的一级反应动力学常数。
为了评估NH2-UiO-66/BiOBr复合光催化剂材料的循环稳定性,将反应结束后的NUB-1静置后回收并用去DMF和无水乙醇充分清洗,在60 ℃烘干后进行循环实验(共进行5次)。
2 结果和讨论
2.1 催化剂的晶相结构
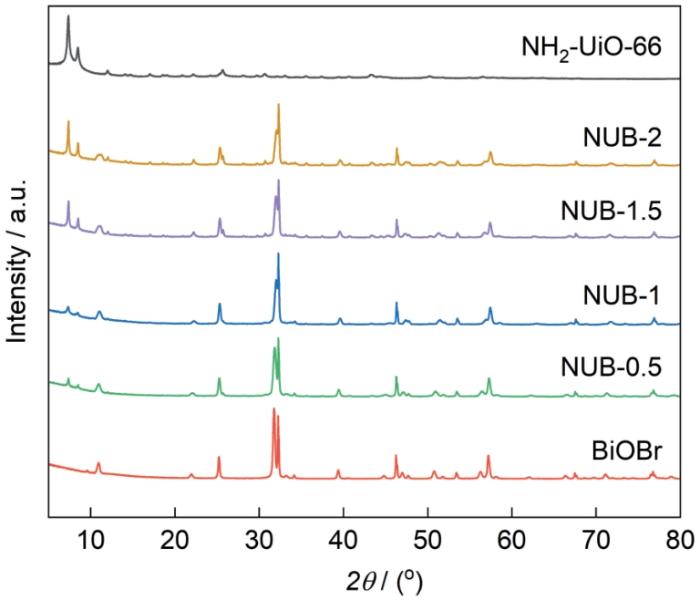
图2给出了催化剂样品的XRD谱。NH2-UiO-66的谱中7.35°、8.50°、25.67°处的特征衍射峰与文献[26, 27]的结果相同。在BiOBr的谱中25.23°、31.76°、32.25°、46.25°和57.18°处的衍射峰分别对应其(101)、(102)、(110)、(200)和(212)晶面[28]。NH2-UiO-66/BiOBr催化剂的谱中保留了NH2-UiO-66、BiOBr各组分的所有衍射峰,表明催化剂的结构完整。与纯BiOBr的谱相比,在NH2-UiO-66/BiOBr谱中31.76°处的(102)面BiOBr峰强降低,而在32.25°处的(110)面BiOBr峰强提高。其原因可能是NH2-UiO-66中的氨基(-NH2)官能团与BiOBr中的Bi3⁺发生配位作用形成了稳定的结构[29],降低了BiOBr晶体高能晶面的表面能,使BiOBr的生长更倾向于沿(110)面定向生长,抑制了(102)面的暴露。这种表面调控了与晶体定向生长的协同效应,使BiOBr纳米片的排列更加规则形成较高晶面选择的结构。
图2
图2
NH2-UiO-66、BiOBr和不同NH2-UiO-66/BiOBr比例的复合催化剂的XRD谱
Fig.2
XRD patterns of NH2-UiO-66, BiOBr, and NH2-UiO-66/BiOBr composites with different ratios
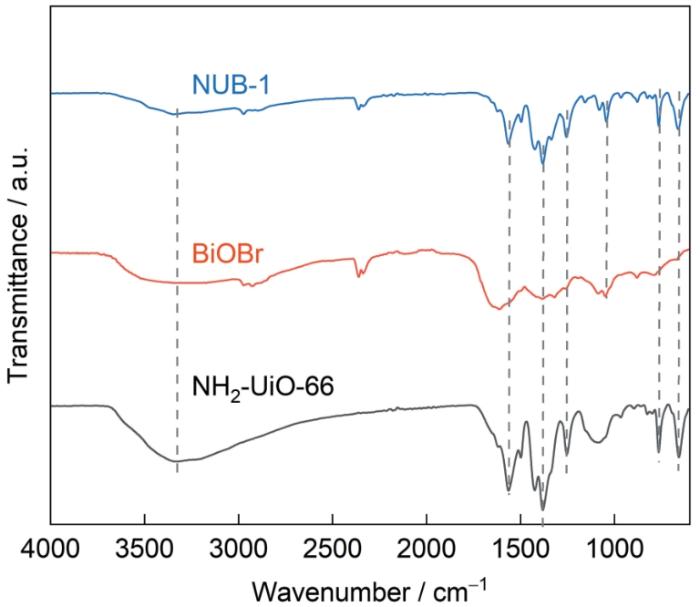
根据FT-IR分析研究了催化剂的化学键和官能团。在NH2-UiO-66的红外谱(图3)中658、766 cm-1处的吸收峰,属于Zr-O的伸缩振动;在1253 cm-1附近的峰对应C-N的伸缩振动,在1380和1564 cm-1处的峰对应C=O的振动峰,与羧基(-COOH)密切相关。在3326 cm-1附近的宽峰对应氨基基团(-NH2)的N-H伸缩振动[30]。这些峰的出现表明,NH2-UiO-66中的官能团和有机框架结构完好地保留在催化剂中。在BiOBr的谱中1049 cm-1处的峰,对应Br-O的伸缩振动[31]。在NUB-1的谱中保留了与NH2-UiO-66和BiOBr对应的特征峰且其没有明显的移位,表明在复合过程中这两种材料结合并形成了稳定的异质结。
图3
图3
NH2-UiO-66、BiOBr和NUB-1复合催化剂的FT-IR光谱
Fig.3
FT-IR spectra of NH2-UiO-66, BiOBr and NUB-1 composite
2.2 催化剂的形貌
图4a给出了NH2-UiO-66的SEM照片,可见均匀的八面体结构,粒径约为120 nm,晶体排列紧密且有序,表明这种催化剂的结晶性良好。这种有序的微观结构有助于光生载流子的传输和分离,从而提高光催化效率。图4b给出了BiOBr的SEM照片,可见一种由约100 nm的纳米片堆叠、交错聚集组成的疏松花簇状结构[32]。这些纳米片排列有序,边缘光滑清晰,形状和尺寸较不规则,厚度为10~20 nm。这种花簇状结构增大了催化剂的比表面积,从而提高了光的吸收和提供了更多的活性位点。颗粒间的空隙有助于反应物的扩散和产物的传输,促进光催化反应。图4c给出了NH2-UiO-66与BiOBr的复合情况。可以看出,催化剂中既存在NH2-UiO-66的多面体结构,也保留了BiOBr的片层结构,两种组分紧密结合。这种复合结构使光催化过程中催化剂能发挥两种材料的优势。NH2-UiO-66和BiOBr的紧密接触促进了界面电荷的转移和分离,抑制了光生电子和空穴的复合,从而提高了光催化效率。EDS结果表明,C、N、O、Zr、Bi、Br六种元素均匀分布。以上结果证实,成功制备出了NH2-UiO-66/BiOBr二元催化剂。
图4
图4
NH2-UiO-66, BiOBr和NUB-1的SEM照片以及NUB-1的EDS图
Fig.4
SEM image of NH2-UiO-66 (a), BiOBr (b) and NUB-1 (c), and EDS elemental mapping images of NUB-1 for C (d), N (e), O (f), Br (g), Zr (h), Bi (i)
图5
图5
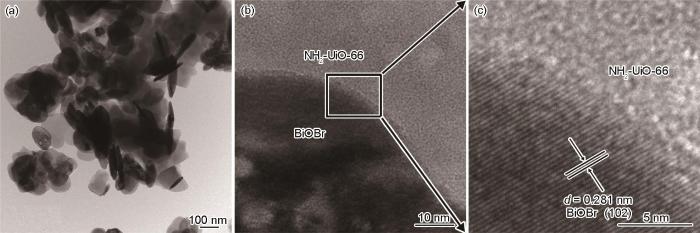
NUB-1的TEM照片
Fig.5
TEM images of NUB-1 (a) low magnification, (b) high magnification, (c) HRTEM image
2.3 催化剂的组成元素
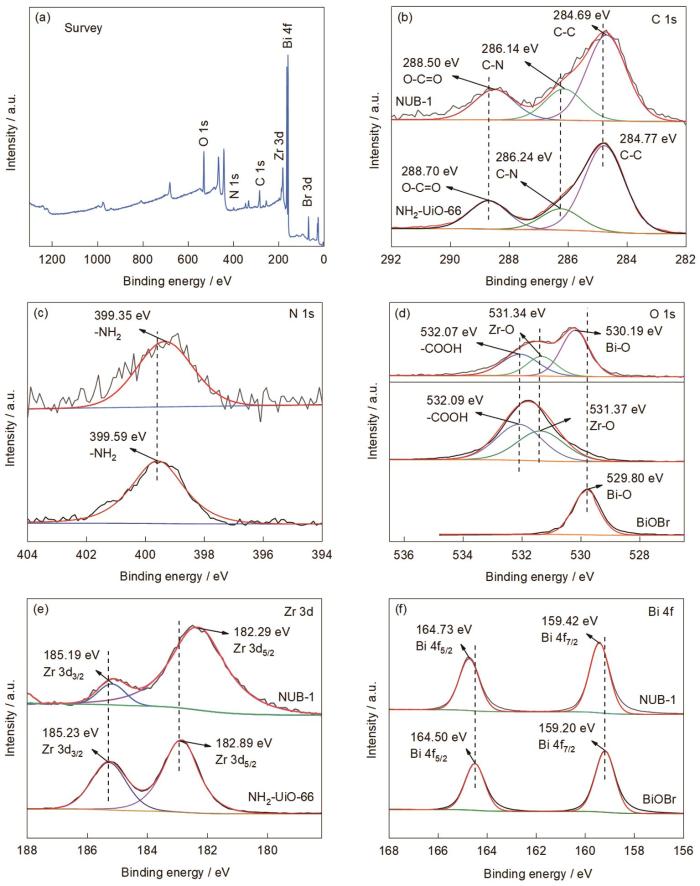
图6a给出了NUB-1的XPS全谱,包含C、O、N、Zr、Bi、Br 6种元素。这表明,在催化剂中成功引入了BiOBr和NH2-UiO-66。从图6b可见C 1s谱中3个典型的结合能峰,284.69 eV处的峰对应C-C,常见于有机分子骨架结构中。286.14 eV处的峰归属于C-N,表明NH2-UiO-66中的氨基(-NH2)官能团与碳结构有关。288.50 eV的峰归属于O-O=C,与NH2-UiO-66结构中的羧基(-COOH)有关。N 1s谱(图6c)中的峰位集中在399.35 eV,对应-NH2官能团。图6d给出了O 1s的峰,3个拟合峰的结合能分别为532.10、531.35和530.08 eV,对应-COOH、Zr-O、Bi-O。这表明,Zr基金属有机框架结构和BiOBr的结构存在于催化剂中[33]。在Zr 3d谱(图6e)中可见两个典型特征峰,185.19 eV处的特征峰对应Zr 3d3/2,182.29 eV处的特征峰对应Zr 3d5/2。在Bi 4f谱(图6f)中可见两个特征峰,分别位于164.73 eV (Bi 4f5/2)和159.42 eV (Bi 4f7/2),表明BiOBr中的铋以Bi3+的形式存在。
图6
图6
NUB-1的XPS全谱以及C 1s, N 1 s, O 1 s, Zr 3d, Bi 4f的高分辨XPS谱
Fig.6
XPS survey spectrum of NUB-1 (a) and high-resolution XPS spectra of C 1s (b), N 1s (c), O 1s (d), Zr 3d (e) and Bi 4f (f)
2.4 催化剂的比表面积和孔径
图7
图7
N2吸附-脱附等温线以及NH2-UiO-66、BiOBr和NUB-1的孔径分布
Fig.7
N2 adsorption-desorption isotherms (a) and pore size distribution of NH2-UiO-66 (b), BiOBr (c) and NUB-1 (d)
2.5 催化剂对光的吸收范围和带隙结构对催化剂的影响
根据UV-vis DRS的测试结果分析了催化剂的光吸收范围和带隙(Eg)。图8a给出了催化剂的紫外-可见吸收光谱。与单一的NH2-UiO-66相比,NUB-1的光吸收强度有所降低。其原因是,NH2-UiO-66与BiOBr之间的Z型异质结的构建促进了光生电荷的转移和分离,进而影响催化剂的性能。同时,催化剂的带隙结构变化以及粗糙度和光散射特性的改变可能影响其吸收特性。
图8
图8
UV-vis DRS图谱、Tauc图以及NH2-UiO-66和BiOBr的Motty-Schottky曲线
Fig.8
UV-Vis diffuse reflectance spectra (a), Tauc plots of different samples (b-d), and Mott-Schottky plots of NH2-UiO-66 (e) and BiOBr (f)
2.6 催化剂的电化学特性
催化剂的光电化学参数定性表征样品中光生载流子的迁移和分离效率[40]。瞬时光电流强度越高表明其电荷传输效率越高,光生电子-空穴对的分离效率越高。EIS图谱中的圆弧半径越小表明其阻抗越小、光生电子-空穴对的分离度越高;PL荧光光谱的峰强度越高,表明光生电子和空穴的复合率越高[41]。图9表明,NUB-1复合催化剂的电荷转移阻抗最小和光电流密度最高,即其光生电子和空穴的分离与传导有显著的优势。复合催化剂NUB-1的荧光强度显著降低,表明异质结抑制了光生载流子的复合,提高了光生电子和空穴的分离效率。NH2-UiO-66与BiOBr的异质结结构降低了电荷传输阻力,提高了光生电荷的分离效率,从而提高了光催化性能。与单一组分的性能相比,NUB-1复合催化剂的光电化学性能和光催化活性优异,即其综合性能优异。
图9
图9
光电流瞬态响应、电化学阻抗谱和PL谱
Fig.9
Transient photocurrent response (a), EIS (b) and PL (c)
2.7 不同复合比催化剂的降解性能
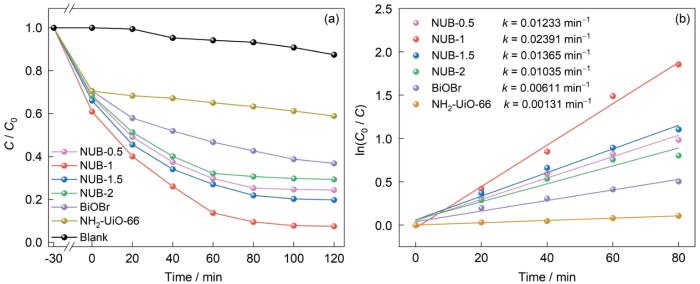
如图10所示,在模拟太阳光照射下,未添加催化剂的空白组120 min对OFX的降解率只有12.6%。BiOBr和NH2-UiO-66在120 min内对OFX的降解率分别为60.63%和41.10%。NUB-0.5、NUB-1、NUB-1.5、NUB-2的降解率为75.60%、92.48%、80.18%、70.62%。NUB-1的降解率最高,并远高于单一的BiOBr和NH2-UiO-66,其一级反应动力学常数k也远高于其他组。这表明,将两种材料复合可提高光催化性能。
图10
图10
不同复合比例的光催化剂对OFX的降解曲线和反应速率常数
Fig.10
Photocatalytic degradation curves (a) and reaction rate constant (b) of ofloxacin over materials with different composite ratios
2.8 催化剂投加量的影响
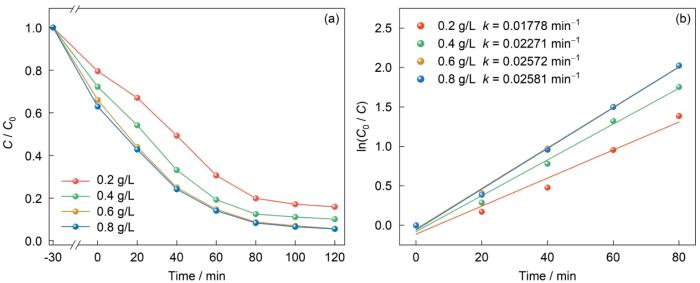
从图11可见,随着NUB-1催化剂投加量的增加(0.2、0.4、0.6、0.8 g/L),降解率显著提高。最低投加量(0.2 g/L)的降解率最低,最高投加量(0.8 g/L)的降解率最高。这表明,催化剂投加量的增加使更多的活性位点参与降解反应,从而提高了降解率。催化剂的投加量为0.6 g/L与0.8 g/L的降解曲线接近,催化剂投加量从0.6 g/L提高到0.8 g/L降解率只提高了0.08%,反应速率常数从0.02572 min-1提高到0.02581 min-1。这表明,投加量提高到一定数值后,再提高催化剂的量效果不大。其原因是,催化剂投加量过高使溶液的浊度提高,颗粒之间相互遮蔽而影响光的透过、散射和吸收。另一方面,OFX的浓度是一定的,投加过量的催化剂也不能充分利用活性点位。为了降低成本,后续实验中催化剂的投加量均为0.6 g/L。
图11
图11
NUB-1不同投加量的降解曲线和反应速率常数
Fig.11
Degradation curves (a) and reaction rate constants (b) of NUB-1 with different dosages
2.9 水体pH值的影响
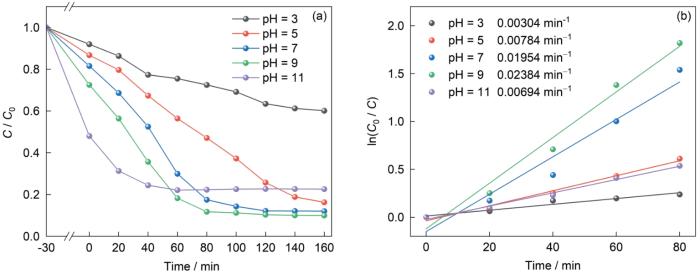
使用稀HCl和NaOH溶液在OFX初始水溶液的pH = 3~11范围内将其调节,研究pH值对光催化剂降解性能的影响。从图12可见,反应体系的pH=3、5、7、9、11时,降解率分别为36.52%、74.27%、87.84%、89.68%和53.57%。在强酸性条件下(pH = 3),催化剂的降解率最低。其原因是,在强酸性条件下NH2-UiO-66中氨基基团的质子化使光催化剂的催化活性降低。在酸性条件下OFX分子以阳离子态(OFXH2+)存在[42],表面带正电荷[43]。于是,与也带正电荷的质子化NH2-UiO-66相互排斥,使催化剂对OFX分子的吸附降低。同时,在酸性条件下溶液中OH⁻离子的浓度较低,限制了羟基自由基(•OH)的生成,也在一定程度上抑制了氧化反应。
图12
图12
pH值对OFX降解性能的影响和反应速率常数
Fig.12
Effect of pH on OFX degradation (a) and reaction rate constant (b)
2.10 阴离子对降解率的影响
废水中的阴离子使溶液的pH值改变,还与反应体系中的污染物竞争活性氧物质,从而降低催化剂对污染物的降解。如图13所示,在反应体系中分别加入浓度为5 mmol/L的HCO
图13
图13
无机阴离子对OFX降解性能的影响和反应速率常数
Fig.13
Effect of inorganic anion on OFX degradation (a) and reaction rate constant (b)
2.11 催化剂的循环稳定性
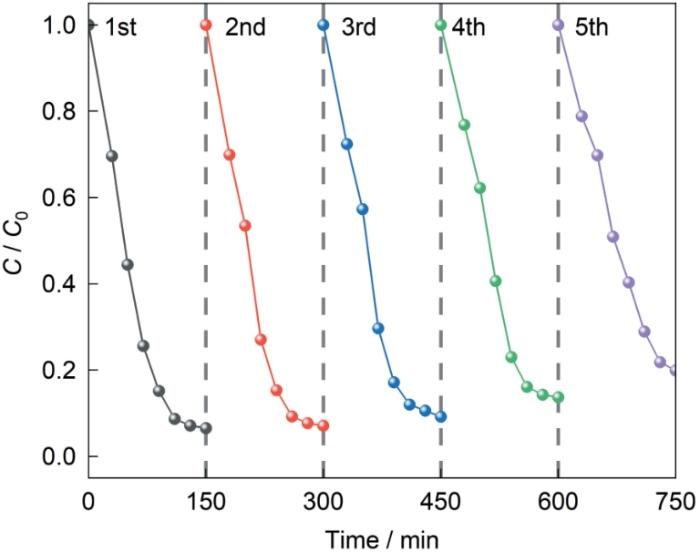
如图14所示,反应120 min催化剂对OFX的降解率分别为92.46%、92.19%、90.80%、87.59%、82.11%,5次循环使用后NUB-1的催化活性仍然较高,降解率高于80%。这表明,催化剂的可重复使用的循环性能较高。
图14
2.12 催化剂对不同种类污染物的降解率
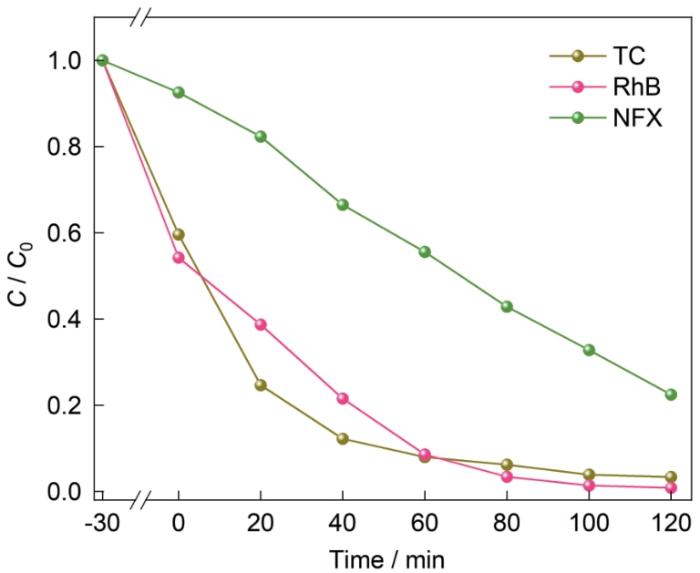
用四环素(TC)、罗丹明B(RhB)和诺氟沙星(NFX)作为模拟污染物研究NUB-1的降解性能。图15表明,光照120 min对TC和RhB的降解率分别为96.63%和99.14%。光照120 min对NFX的降解率也达到77.53%。这表明,NUB-1催化剂对不同种类污染物的降解性能都比较高。
图15
图15
NUB-1对不同污染物的降解曲线
Fig.15
Degradation curves of NUB-1 to different pollutants
2.13 自由基猝灭剂的影响及其机理
图16
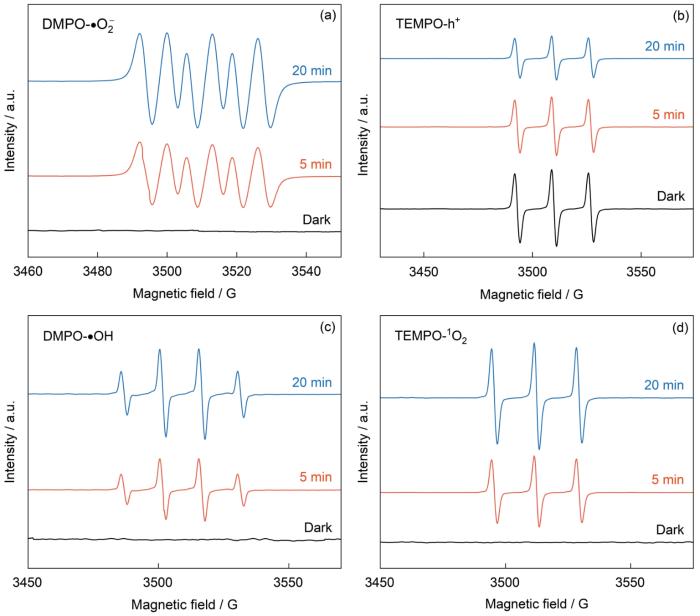
为了证明光催化反应体系中存在•OH、1O2、h+、•O
图17
图17
NUB-1不同自由基的ESR信号捕获曲线
Fig.17
ESR spectra of DMPO-•O2- (a), TMEPO-h+ (b), DMPO-•OH (c), and TMEPO-1O2 (d)
根据以上结果,提出NH2-UiO-66/BiOBr复合光催化剂降解OFX的机理(图18)。光照使催化剂价带上的电子跃迁至导带,在价带留下空穴。NH2-UiO-66与BiOBr形成Z型异质结,二者之间的局部电场使BiOBr导带上的电子与NH2-UiO-66价带上的空穴复合,剩余的光生电子集中在NH2-UiO-66的导带,而空穴则集中在BiOBr的价带,即光生电子与空穴分离。NH2-UiO-66导带的电位(-0.68 eV)比O2/•O
图18
图18
NH2-UiO-66/BiOBr Z型异质结光催化剂降解OFX的机理
Fig.18
Mechanism of the photocatalytic degradation of OFX by the NH2-UiO-66/BiOBr Z-scheme heterojunction photocatalyst
于是,NH2-UiO-66/BiOBr复合光催化剂降解OFX的机理可表示为
3 结论
(1) NH2-UiO-66与BiOBr复合形成的Z型异质结结构,使光生电子与空穴的分离效率和催化剂的光催化性能显著提高。
(2) 与单一组分相比NUB-1的电荷转移阻抗最低和光电流密度较高,具有优异的电荷分离和传输性能。
(3) 超氧自由基(•O
(4) NUB-1复合催化剂5次循环后保持了较高的光催化效率,表明其具有优异的稳定性和循环能力,对TC、RhB以及NFX等多种有机污染物的光催化降解性能较高。
参考文献
Recent advances in sorption-based photocatalytic materials for the degradation of antibiotics
[J].
Potential of solar photodegradation of antibiotics in shallow ditches: Kinetics, the role of dissolved organic matter and prediction models
[J].
Swine wastewater co-exposed with veterinary antibiotics enhanced the antibiotic resistance of endophytes in radish (Raphanus sativus L.)
[J].
Continuous flow column adsorption and desorption for the study of interaction of fluoroquinolone antibiotic ofloxacin hydrochloride with ZnO and CuO nanometal oxides in aqueous solution: (Continuous column adsorption desorption)
[J].
Fe-O-Bi efficient electron transfer channels and photo-Fenton synergy in S-scheme heterojunctions: insights into interfacial interactions and ofloxacin degradation
[J].
Progress on mechanism and efficacy of heterogeneous photocatalysis coupled oxidant activation as an advanced oxidation process for water decontamination
[J].
Visible-light-response Fe-doped BiOCl microspheres with efficient photocatalysis-Fenton degradation of antibiotics
[J].
Optimizing ligand-to-metal charge transfer in metal-organic frameworks to enhance photocatalytic performance
[J].
Trends in the energy and environmental applications of metal-organic framework-based materials
[J].
Functionalized Zr-MOFs for Enhancing flame retardancy and smoke suppression performance on TPU
[J].
Development of Ce-doped NH2-UiO-66(Zr) photocatalysts for efficient CO2 reduction in an aqueous system
[J].
Activation and Zr precursor influence on UiO-66-NH2 composites for efficient cationic and anionic dye removal
[J].
Fe(III)-incorporated UiO-66(Zr)-NH2 frameworks: Microwave-assisted scalable production and their enhanced photo-Fenton degradation catalytic activities
[J].
Elaborate construction of a MOF-derived novel morphology Z-scheme ZnO/ZnCdS heterojunction for enhancing photocatalytic H2 evolution and tetracycline degradation
[J].
Regulating electron transfer between valence-variable cuprum and cerium sites within bimetallic metal-organic framework towards enhanced catalytic hydrogenation performance
[J].
A theoretical and experimental approach for photocatalytic degradation of caffeic acid using BiOBr microspheres
[J].
The photocatalytic potential of BiOBr for wastewater treatment: A mini-review
[J].
Recent advances in BiOBr-based photocatalysts for environmental remediation
[J].
Novel BiOBr by compositing low-cost biochar for efficient ciprofloxacin removal: the synergy of adsorption and photocatalysis on the degradation kinetics and mechanism insight
[J].
Core-shell-like BiOBr@BiOBr homojunction for enhanced photocatalysis
[J].
Photocatalytic degradation of tetracycline by using a regenerable (Bi) BiOBr/rGO composite
[J].
BiOBr/MXene/gC3N4 Z-scheme heterostructure photocatalysts mediated by oxygen vacancies and MXene quantum dots for tetracycline degradation: Process, mechanism and toxicity analysis
[J].
Stable self-assembly AgI/UiO-66(NH2) heterojunction as efficient visible-light responsive photocatalyst for tetracycline degradation and mechanism insight
[J].
Fabrication of novel ZnO@BiOBr/UiO-66-NH2 core-shell heterojunction for improved tetracycline degradation
[J].
BiOBr/protonated graphitic C3N4 heterojunctions: Intimate interfaces by electrostatic interaction and enhanced photocatalytic activity
[J].
Selective adsorption of cationic dyes by UiO-66-NH2
[J].
High-efficiency adsorption of norfloxacin using octahedral UIO-66-NH2 nanomaterials: Dynamics, thermodynamics, and mechanisms
[J].
One-pot hydrothermal synthesis of g-C3N4/BiOBr/Bi2MoO6 as a Z-scheme heterojunction for efficient photocatalytic degradation of ciprofloxacin (CIP) antibiotic and Rhodamine B (RhB) dye
[J].
A novel visible-light-induced double Z-scheme photocatalytic system: NH2-UiO-66/BiOBr/Bi2S3 for degradation of tetracycline hydrochloride and rhodamine B
[J].
Synthesis of NH2-UiO-66/Bi4O5I2 heterojunction for high tetracycline reduction under visible light
[J].
Construction of Type-II Zn-SnO2/BiOBr heterojunctions with dual-oxygen vacancies for enhanced photocatalytic degradation
[J].
A novel persulfate activation strategy by double Z-scheme Bi2O3/CuBi2O4/BiOBr heterojunction: Non-radical dominated pathway for levofloxacin degradation
[J].
Bi2Sn2O7/UiO-66-NH2 heterojunction photocatalyst simultaneously adsorbed and photodegraded tetracycline
[J].
Z-scheme heterojunction Bi2MoO6/NH2-UiO-66(Zr/Ce) for efficient photocatalytic degradation of oxytetracycline: Pathways and mechanism
[J].
Direct Z-scheme Bi2MoO6/UiO-66-NH2 heterojunctions for enhanced photocatalytic degradation of ofloxacin and ciprofloxacin under visible light
[J].
Synthesis of UiO-66-NH2@PILs core-shell composites for CO2 conversion into cyclic carbonates via synergistic catalysis under solvent- and additive-free conditions
[J].
Use and misuse of the Kubelka-Munk function to obtain the band gap energy from diffuse reflectance measurements
[J].
Synergetic photocatalytic degradation of the tetracycline antibiotic over S-scheme based BiOBr/CuInS2/WO3 ternary heterojunction photocatalyst
[J].
One-pot synthesis of Ni(Ⅱ)-doped UiO-66-NH2 for enhanced photocatalytic CO2 reduction to CO with efficient charge transfer
[J].
A novel 0D/3D Z-Scheme heterojunction ZnS/MIL-88(A) with significantly boosted photocatalytic activity toward tetracycline
[J].
Preparation of g-C3N4/Ag/TiO2 NTs and photocatalytic degradation of ceftazidine
[J].
g-C3N4/Ag/TiO2NTs的制备及其对西维因的光催化降解
[J].
Influence of pH and DO on the ofloxacin degradation in water by UVA-LED/TiO2 nanotube arrays photocatalytic fuel cell: mechanism, ROSs contribution and power generation
[J].
Host-guest interaction between Ofloxacin-β-Cyclodextrin complexes in acidic and neutral pH: A fluorescence quenching study
[J].
Potentiometric determination of acid dissociation constants (pKa) for human and veterinary antibiotics
[J].
Distinct photolytic mechanisms and products for different dissociation species of ciprofloxacin
[J].
Study on the influence of pH and DO inphotocatalytic fuel cell degrading ofloxacin
[D].
pH和DO对光催化燃料电池降解氧氟沙星影响研究
[D].
Preparation of S-scheme heterojunction photocatalyst Y2O3/BiOCl and visible light degradation of ofloxacin: Photocatalytic mechanism, DFT calculation, degradation pathway, and toxicity evaluation
[J].
ZIF-67-derived monolithic bimetallic sulfides as efficient persulfate activators for the degradation of ofloxacin
[J].
Construction of S-scheme BiOCl/Bi24O31Cl10 heterojunction by thermally induced in-situ phase transition strategy for photocatalytic degradation of ciprofloxacin
[J].
Transforming goat manure into surface-loaded cobalt/biochar as PMS activator for highly efficient ciprofloxacin degradation
[J].
A novel electrochemically enhanced homogeneous PMS-heterogeneous CoFe2O4 synergistic catalysis for the efficient removal of levofloxacin
[J].
Construction dual active sites on SnO2 via Fe doping for effective ciprofloxacin photodegradation
[J].
Visible light photocatalytic degradation of MB using UiO-66/g-C3N4 heterojunction nanocatalyst
[J].
Efficient photocatalytic degradation of tetracycline under visible light by Z-scheme Ag3PO4/mixed-valence MIL-88A(Fe) heterojunctions: Mechanism insight, degradation pathways and DFT calculation
[J].
Design of UiO-66@BiOIO3 heterostructural composites with remarkable boosted photocatalytic activities in removing diverse industrial pollutants
[J].
Construction of solar light-driven dual Z-scheme Bi2MoO6/Bi2WO6\AgI\Ag photocatalyst for enhanced simultaneous degradation and conversion of nitrogenous organic pollutants
[J].
Fabrication of the flower-like Z-scheme heterojunction photocatalyst Bi-BiOI/UiO 66 for enhanced photodegradation of acetaminophen in simulated wastewater
[J].
Intraligand charge transfer boosts visible-light-driven generation of singlet oxygen by metal-organic frameworks
[J].
In-situ-construction of BiOI/UiO-66 heterostructure via nanoplate-on-octahedron: A novel p-n heterojunction photocatalyst for efficient sulfadiazine elimination
[J].
Construction of efficient g-C3N4/NH2-UiO-66 (Zr) heterojunction photocatalysts for wastewater purification
[J].