金属-海水溶解氧半燃料电池大规模应用的主要困难,是氧催化正极材料的制备。在正极上发生的氧还原(ORR)反应是一个缓慢的动力学反应。海水中的氧含量较低,因此需要稳定高效的催化正极还原海水中的溶解氧[8~10]。海水溶解氧燃料电池的正极使用碳基材料,如碳毡、碳布或碳刷。这类材料具有良好的柔性、抗压性和耐腐蚀性,但是其ORR催化性能较差而需要负载催化剂[11]。Pt基催化剂在燃料电池ORR反应中的性能优异,但是Pt基催化剂的价格昂贵且易被毒化[12,13]。因此,需要研制一种低Pt含量的催化剂。为此,可将Pt与过渡金属合金化或用非金属元素掺杂。Liang[14]以商业Pt/C催化剂为原料,研究了Pt/Co的合金化程度、粒径和退火温度与ORR活性之间的关系。结果表明,催化剂的合金化程度随着温度的提高而提高。退火温度为600℃的Pt/Co催化剂的平均粒径达到2.1 nm,质量活度高达1.04 A·mg-1。将Pt3Co合金高温退火使Pt偏析到合金表面,可显著提高Pt催化位点的利用率[15]。还需要提高Pt的分散性或设计Pt单原子催化剂,以降低Pt的用量。Zhai等[16]发现,将B原子和Pt同时掺杂到石墨烯中使Pt的氧吸附性能降低,而N和O掺杂可提高Pt对氧的吸附性能。海水中的Cl-与氧分子在Pt基催化剂上发生吸附竞争,使催化剂的效率降低[17]。因此,需要抗毒化且高活性的ORR催化阴极[8,10,16,18~20]。
金属有机框架(MOF),是由金属离子(或金属蔟)与有机配体通过配位键连接构成的。沸石咪唑酯骨架结构(ZIF)材料是一种典型的多孔晶体材料,其稳定性和孔隙率都比较高。ZIF系材料可将配体溶于去离子水中缓慢生成,在原位生长过程中能均匀、牢固的附着在电极表面,ZIF材料高温碳化后形成的M-N-C结构具有对溶解氧的催化活性。与Pt元素合理负载,可协同提高其ORR催化活性和稳定性[21~23]。本文采用原位生长的方法在碳布上生长出一层ZIF67/ZIF8复合材料。随后,通过煅烧和电沉积方法将Pt以雪花状的纳米颗粒的形式沉积在ZIF碳化层上制备碳基Pt@Co多层次复合催化阴极,研究其对海水中氧的还原性能。
1 实验方法
1.1 催化材料的制备
使用碳布基体制备海水溶解氧燃料电池复合正极材料。先将碳布在80℃用浓硫酸和浓硝酸(体积比为3∶1)混合液刻蚀6 h,然后用超纯水和无水乙醇充分超声清洗,最后将其放入烘箱中干燥。
将6.632 g的二甲基咪唑(2-MIM)溶于250 mL超纯水中得到溶液A并将其超声处理20 min,将1.455 g的硝酸钴(Co(No3)2,6H2O)和3.4 g的硝酸锌(Zn(No3)2,4H2O)溶于250 mL超纯水中得到溶液B并将其超声分散20 min。将预处理后的碳布裁剪成尺寸为6 cm × 6 cm的正方体,将其充分浸泡在溶液A中并将溶液B倒入,在常温常压下静置8 h以使ZIF8/ZIF67复合材料充分生长在碳布上。取出碳布并将其在纯水中充分超声清洗,然后在60℃的烘箱中烘干6 h。将烘干的碳布放入管式炉中,在氩气分中以5℃/min的速率将温度升至950℃保持120 min。冷却后将其用清水和乙醇充分清洗后烘干。将1 mL的氯铂酸(H2PtCl6)溶液溶于500 mL的超纯水中,将碳布与恒流仪(Maisheng Depower Supply,MS-605DS)的阴极相连,将两块置于碳布两侧的钛网与阳极相连,在2 V恒电压下电沉积5 min,然后用超纯水和无水乙醇分别超声清洗碳布并将其烘干,将得到的材料命名为Pt@Co-N-C@CC。将煅烧后不进行电沉积的材料命名为Co-N-C@CC,在碳布上用相同的方法生长ZIF8,得到Pt@N-C@CC。
1.2 性能表征
用场发射扫描电子显微镜(FESEM)和透射电子显微镜(TEM)观察催化材料的微观形貌。用拉曼光谱仪(Raman)分析材料的无序度。用X射线光电子能谱仪(XPS)和X射线衍射仪(XRD)分析材料的表面化学状态。
使用电化学工作站(Bio-Logic VMP3)测试材料的电化学性能。将所得材料裁剪成尺寸为1 cm × 1.2 cm的长方形,以海水为电解质组装三电极体系体系,用铂片电极夹固定材料作为工作电极,尺寸为10 mm × 10 mm × 0.2 mm的铂片作为对电极,饱和Ag/AgCl电极作为参比电极。将体系分别在饱和氧气和饱和氮气分中用循环伏安(CV)法进行电化学测试,扫描速率为5 mV/s,扫描范围为0.2 V~-1 V vs. Ag/AgCl。电化学活性表面积(ECSA)与双电层电容值(Cdl)成正相关,在开路电位的上下50 mV范围内使用不同的扫速进行CV测试,计算拟合后可得材料的Cdl。将材料与镁合金(AZ31)金属板组装成电池,进行抗极化性能测试。用4 mg的20%Pt/C催化剂、0.375 mL超纯水、0.125 mL乙醇和50 μL的5%萘酚配成油墨,以100 μL/cm2的密度滴到预处理后的碳布上作为参照组。将制备的材料裁剪成圆型,用萘酚溶液将其粘贴在旋转圆盘电极的铂碳电极上进行线性扫描伏安(LSV)的测试,用测试结果计算转移电子数和过氧化氢产率。
2 结果和讨论
2.1 材料的形貌
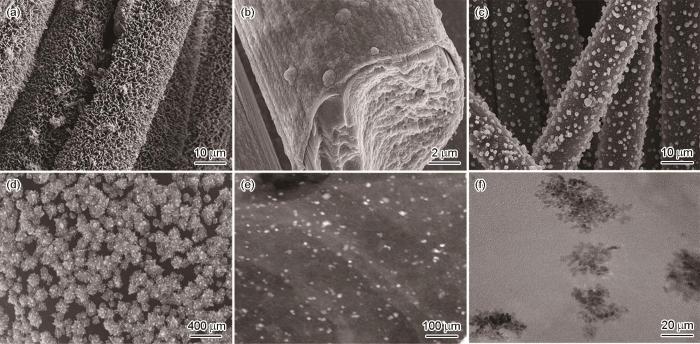
图1a表明,在碳布基底上均匀生长出的是ZIF8/67纳米片,夹杂着少量的纳米花。碳布被完整包裹无裸露,用超声清洗后依旧完整附着,表明ZIF与碳布之间的结合力较强。图1b给出了煅烧后材料的SEM照片。从碳布纤维的截面可观察到ZIF8/67复合材料在高温下坍塌均匀地覆盖和在包覆碳布表面,碳化层上的小凸起可能是纳米花高温煅烧后形成的。图1c和图1d给出了材料高温碳化和电沉积Pt后的SEM形貌。从图1c中的低倍SEM图像可见,电沉积后Pt以微米级颗粒状形貌存在于最外层(Pt负载量为1.23 mg/cm2)。从图1d中的高倍SEM图像可见,Pt以纳米花的形貌均匀覆盖在碳化层表面,尺寸约为200 nm。高曲率的纳米锥形貌具有尖端效应,有利于传质[24]。从图1e和图1f中更高倍率的TEM照片可见Pt在碳化层表面的纳米花形貌。Pt在碳化层表面的沉积过程可能以纳米花的形貌长大,形成较为致密的纳米花后以颗粒形貌继续均匀地沉积在纳米花表面,没有明显的团聚。均匀有序的形貌,有利于材料在海水中ORR催化性能的稳定。
图1
图1
ZIF8/67@CC和Co-N-C@CC的SEM照片、Pt@Co-N-C@CC的SEM照片以及Pt@Co-N-C@CC的TEM照片
Fig.1
SEM images of different composite materials (a) ZIF8/67 grown on carbon cloth; (b) ZIF8/67@CC after calcination, (c, d) electrodeposited Pt nanoclusters and (e, f)TEM images of Pt@Co-N-C@CC
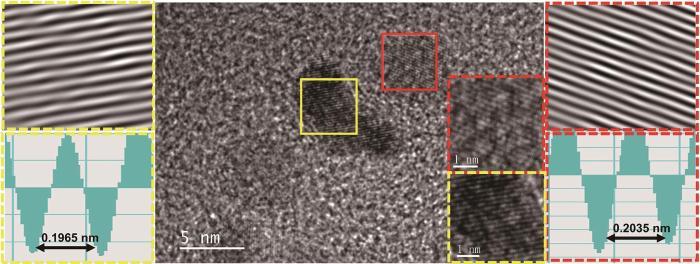
图2给出了Pt@Co-N-C@CC的高分辨率投射电镜(HRTEM)图像,在更微观的角度观察到催化材料表面晶格状态。根据傅里叶变换,确定了材料表面两处区域的晶面间距分别为0.1965 nm和0.2035 nm。这两个值可能分别对应Pt的(200)晶面和C的(101)晶面。标准Pt的(200)晶面间距为1.9690(PDF#87-0642)。图2中Pt的晶面间距更小,表明Pt与碳化层之间存在微观应力。这种应力可能影响Pt的电子构型,使其d带中心下降而使ORR的反应活性提高。在图中未找到Co元素对应的晶面,可能是材料表面均匀覆盖的铂纳米花掩盖了Co元素,Pt与Co不是以合金的形式存在于碳布表面,而是形成了Pt@Co-N-C@CC多壳层结构。
图2
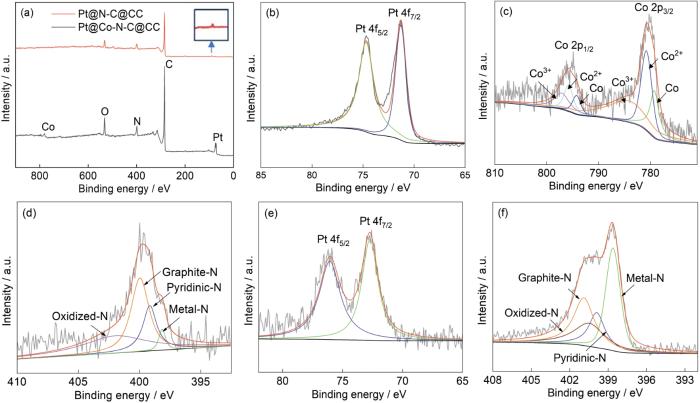
图3给出了用XPS对Pt@Co-N-C@CC和Pt@N-C@CC复合阴极表面的化学元素组成和化学键状态的分析结果。图3a表明,Pt@Co-N-C@CC复合阴极含有Pt、C、N、O、Co等元素,Pt@N-C@CC复合阴极含有Pt、C、N等元素。ZIF8/67材料中的Zn元素在高温下气化蒸发,只剩下Co元素。图3b和图3e分别给出了Pt@Co-N-C@CC和Pt@N-C@CC的Pt 4f精细谱。与标准Pt 4f7/2(70.90 eV) 相比,图中Pt的峰发生了左移,Pt与碳化层中不同元素相互作用或碳化层表面的缺陷都影响XPS中Pt的峰位。可以看出,Pt以单质的形式存在,正向偏移表明材料中Pt原子的d带中心下移[25,26]。与图2给出的信息相同,表明碳化层改变了Pt原子的电子结构。图3c给出了Pt@Co-N-C@CC复合阴极中Co元素的精细谱,Co 2p谱图中出现了六个峰,其中796.50 eV和780.90 eV处的峰分别对应Co的两个卫星峰Co 2p1/2与Co 2p3/2的结合能,其余的卫星峰源于为Co0和Co3+呈现出正电性。在Pt纳米颗粒连接处的电荷效应使两层级之间产生更强的作用力,可优化传质效率[27]。图3d和图3f分别给出了Pt@Co-N-C@CC和Pt@N-C@CC复合阴极中N的精细谱。可以看出,两种材料中的N以吡啶N、吡咯N、石墨N和金属N的形式存在。吡啶N有利于降低ORR反应的激活能,且稳定不易变性分解[28]。图3d中吡啶N的相对含量比图3f中的更高,有利于ORR的催化。这表明,与ZIF8相比,ZIF67/ZIF8复合材料在高温下煅烧后更容易产生吡啶N,有利于ORR的催化。
图3
图3
Pt@N-C@CC与Pt@Co-N-C@CC的XPS总谱图、Pt@Co-N-C@CC的Pt,Co,N的精细谱图以及Pt@N-C@CC的Pt,N精细谱
Fig.3
XPS full spectrum of Pt@N-C@CC and Pt@Co-N-C@CC (a); XPS fine spectra of Pt 4f, Co 2p and N 1s for Pt@Co-N-C@CC (b~d); XPS fine spectra of Pt 4f for Pt@N-C@CC (e) and XPS fine spectra of N 1s for Pt@N-C@CC (f)
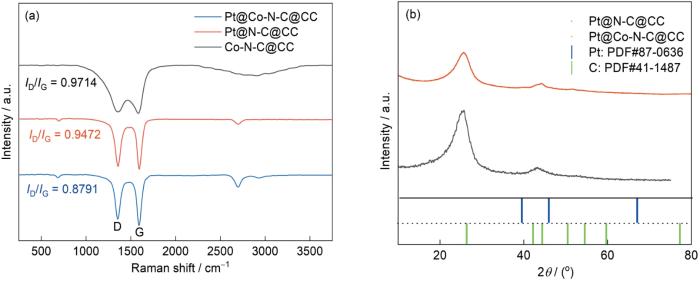
图4a给出了Pt@Co-N-C@CC、Pt@N-C@CC和Co-N-C@CC材料的拉曼光谱。可以看出,D带分别出现在波数1591 cm-1、1594 cm-1和1583 cm-1处,而G带分别出现在的波数1353 cm-1、1359 cm-1和1360 cm-1处。镀Pt后材料的D带和G带都向低波数位移,即发生了红移。出现红移的原因,可能是电沉积后产生共振力或者量子化能。ID/IG值的降低表明,材料镀铂使缺陷减少,Pt优先电沉积在碳化层的缺陷处。Pt@Co-N-C@CC和Pt@Co-N-C@CC在波数695 cm-1处出现的峰,是Pt与碳化层上的元素键合后发生对称伸缩振动所致。Co-N-C@CC在2800 cm-1处的宽峰与C-H的伸缩振动有关,高温煅烧后消失。在2600 cm-1处出现的新峰,可能与晶格振动有关。图4b给出了Pt@Co-N-C@CC和Pt@Co-N-C@CC的XRD谱。与C的XRD标准卡片比较,出现在2θ =26.4°的衍射峰对应C的(002)晶面,42.2°处的峰对应C的(100)晶面。未出现明显Pt的衍射峰,可能是Pt纳米颗粒中同时出现非晶态或者单晶态区域所致[29,30]。
图4
图4
Pt@Co-N-C@CC, Pt@N-C@CC,Co-N-C@CC的拉曼图像以及Pt@Co-N-C@CC和Pt@N-C@CC的XRD谱
Fig.4
Raman spectra of Pt@Co-N-C@CC, Pt@Co-N-C@CC, Co-N-C@CC (a) and XRD patterns of Pt@Co-N-C@CC and Pt@N-C@CC (b)
2.2 材料的电化学活性
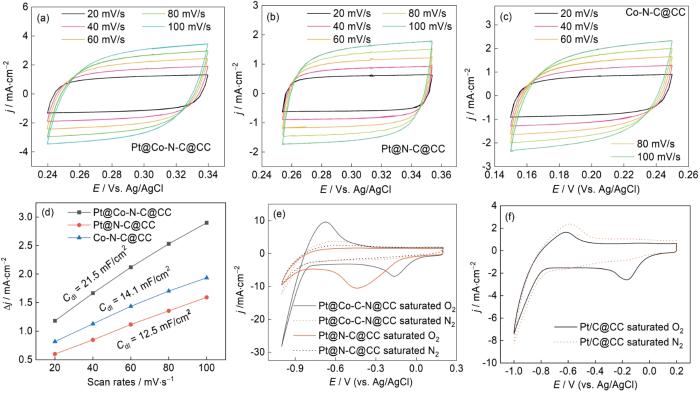
电化学活性表面积(ECSA)是表征材料催化性能的关键参数之一。为了简化ECSA的测量,用材料的双点层电容值(Cdl)与ECSA呈现正相关的关系定性分析ECSA。在开路电位上下50 mV范围内以不同的扫速进行CV测试,在开路电位下找到两个对应的电流密度绝对值之和除以2,以此值为纵坐标和以扫描速度为横坐标进行拟合,可求得Cdl。图5a给出了Pt@Co-N-C@CC在不同扫速下的CV图像[31]。对比图5b与图5c可见,催化材料Pt@N-C@CC的开路电位最高,约为0.3 V,Pt@Co-N-C@CC的开路电位为0.28 V。但是,Pt@Co-N-C@CC在较低的开路电位下激发的电流更大。Co/N/C@CC的开路电位最低,约为0.2 V,表明Pt的沉积使材料的开路电位提高。较高的开路电位有利于海水电池输出更高的电压。图5d给出了三种材料的Cdl,可见Pt@Co-N-C@CC的ECSA明显比Pt@N-C@CC和Co-N-C@CC的高。Pt与Co碳化层间良好的协同作用使Pt@Co-N-C@CC的活性位点更多。图5e和图5f分别给出了Pt@Co-N-C@CC和Pt@N-C@CC在饱和O2和饱和N2下的CV曲线。可以看出,两种催化剂在饱和O2条件下CV图的面积均比饱和N2条件下的大,表明材料具有出色的电容特性。在饱和N2条件下未观察到氧还原峰,且激发电流密度较小。Pt@Co-N-C@CC的氧还原电位约为-0.2 V,Pt@N-C@CC的氧还原电位为-0.45 V。Pt@Co-N-C@CC的氧还原电位更接近商业Pt/C催化剂的值(-0.16 V vs.Ag/AgCl)。Pt@N-C@CC的氧还原电流密度最大,其原因可能是在成分复杂的海水中在-0.45的电位下可能发生了其他副反应。两种材料在高负电位下都发生了析氢反应,但是Pt@Co-N-C@CC降低了析氢电位,且电流密度更大,表明Pt@Co-N-C@CC的活性更高。
图5
图5
Pt@Co-N-C@CC,Pt@N-C@CC, Co-N-C@CC在不同扫速下的CV图像、拟合所得的Cdl值以及Pt@Co-N-C@CC,Pt@N-C@CC与Pt/C-20%在饱和O2与饱和N2下的CV图像
Fig.5
CV plots of Pt@Co-N-C@CC, Pt@N-C@CC and Co-N-C@CC with different scanning speeds (a~c); double-layer capacitance values for the three materials (d) and CV plots of Pt@Co-N-C@CC, Pt@N-C@CC and Pt/C-20% in saturated oxygen and saturated nitrogen environments (e, f)
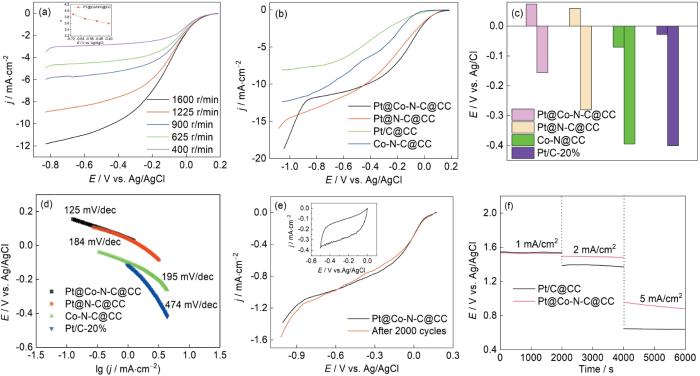
可计算此时电化学过程转移的电子数。式中j为测量的电流密度,jk,jl分别为动力学限制和扩散电流密度,ω为角速度,F为法拉第常数,C0为氧气在海水中的体积浓度,D为氧气在海水中的扩散系数[32]。根据K-L方程可确定Pt@Co-N-C@CC在ORR反应时的转移电子数。ORR反应涉及双电子过程和四电子过程,其中双电子过程形成中间产物过氧化氢,对反应不利,因此四电子过程是理想的反应途径。Pt@Co-N-C@CC在低于-0.7 V范围的反应电子数较高,大于3.9。图6b,c给出了四种不同的催化阴极在1600 r/min转速下的LSV曲线和对应的起始电位与半波电位。根据其结构,Pt@Co-N-C@CC的起始电位和半波电位分别为0.075 V和-0.156 V,商业Pt/C@CC的起始电位和半波电位分别为-0.028 V和-0.401 V。较高的起始电位和半波电位意味着其具有更优异的ORR催化性能。在高于-0.8 V时Pt@Co-N-C@CC材料的电流密度急剧提高,表明此时发生了与CV图像给出的结果相同的析氢反应。图6d给出了根据LSV曲线计算出的Tafel斜率。Pt@Co-N-C@CC的Tafel斜率最低,表明ORR催化性能更高。商业Pt/C催化剂虽然其ORR催化性能优异,但是海水中大量的Cl-使其效率降低和耐久性受到毒化。相比之下,Pt@Co-N-C@CC中的Pt元素以从纳米级到微米级的多晶态纳米花的形式均匀覆盖在碳化层上并与碳化层复合,减少了Cl-的吸附毒害作用,且碳化层耐腐蚀且有M-N-C催化位点,从而使Pt@Co-N-C@CC在海水中的电化学性能显著提高。图6e给出了Pt@Co-N-C@CC在饱和氧气和零转速条件下旋转圆盘电极的LSV曲线。可以看出,经过2000次的CV循环起始电位几乎没有变化只是半波电位略微降低,表明其耐久性很好。图6f给出了Pt@Co-N-C@CC与镁合金组装的海水电池的恒电流极化曲线。可以看出,在电流密度为1 mA/cm2的条件下Pt@Co-N-C@CC与Pt/C@CC的抗极化性能相似,其电压能稳定在1.5 V以上。随着电流密度提高到5 mA/cm2用Pt/C@CC组装的海水电池的电压锐减到0.5 V,Pt@Co-N-C@CC阴极的电压保持在0.8 V以上。这表明,Pt@Co-N-C@CC在高电流密度下的抗极化性能更优异。
图6
图6
Pt@Co-N-C@CC在不同扫速下的LSV、不同催化材料在1600 r/min下的LSV图像、不同催化材料的氧还原起始电位与半波电位、催化材料拟合所得的的Tafel斜率、Pt@Co-N-C@CC的稳定性测试以及Pt@Co-N-C@CC和Pt/C-20%@CC的恒电流极化测试
Fig.6
LSV plots of Pt@Co-N-C@CC at different speeds (a); LSV plots of four kinds of catalytic cathodes at 1600 r/min (b); onset potential and half-wave potential of catalytic cathode (c); Tafel slopes calculated from LSV of four catalytic cathodes (d); stability tests of Pt@Co-N-C@CC (e) and constant current polarization curves of Pt@Co-N-C@CC and Pt/C-20%@CC (f)
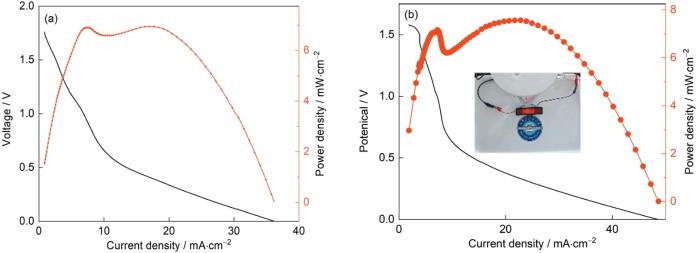
图7
图7
Pt/C-20%@CC和Pt@Co-N-C@CC的功率密度曲线
Fig.7
Power density of seawater battery assembled with Pt/C-20%@CC as cathode (a) and power density (b) of seawater battery assembled with Pt@Co-N-C@CC as cathode
3 结论
(1) 用生长-碳化煅烧-电沉积方法制备的Pt@Co-N-C@CC复合电极在海水中的ORR催化效果良好,能在一定程度上抑制Cl-对Pt基催化剂的毒化,还具有良好的抗极化性能。
(2) 用Pt@Co-N-C@CC复合电极组装的海水电池在5 mA/cm2的电流密度下的电压高于0.8 V,且具有较高的功率密度。合适设计海水溶解氧燃料电池的结构,可提高其输出电压。
参考文献
Marine chemical technology and sensors for marine waters: Potentials and limits
[J].
Power sources for autonomous underwater vehicles
[J].
Progress and applications of seawater-activated batteries
[J].
Microstructure and electrochemical corrosion behavior of extruded Mg-Al-Pb-La alloy as anode for seawater-activated battery
[J].
Pyridinic-nitrogen-containing carbon cathode: efficient electrocatalyst for seawater batteries
[J].
Rechargeable seawater batter-ies—from concept to applications
[J].
A high-specific-energy magnesium/water battery for full-depth ocean application
[J].
Strain engineering to enhance the oxidation reduction reaction performance of atomic-layer Pt on nanoporous gold
[J].
Kinetics of oxygen reduction reaction on three different Pt surfaces of Pt/C catalyst analyzed by rotating ring-disk electrode in acidic solution
[J].
Platinum stabilized by defective activated carbon with excellent oxygen reduction performance in alkaline media
[J].
Optimized hard carbon derived from starch for rechargeable seawater batteries
[J].
Electrochemical kinetics and X-ray absorption spectroscopy investigations of select chalcogenide electrocatalysts for oxygen reduction reaction applications
[J].
Use of carbon monoxide and cyanide to probe the active sites on nitrogen-doped carbon catalysts for oxygen reduction
[J].
Annealing-temperature-dependent relation between alloying degree, particle size, and fuel cell performance of PtCo catalysts
[J].
Adsorbate-induced surface segregation for core-shell nanocatalysts
[J].
Heteroatom doping regulates the catalytic performance of single-atom catalyst supported on graphene for ORR
[J].
The oxygen reduction reaction on a Pt/carbon fuel cell catalyst in the presence of chloride anions
[J].
Active sites engineering of Pt/CNT oxygen reduction catalysts by atomic layer deposition
[J].Understanding carbon-supported Pt-catalyzed oxygen reduction reaction (ORR) from the perspective of the active sites is of fundamental and practical importance.In this study,three differently sized carbon nanotube-supported Pt nanoparticles (Pt/CNT) are prepared by both atomic layer deposition (ALD) and impregnation methods.The performances of the catalysts toward the ORR in acidic media are comparatively studied to probe the effects of the sizes of the Pt nanoparticles together with their distributions,electronic properties,and local environments.The ALD-Pt/CNT catalysts show much higher ORR activity and selectivity than the impregnation-Pt/CNT catalysts.This outstanding ORR performance is ascribed to the well-controlled Pt particle sizes and distributions,desirable Pt<sup>0</sup> 4<i>f</i> binding energy,and the Cl-free Pt surfaces based on the electrocatalytic measurements,catalyst characterizations,and model calculations.The insights reported here could guide the rational design and fine-tuning of carbon-supported Pt catalysts for the ORR.
First-principles screening of Pt doped Ti2CNL (N = O, S and Se, L = F, Cl, Br and I) as high-performance catalysts for ORR/OER
[J].
Facet-orientated Pd core impels quasi-monolayer Pt shell to boost the oxygen-reduction electrocatalysis
[J].
Metal-organic framework (MOF)-derived catalysts for fine chemical production
[J].
Co porphyrin-based metal-organic framework for hydrogen evolution reaction and oxygen reduction reaction
[J].
Synergetic dual-ion centers boosting metal organic framework alloy catalysts toward efficient two electron oxygen reduction
[J].
Regulating the tip effect on single-atom and cluster catalysts: Forming reversible oxygen species with high efficiency in chlorine evolution reaction
[J].
One-pot synthesis of reduced graphene oxide supported hollow Ag@Pt core-shell nanospheres with enhanced electrocatalytic activity for ethylene glycol oxidation
[J].
Platinum-cobalt alloy networks for methanol oxidation electrocatalysis
[J].
Biomimetic and bioinspired synthesis of nanomaterials/nanostructures
[J].
The role of iron nitrides in the Fe-N-C catalysis system towards the oxygen reduction reaction
[J].
Covalent phenanthroline framework derived FeS@Fe3C composite nanoparticles embedding in N-S-codoped carbons as highly efficient trifunctional electrocatalysts
[J].
In-situ coupling FeN nanocrystals with Fe/Fe3C nanoparticles to N-doped carbon nanosheets for efficient oxygen electrocatalysis
[J].
Correlation between electrochemical performance degradation and catalyst structural parameters on polymer electrolyte membrane fuel cell
[J].
An oxygenophilic atomic dispersed Fe N C catalyst for lean-oxygen seawater batteries
[J].