MnZn铁氧体是典型的软磁材料,其磁导率高、矫顽力和功率损耗低,得到了广泛的应用[1~3]。Mn-Zn铁氧体具有尖晶石结构,锌离子倾向占据四面体间隙的A位,铁离子和锰离子通常占据四面体间隙的A位和八面体间隙位置的B位[4,5]。MnZn铁氧体金属离子之间的超交互作用和金属离子的含量,都影响其性能。Zheng等[6]用高能球磨工艺制备了不同Zn含量的MnZn铁氧体纳米晶粉末,其晶格常数随着Zn含量的降低而增大。Li等[7]研究了原料中的Fe2+含量对MnZn铁氧体磁性能的影响。Zhang等[8]用固态反应法制备了不同Zn含量的Mn1-xZnxFe2O4 (x = 0.46,0.47,0.48,0.50,0.51)。结果表明:在设定的成分范围内,只生成了尖晶石单相MnZn铁氧体;功率损耗随着Zn含量的提高而降低;x = 0.48时,在150 kHz条件下,Mn1 - x Zn x Fe2O4的功率损耗仅为115.2 mW/cm3;随着Zn含量的提高,复数磁导率增大。Shang等[9]用溶剂热反应方法先制备Mn x Zn1 - x Fe2O4 (x = 0.3,0.5,0.7)纳米纤维,然后以纳米纤维为增强相制备同质增强MnZn铁氧体材料。结果表明:随着Mn含量的提高,纳米纤维的晶格常数降低,饱和磁化强度提高;控制Mn2+和Zn2+的比例可优化纳米纤维的尺寸和排列;因为MnZn铁氧体纳米纤维同时具有小尺寸和优异的性能,使同质增强MnZn铁氧体材料具有优异的磁性能和力学性能。Serkol等[10]用超声处理法合成了Mn0.5Zn0.5Dy x Eu x Fe2 - 2x O4 (x ≤ 0.1)纳米级尖晶石铁氧体,随着Eu3+和Dy3+含量的提高,饱和磁化强度Ms降低,矫顽力Hc增大,TB稍有增大。同时,所有样品在较低的Ts下都出现类似自旋玻璃态行为,表明磁矩之间具有强烈的交互作用。Wu等[11]用传统氧化物陶瓷法制备了MnZn铁氧体Mn0.670 - x Zn0.213Sn x Fe2.117O4 (x = 0.000-0.015)。结果表明:主要位于晶界的Sn4+极大地影响其性能;随着Sn含量的提高,初始磁导率和饱和磁感应强度单调降低,且x > 0.006时降低的幅度更为显著;随着Sn含量的提高,这种铁氧体的高频功率损耗先降低然后提高;x = 0.003的这种MnZn铁氧体的涡流损耗和磁滞损耗受到抑制,磁芯损耗降低至457 kW/m3 (3 MHz,30 mT,25 ℃)且具有优异的综合性能(μi = 904,Bs = 469 mT)。
上述研究结果表明,无论是采用湿法还是干法都能制备MnZn铁氧体,而且优化成分显著影响其特性。但是,用传统的干法制备的铁氧体成分不均匀,颗粒尺寸大且分布不均匀。湿法的工艺过程繁琐,制备条件苛刻,难以制备出高品质产品。鉴于此,本文用“化学溶胶-喷雾干燥-煅烧”的法制备MnZn铁氧体并优化其主要成分,研究Zn含量对其性能的影响。
1 实验方法
1.1 MnZn铁氧体材料的制备
按照配方Mn1 - x Zn x Fe2O4 (x = 0.1,0.3,0.5,0.7,0.9)称取Fe(NO3)3·9H2O、Zn(NO3)2·6H2O、50%Mn(NO3)2以及十六烷基三甲基溴化铵(CTAB)并将其混合在去离子水中,磁力搅拌30 min后得到均匀的溶胶。将溶胶泵入高速旋转(28000 r/min)的喷雾干燥机(MDR-5)中,喷雾干燥机的进风口温度为250 ℃,进料速率为30 mL/min。将喷雾干燥获得的MnZn铁氧体前驱体粉末置于温度为1060 ℃的马弗炉(KSY-4-10),在空气中煅烧3 h后取出在空气中冷却,得到不同Zn含量的MnZn铁氧体粉末样品。
1.2 MnZn铁氧体粉末样品性能的表征
用X射线衍射仪(Advance D8,Bruker,Germany)测定样品的XRD谱。用Cu靶Kα射线(λ = 0.15406 nm),管压为40 kV,管流为40 mA,2θ为5°~80°,步长为0.02°。用红外光谱仪(Nicolet-6700,ThermoFisher Scientific, Waltham, MA, USA),用KBr压片法在400~4000 cm-1范围内测试样品的红外吸收光谱。用拉曼光谱仪(LabRAM HR800,Horiba Jobin Yvon, Palaiseau, France)在100~2000 cm-1范围内测试样品的拉曼光谱。激发波长为632.8 nm,激光功率为0.4 mW,曝光时间为10 s,曝光循环次数为2次。用X射线光电子能谱仪(XPS,ESCALAB250Xi,ThermoFisher Scientific, USA)测定元素价态,真空度为1.333 × 10-7 Pa,选用AlKα X射线源。使用Thermo Avantage软件分析数据,参照C1s的结合能(284.8 eV)进行电荷校正。用扫描电镜(Nova NanoSEM 230,FEI, Brno, Czech Republic)观察样品的形貌,用二次电子模式,加速电压为15 kV。用透射电镜(Tecnai G2 F20,FEI, Brno, Czech Republic)观察样品的明场像形貌和高分辨形貌,加速电压为200 kV。用超导量子干涉磁测量系统(MPMS XL-7,Quantum Design, San Diego, CA, USA)测试样品的室温磁性,外加磁场为30000 Oe。
2 实验结果和讨论
2.1 样品的物相
图1给出了不同Zn含量粉末的XRD谱。可以看出,随着Zn含量的提高,谱中衍射峰的强度和样品中物相均发生了变化。在x = 0.1和x = 0.3样品的谱中除了MnZn铁氧体相的峰,还有少量杂质相α-Fe2O3的峰。其原因可能是Fe含量过量高生成了α-Fe2O3相。Zn的含量继续提高,则α-Fe2O3消失。这表明,只有Zn的含量适当,才能制备出单相的MnZn铁氧体粉末。
图1
图1
不同Zn含量粉末样品的XRD谱
Fig.1
XRD patterns of powders with different zinc concentration
以尖晶石MnZn铁氧体相的(311)晶面为基础,用Scherrer公式估算样品的平均晶粒尺寸。表1列出了不同Zn含量粉末样品的平均晶粒尺寸和晶格常数。可以看出,即使所有样品的制备条件都相同,其晶粒尺寸并没有随着Zn含量的变化而单调变化,而是先增大再减小,其原因可能与反应状态有关。在该制备条件下,不同的Zn含量使MnZn铁氧体相生成的速率不同,因此晶粒尺寸不同[12,13]。在0.5 ≤ x≤ 0.9范围内晶格常数减小,因为Zn2+的半径比Mn2+的小,且Zn2+替代Mn2+的量增加。当0.1 ≤ x ≤ 0.3时,少量α-Fe2O3相的析出使非纯相样品中的MnZn铁氧体相的晶格常数不同。而且,在本文制备的MnZn铁氧体中Mn有Mn2+、Mn3+、Mn4+三种价态(图4),离子半径大小的排序为:Mn4+(0.052 nm) < Mn3+(0.070 nm) < Zn2+(0.082 nm) < Mn2+(0.091 nm)。因为Zn2+替代不同价态Mn离子的量不同,在0.1 ≤ x ≤ 0.9范围内晶格常数没有随着Zn含量的提高而单调降低,而是先减小再增大最后再减小。
表1 不同Zn含量粉末样品的平均晶粒尺寸和晶格常数
Table 1
| Samples | Average crystallite size / nm | Lattice parameter / nm |
|---|---|---|
| x = 0.1 | 81.0 | 0.84549 ± 0.00039 |
| x = 0.3 | 95.1 | 0.84404 ± 0.00033 |
| x = 0.5 | > 100 | 0.84534 ± 0.00010 |
| x = 0.7 | > 100 | 0.84490 ± 0.00014 |
| x = 0.9 | 62.7 | 0.84413 ± 0.00015 |
图2
图2
不同Zn含量粉末样品的FTIR谱
Fig.2
FTIR spectra of powders with different zinc concentration
图3
图3
不同Zn含量粉末样品的Raman谱
Fig.3
Raman spectra of powders with different zinc concentration
图4
图4
不同Zn含量粉末样品的XPS谱
Fig.4
XPS spectra of powders with different zinc concentration (a~d) x = 0.3, (e~h) x = 0.9
2.2 不同Zn含量铁氧体粉末样品的红外光谱
图2给出了不同Zn含量铁氧体粉末样品的FTIR谱。可以看出,所有样品的谱中都出现了四个明显的吸收峰。位于高波数区域的两个吸收峰(3431~3449 cm-1和1628~1633 cm-1),是样品吸附的水汽所致。位于低波数区域的MnZn铁氧体相的两个吸收峰(422~438 cm-1和544~557 cm-1),随着Zn含量的提高单调地红移。其原因是,Zn2+替代Mn2+影响了Fe3+-O2-的振动而使峰位移动[14]。同时,Mn的三种价态(Mn2+、Mn3+、Mn4+)(图4)使Zn含量的提高引起不同价态的Mn离子含量变化,离子所处环境的改变影响了化学健的振动。Zn含量较低的样品中含有α-Fe2O3相,其与MnZn铁氧体相中的Fe3+-O2-的振动频率相近,使相应的吸收峰重合,也可能是α-Fe2O3的含量较低而没有出现显著的吸收峰。
2.3 MnZn铁氧体粉末的拉曼光谱
图3给出了优化Zn含量的铁氧体粉末样品的Raman光谱。可以看出,所有样品的谱中都出现了MnZn铁氧体相的衍射峰,谱中317~359 cm-1处的峰对应Eg振动模,451~458 cm-1处的峰对应T2g(2),510~561 cm-1处的峰对应T2g(3),596~633 cm-1处的峰对应A1g,表明样品中有MnZn铁氧体相。图中A1g峰的半高宽较大,可能是四面体间隙中不同Me-O键的对称伸缩振动所致[15,16]。同时,x = 0.1样品的谱中位于497 cm-1的微弱衍射峰对应α-Fe2O3的A1g振动模,是Fe-O的振动引起的[17,18]。但是,x = 0.3样品的谱中没有出现明显的α-Fe2O3拉曼衍射峰,因为其含量极少。随着Zn含量的提高谱中衍射峰的强度提高,表明对应的MnZn铁氧体的含量较高。这个结果,与XRD谱给出的结果吻合。
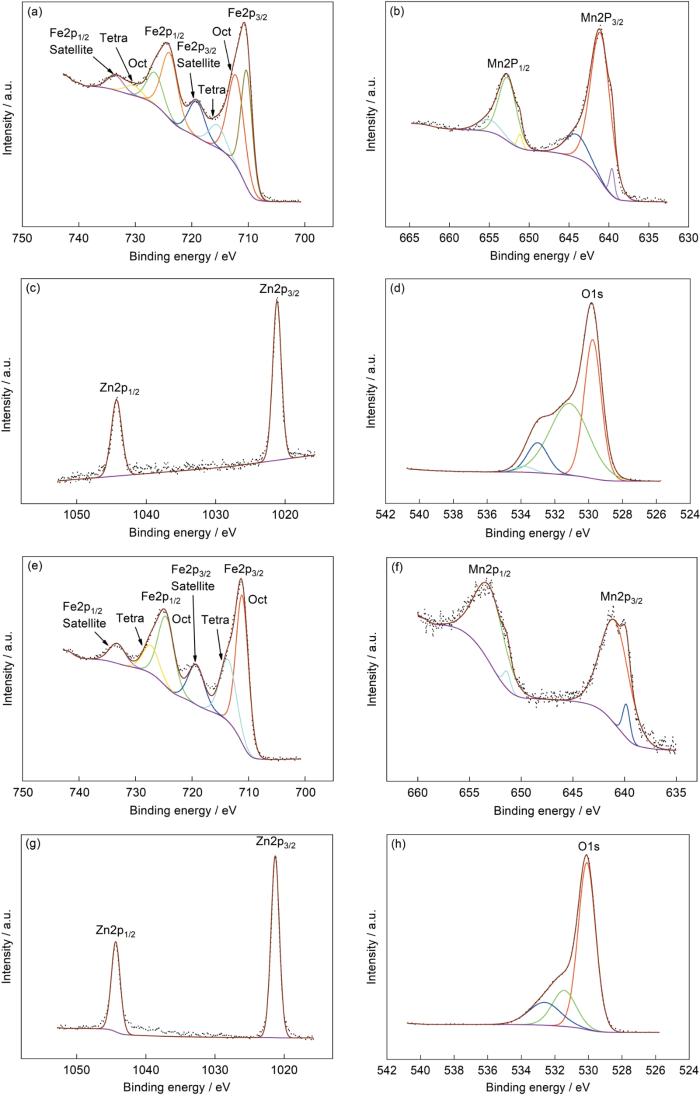
2.4 典型Zn含量粉末样品的XPS谱
图4给出了典型Zn含量粉末样品的XPS精细谱。图4a和图4e给出了x = 0.3和x = 0.9样品的Fe2p谱,可见谱峰都分裂为Fe2p3/2和Fe2p1/2峰且都有卫星峰。x = 0.3样品的两个峰对应的结合能分别为710.80和724.09 eV,双重线的距离为13.29 eV,Fe2p3/2对应的卫星峰其结合能为719.16 eV。x = 0.9样品的劈裂峰其结合能则分别为711.28和724.54 eV,双重线的距离为13.26 eV,Fe2p3/2对应的卫星峰其结合能为719.25 eV。这些结果与文献[19~21]的结果吻合,而且卫星峰都完整清晰,并不是肩峰,都表明样品中都只有Fe3+。样品中不同物相的结构不同,其中化学环境不同的Fe3+峰对应不同的结合能。x = 0.3样品的Fe2p3/2有三个子峰,其结合能为710.29,712.17和715.49 eV,分别对应α-Fe2O3和MnZn铁氧体中八面体间隙和四面体间隙中的Fe3+[15,20]。这些结果,也与上述结果吻合。不同的是,x = 0.9样品的Fe2p3/2只有2个子峰,其结合能分别为711.11和713.63 eV,是MnZn铁氧体处于间隙位置的Fe3+引起的。图4b、f给出了x = 0.3和x = 0.9样品的Mn2p精细谱,Mn的本征特性使XPS谱峰劈裂成Mn2p3/2和Mn2p1/2。其中x = 0.3样品对应的结合能为641.07和652.74 eV,x = 0.9样品对应的结合能为640.68和652.84 eV。氧化状态不同的Mn其离子价态不同,因此本文制备的样品中Mn元素也可能呈不同价态。将x = 0.3样品的Mn2p3/2峰分解,得到结合能分别为639.61,641.05和644.08 eV的三个子峰。根据文献[22~24]的结果,可以判定这三个子峰分别对应Mn2+,Mn3+,Mn4+。x = 0.9样品的Mn2p3/2峰可分解为结合能分别为639.84和640.95 eV的两个子峰,分别对应Mn2+和Mn3+。这表明,Zn含量的提高阻碍Mn3+进一步氧化为Mn4+。x = 0.3样品的Zn2p3/2和Zn2p1/2的结合能为1021.17和1044.28 eV,x = 0.9样品的结合能则为1021.29和1044.35 eV。结果表明,两种样品中都只有Zn2+,且均位于A位[15]。图4d中O1s的峰分解为结合能分别为529.76,531.14,533.01和533.81 eV的四个子峰。图4h中的O1s峰的三个子峰的结合能则分别为530.09,531.45,532.61 eV。因为x = 0.3和x = 0.9样品的物相不同,不同晶格中的O2-其衍射峰对应的结合能不同。但是,晶格氧对应的结合能低,而高结合能533.81和532.61 eV的衍射峰则与表面吸附的氧有关[19]。
2.5 不同Zn含量铁氧体粉末样品的形貌
图5给出了在1060 ℃煅烧3 h的不同Zn含量的铁氧体粉末样品的SEM照片。可以看出,各个粉末样品的宏观形貌呈空心球壳状,有破碎的,有多孔的,有近完整球形的,颗粒尺寸也各不相同。组成立方形貌球壳的细小颗粒尺寸均匀,没有出现异常长大的颗粒。如上所述,在x = 0.1和x = 0.3的样品中除了MnZn铁氧体相,还有杂质相α-Fe2O3。但是,在SEM照片的视场中并没有发现明显不同的形貌,其原因可能是α-Fe2O3的含量较低。随着Zn含量的提高颗粒之间没有烧结在一起,表明Zn含量的高低不影响颗粒之间的烧结行为。
图5
图5
不同Zn含量粉末样品的SEM照片
Fig.5
SEM images of powders with different zinc concentration
(a) x = 0.1, (b) x = 0.3, (c) x = 0.5, (d) x = 0.7, (e) x = 0.9
粉末呈空心球壳形貌,且球壳是许多细小颗粒组成的。图6a、6c和6e给出了粉末的TEM明场照片,可见有不同的立方小颗粒。图6b和图6d给出了x = 0.3样品的高分辨图像。图6b中的晶面间距为0.491 nm,对应MnZn铁氧体相的(111)晶面;而图6d中的晶面间距为0.251 nm,对应α-Fe2O3的(110)晶面。在x = 0.9样品的高分辨图像中,晶面间距为0.487 nm,与MnZn铁氧体相的(111)晶面匹配。将高分辨图像进行FFT转变,结果如插图所示,可见明显的单晶衍射花样,也表明所选区域为单物相。如上所述,x = 0.3样品含有MnZn铁氧体相和α-Fe2O3,而x = 0.9样品则只有MnZn铁氧体单相,与XRD谱的结果吻合。文献[25]报导的x = 0.5样品也只含有MnZn铁氧体单相。这些结果都表明,不低于0.5的Zn含量才有利于生成单相铁氧体。
图6
图6
不同Zn含量粉末样品的明场像和高分辨图像
Fig.6
Bright field images and high resolution images of powders with different zinc concentration (a~d) x = 0.3, (e, f) x = 0.9
2.6 铁氧体粉末样品的磁性能
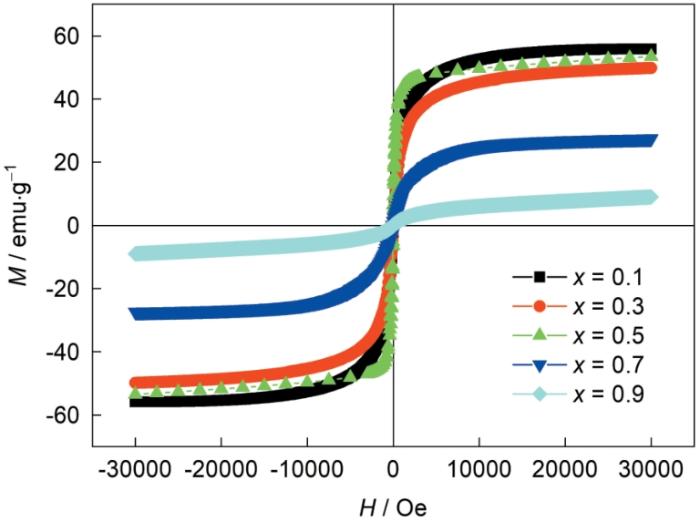
图7给出了不同Zn含量铁氧体粉末样品的室温磁滞回线,其磁性参数列于表2。可以看出,除了x = 0.5,随着Zn含量的提高样品的饱和磁化强度Ms减小,因为Zn倾向于占据A位,Zn含量的进一步提高使A-B位之间的超交互作用降低,从而降低了Ms[26]。而x = 0.5的样品中金属离子在A位和B位的分布发生变化,从而使其Ms没有按单调递减规律变化。测试结果表明,其Mr和Hc分别为0.24~6.50 emu/g和28.03~107.63 Oe,且Mr和Hc的变化没有规律。Mr与Ms不同,其大小受磁畴尺寸、晶粒尺寸和组织的影响,尤其是磁畴尺寸近单畴尺寸的样品[27]。本文制备的铁氧体矩形比Mr/Ms为0.02~0.12,远小于0.5,表明样品具有多磁畴结构[27,28],其磁化方式主要是畴壁移动。所有粉末样品的磁结构相同,Mr,Hc和Mr/Ms等参数主要受其形貌、组织结构和相组成等因素的影响[29]。本文制备的所有样品其宏观形貌都是空心球壳状,没有影响磁性能的特殊形貌。Zn含量的不同使样品物相组成不同,对磁性能有一定的影响。
图7
图7
不同Zn含量粉末样品的磁滞回线
Fig.7
Hysteresis loops of powders with different zinc concentration
表2 不同Zn含量粉末样品的磁性参数
Table 2
| Samples | Ms / emu·g-1 | Mr / emu·g-1 | Hc / Oe | Mr/Ms |
|---|---|---|---|---|
| x = 0.1 | 55.87 | 2.25 | 48.86 | 0.04 |
| x = 0.3 | 49.85 | 1.09 | 28.55 | 0.02 |
| x = 0.5 | 53.49 | 6.50 | 28.03 | 0.12 |
| x = 0.7 | 27.45 | 1.25 | 107.63 | 0.05 |
| x = 0.9 | 8.99 | 0.24 | 101.94 | 0.03 |
3 结论
(1) 用“化学溶胶-喷雾干燥-煅烧”方法可制备Mn1 - x Zn x Fe2O4 (x = 0.1, 0.3, 0.5, 0.7, 0.9)软磁铁氧体。x大于或等于0.5的MnZn铁氧体粉末是单相的,反之出现杂质相α-Fe2O3。
(2) MnZn铁氧体粉末均呈细小颗粒组成的空心球壳形貌,且Zn含量变化细小颗粒之间也没有出现烧结。x = 0.5的MnZn铁氧体其综合性能最优。
参考文献
Fabrication and characterization of Manganese-Zinc Ferrite nanoparticles produced utilizing heat treatment technique
[J].
Electromagnetic properties of nanocrystalline Mn-Zn ferrite synthesized from spent Zn-C battery via egg-white route
[J].
Dose dependent modifications in structural and magnetic properties of γ-irradiated nanocrystalline Mn0.5Zn0.5Fe2O4 ceramics
[J].
Influence of pH on nanosized Mn-Zn ferrite synthesized by sol-gel auto combustion process
[J].
Effect of nickel on the electrical properties of nanostructured MnZn ferrite
[J].
Synthesis, structure and magnetic properties of nanocrystalline Zn x Mn1 - x Fe2O4 prepared by ball milling
[J].
Effect of Fe2+content in raw materials on Mn-Zn ferrite magnetic properties
[J],
Effects of Zn content on microstructure and magnetic properties of MnZn ferrite
[J].
Fabrication and property analysis of Mn x Zn1 - x Fe2O4 nanofibers and homogeneous-fiber-reinforced MnZn ferrite materials
[J].
Magnetic and optical characterizations of Dy-Eu co-substituted Mn0.5Zn0.5Fe2O4 nanospinel ferrites
[J].
Effects of Sn substitution on the microstructural and electromagnetic properties of MnZn ferrite for high-frequency applications
[J]. J.
Magnetic and optical properties of Mn1 - x Zn x Fe2O4 nanoparticles
[J].
Structural and magnetic properties of Mn1 - x Zn x Fe2O4 ferrite nanoparticles
[J].
Nanostructural, morphological and magnetic studies of PEG/Mn(1 - X)Zn( X)Fe2O4 nano-particles synthesized by co-precipitation
[J].
Cation distribution in Ni-substituted Mn0.5Zn0.5Fe2O4 nanoparticles: A Raman, Mössbauer, X-Ray diffraction and electron spectroscopy study
[J].
Micro Raman, Mossbauer and magnetic studies of manganese substituted zinc ferrite nanoparticles: role of Mn
[J].
Size-dependent structural transformations of hematite nanoparticles. 1. Phase Transition
[J].Using Fourier Transform InfraRed (FTIR) spectroscopy, Raman spectroscopy, X-ray diffraction (XRD), and Transmission Electron Microscopy (TEM), we characterize the structure and/or morphology of hematite (alpha-Fe(2)O(3)) particles with sizes of 7, 18, 39 and 120 nm. It is found that these nanoparticles possess maghemite (gamma-Fe(2)O(3))-like defects in the near surface regions, to which a vibrational mode at 690 cm(-1), active both in FTIR and Raman spectra, is assigned. The fraction of the maghemite-like defects and the net lattice disorder are inversely related to the particle size. However, the effect is opposite for nanoparticles grown by sintering of smaller hematite precursors under conditions when the formation of a uniform hematite-like structure throughout the aggregate is restricted by kinetic issues. This means that not only particle size but also the growth kinetics determines the structure of the nanoparticles. The observed structural changes are interpreted as size-induced alpha-Fe(2)O(3)<-->gamma-Fe(2)O(3) phase transitions. We develop a general model that considers spinel defects and absorbed/adsorbed species (in our case, hydroxyls) as dominant controls on structural changes with particle size in hematite nanoparticles, including solid-state phase transitions. These changes are represented by trajectories in a phase diagram built in three phase coordinates-concentrations of spinel defects, absorbed impurities, and adsorbed species. The critical size for the onset of the alpha-->gamma phase transition depends on the particle environment, and for the dry particles used in this study is about 40 nm. The model supports the existence of intermediate phases (protohematite and hydrohematite) during dehydration of goethite. We also demonstrate that the hematite structure is significantly less defective when the nanoparticles are immersed in water or KBr matrix, which is explained by the effects of the electrochemical double layer and increased rigidity of the particle environment. Finally, we revise the problem of applicability of IR spectroscopy to the lattice vibrations of hematite nanoparticles, demonstrating that structural comparison of different samples is much more reliable if it is based on the E(u) band at about 460 cm(-1) and the spinel band at 690 cm(-1), instead of the A(2u)/E(u) band at about 550 cm(-1) used in previous work. The new methodology is applied to analysis of the reported IR spectra of Martian hematite.
Infrared-and Raman-Active Phonons of magnetite, maghemite, and hematite: A computer simulation and spectroscopic study
[J].
Synthesis and characterization of MnZn ferrite nanoparticles with improved saturation magnetiza-tion
[J].
Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials
[J].
In situ ambient pressure XPS observation of surface chemistry and electronic structure of α-Fe2O3 and γ-Fe2O3 nanoparticles
[J].
Effect of Mn substitution on the promoted formaldehyde oxidation over spinel ferrite: Catalyst characterization, performance and reaction mMechanism
[J].
Elemental mercury capture from flue gas by magnetic Mn-Fe spinel: Effect of chemical heterogeneity
[J].
Interpretation of XPS Mn(2p) spectra of Mn oxyhydroxides and constraints on the mechanism of MnO2 precipitation
[J].
Microstructure and magnetic properties of MnZn ferrite powders prepared by nano-in-situ composite method
[J]. J.
Effect of zinc concentration on the microstructure and relaxation frequency of Mn-Zn ferrites synthesized by solid state reaction
[J].
Influence of Mg substitution on structural, magnetic and dielectric properties of X-type barium-zinc hexaferrites Ba2Zn2 - x Mg x Fe28O46
[J].
Structural, magnetic and dielectric properties of Co-Zr substituted M-type calcium hexagonal ferrite nanoparticles in the presence of α-Fe2O3 phase
[J].
Structural characterization of nano-crystalline BaFe12O19 powders synthesized by sol-gel combustion route
[J].