离子交换膜是膜电解、电渗析、扩散渗析等膜分离技术的核心部件,利用其对阴/阳离子选择透过能力的不同可实现不同组分的分离、提纯、浓缩,广泛应用于海水淡化、电解水制氢、燃料电池以及有色金属回收等方面[1,2]。使用双膜三室电解法电沉积回收钴,含氧化剂的酸性环境对阳离子交换膜的抗氧化性和选择透过性要求较高[3,4]。传统阳离子交换膜(阳膜)的耐污染性低、选择透过性差,使其使用寿命和分离效率降低、成本和能耗提高[5]。为了提高阳膜的性能需将其改性,有掺混改性[6]和表面改性等方法[7]。表面改性,是在成品膜的基础上借助化学沉积、辐射或等离子体聚合等技术修饰膜表面,增加膜表面的功能基团或在阳膜表面生成改性层以提高其分离性能[8]。但是,采用表面改性制备的阳膜,其改性层不稳定和容易脱落[9]。
贻贝分泌的贻贝黏附蛋白能通过原位交联或硬化反应形成坚固的防水层,受到了极大的关注[10]。多巴胺(DA)能牢固地粘附在各种无机和有机材料上,因为DA中的儿茶酚和胺基官能团能模拟贻贝的粘附性并能在碱性溶液和氧气环境中发生自聚反应[11]。但是,DA在阳膜表面自聚合和沉积需要8~12 h甚至更长时间才能生成聚多巴胺(PDA)涂层[12]。同时,PDA长期沉积容易通过非共价键形成较大的聚集体而使阳膜的表面粗糙[13]。因此,较快地生成具有结构均匀性和界面亲水性的PDA表面层仍然较为困难。引入FeCl3[14]、CuSO4/H2O[15]和(NH4)2S2O8[16]等氧化剂,能较快地生成均匀的PDA层。氧化系统(如CuSO4/H2O2体系)能在碱性介质中产生活性氧(如羟基自由基),在DA聚合和随后PDA涂层的生成中起关键作用[17]。同时,Michael加成和Schiff碱反应使PDA易与聚乙烯亚胺(PEI)发生共价交联。引入PEI可使改性层更均匀,因为PEI能干扰PDA中非共价作用产生的聚集[18]。WANG[19]等进行CuSO4/H2O2的催化氧化在膜表面较快地生成了均匀的PDA-PEI层,但是这类芬顿反应的共沉积过程难以精确调控。CAO[20]等进行反向电沉积在膜表面交替构建4-苯乙烯磺酸钠和DA/PEI改性层,但是这个过程较为复杂且受电极反应的影响较大。本文以FeCl3为氧化剂沉积DA-PEI改性阳膜,研究DA与PEI的浓度比对其物化性能的影响。
1 实验方法
1.1 实验材料和仪器设备
实验材料:无水三氯化铁(FeCl3)、聚乙烯亚胺(PEI)、多巴胺(DA)、三(羟甲基)氨基甲烷(Tris);浓盐酸(HCl)、氯化钾(KCl)、氯化钠(NaCl)、30%过氧化氢(H2O2),均为分析纯。实验用水为纯水。商用阳膜的性能参数,列于表1。
表1 商用阳膜的性能参数
Table 1
| Membrane type | Thickness / mm | Moisture content / % | Ion exchange capacity / mol·kg-1 | Selective permeability / % | Membrane resistance / Ω·cm2 |
|---|---|---|---|---|---|
| IONSEP-MC-C | 0.42 | < 40 | > 2.4 | > 92 | < 10 |
仪器设备:VERTEX70型红外-拉曼光谱(FTIR),JSM6700F型扫描电子显微镜(SEM);DT-830型数字万用表;PGSTAT128N型Autolab电化学工作站;UV2600A型紫外可见分光光度计。
1.2 商用基膜的改性
(1) 将尺寸为5 cm × 5 cm的商用基膜浸在3%NaCl溶液中24 h,用纯水冲洗干净后置于浓度为0.001 mol/L的FeCl3溶液中,磁力搅拌1 h使Fe3+连接到磺酸官能团。
(2) 配制Tris-PEI溶液:分别向体积为100 mL浓度为100 mmol/L 的Tris溶液中加入0.05 g、0.1 g、0.2 g及0.4 g的PEI,调节溶液的pH值为8.5,然后加入0.2 g的DA得到不同DA与PEI浓度比的DA-PEI溶液。
(3) 将预处理后的商用基膜浸入DA-PEI溶液中,磁力搅拌30 min在阳膜表面生成PDA-PEI改性层。将原基膜命名为M0,将用DA单独改性的膜命名为M1,将引入PEI改性的膜按浓度从低到高依次命名为M2、M3、M4、M5。
1.3 膜样品性能的表征
测试阳膜的迁移数及选择透过性:(1) 将阳膜裁剪成尺寸为5 cm × 5 cm的样品,将其浸泡于0.15 mol/L KCl溶液中使其转变为K型膜。(2) 将阳膜夹入测试装置(图1)中,两侧的电极均为Ag-AgCl电极。(3)在A室和B室分别充满0.1 mol/L和0.2 mol/L KCl溶液,用数字万用表测量两电极间的电位。
图1
阳膜的迁移数为
式中t+ 为阳离子迁移数,Em为数字万用表读数(mV);E0 (取25℃时的16.08 mV)为0.1 mol/L与0.2 mol/L KCl溶液理论电位值。
阳膜的选择透过性为
式中P为阳膜选择透过性,t为无浓度变化时通过待测膜的迁移数[23]。
测试阳膜的抗氧化性:(1) 剪裁表面没有针孔和裂纹的膜样品,使其尺寸为3 cm × 2 cm;将其表面用纯水洗净后置于温度为60℃的真空烘箱中24 h,取出后称其质量(记为M1)。(2) 将膜放入Fenton试剂中并置于温度为60℃的恒温水浴中72 h,用纯水洗净其表面后烘干至恒质量,将其记为M2。膜的抗氧化性为
式中ε为膜的氧化百分比,M1为氧化前干膜的质量,M2为氧化后干膜的质量。
2 结果和讨论
2.1 DA-PEI共沉积改性阳膜的机理
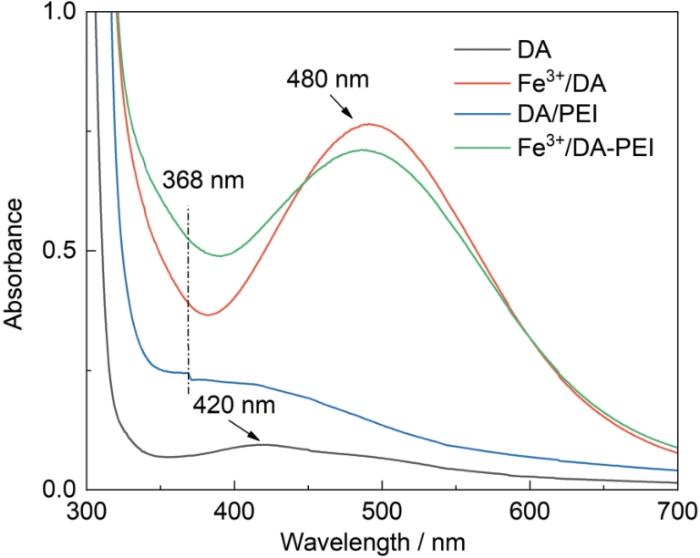
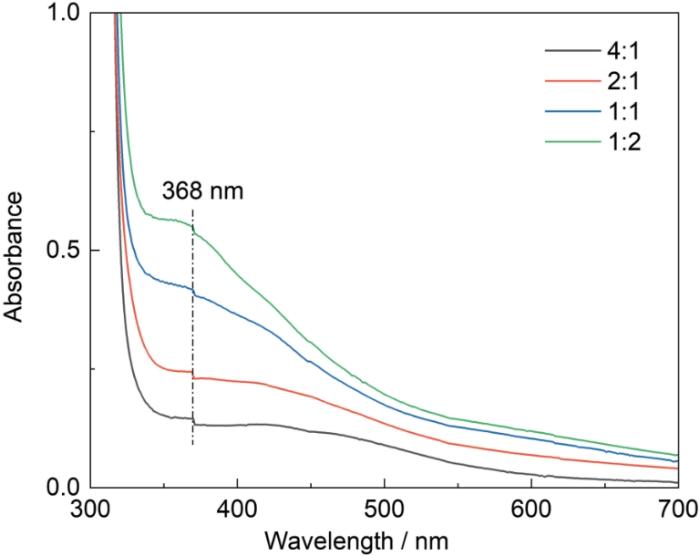
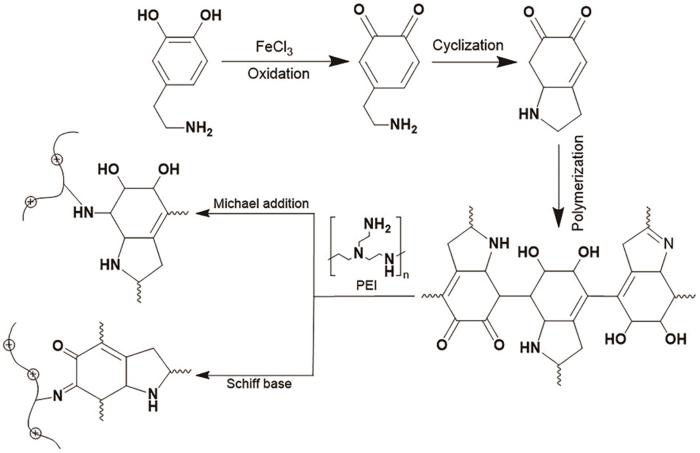
为验证氧化剂在改性过程中的氧化作用,测定了不同改性剂氧化10 min后的紫外-可见光谱,如图2所示。在DA溶液的谱中420 nm处出现一个较弱的特征吸收峰,可归因于DA聚合成PDA后分子中的C=C-C=O结构[24,25]。在DA溶液中加入FeCl3,在Fe3+/DA溶液谱中的480 nm处出现一个很强吸收峰,表明FeCl3氧化剂可诱导DA氧化聚合。DA和Fe3+/DA溶液的紫外吸收带红移,可归结为Fe3+/DA溶液中产生了Fe3+-PDA螯合物[26,27]。在DA/PEI溶液的紫外-可见光谱中368 nm处出现一个较强的肩峰,与溶液中的C=C-C=N结构有关。在DA/PEI溶液中加入氧化剂,使Fe3+/DA-PEI溶液的谱中在368 nm处的吸光度增大,表明FeCl3可氧化加速共沉积反应[25]。保持DA浓度2 g/L不变且不加氧化剂,依次改变PEI浓度得到图3给出的紫外-可见光谱。可以看出,随着PEI浓度的提高谱中368 nm处的吸光度增大。这表明,改变PEI的浓度可调节多巴胺的聚合速率。DA-PEI共沉积的反应原理,如图4所示。
图2
图3
图3
不同DA与PEI浓度比溶液的紫外-可见光谱
Fig.3
UV-Vis spectra of solutions with different concentration ratios of DA and PEI
图4
2.2 DA-PEI共沉积改性膜的含水率和离子交换容量
图5
图5
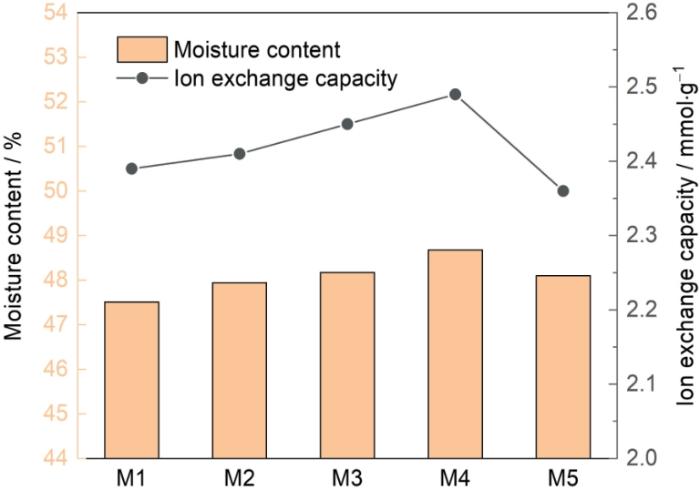
不同DA与PEI浓度比对改性膜含水率和离子交换容量的影响
Fig.5
Influence of different concentration ratios of DA and PEI on water content and ion exchange capacity
PEI的浓度提高,使离子交换容量呈现先上升后下降的趋势。浓度比为1∶1的膜离子交换容量最高。其原因是,随着PEI含量的提高阳膜表面沉积的改性层增加,DA中丰富的酚羟基增加了可交换基团的数量,使荷电密度和离子交换容量提高。但是低于1∶1的浓度比使DA与PEI聚合沉积效果较差和改性层疏松,从而使阳膜的离子交换容量降低[29]。
2.3 DA-PEI共沉积改性膜的迁移数和选择透过性
图6
图6
DA与PEI浓度比对改性膜迁移数和选择透过性的影响
Fig.6
Effects of different concentration ratios of DA and PEI on migration number and selective permeability
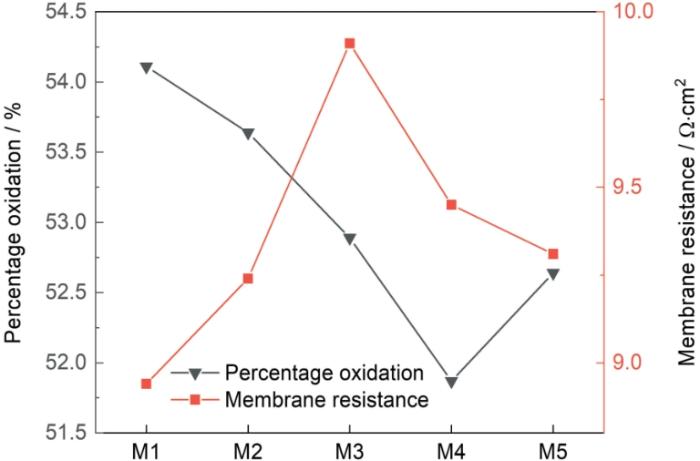
2.4 DA-PEI共沉积改性膜的膜电阻和抗氧化性
图7
图7
DA与PEI浓度比对改性膜抗氧化性和膜电阻的影响
Fig.7
Effects of different concentration ratios of DA and PEI on oxidation resistance and membrane resistance
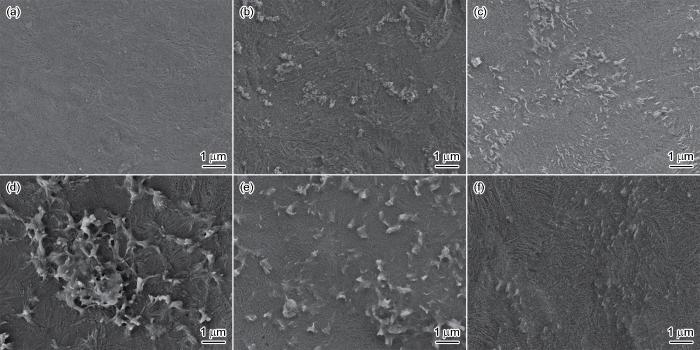
图8
图8
原膜和不同浓度比的改性膜的SEM照片
Fig.8
SEM images of original membrane and modified membrane with different concentration ratio (a) M0, (b) M1, (c) M2, (d) M3, (e) M4 and (f) M5
2.5 DA-PEI共沉积改性膜的表面形貌
图8给出了原膜和用不同浓度的改性剂处理30 min后改性膜的表面形貌。可以看出,原膜的表面较为平整,而DA单独改性的膜表面黏附少量的纳米级球形聚集体。这表明,在Fe3+的作用下DA沉积聚合生成PDA,PDA的非共价作用产生的团聚效应使膜的表面更加粗糙。PEI的引入,使球形团块转变为PDA和PEI聚集体(图8c、8d)。其原因是,具有亲水性胺基基团的PEI分子通过Michael加成或Schiff碱反应与PDA交联,使其聚集产生更多的分支结构[32,33]。继续提高PEI浓度,则改性膜表面出现峰谷形貌(图8e),沉积物颗粒在膜表面的分布更加均匀。这表明,改变PEI的浓度可调控膜表面形貌,从而改变膜的选择透过性。
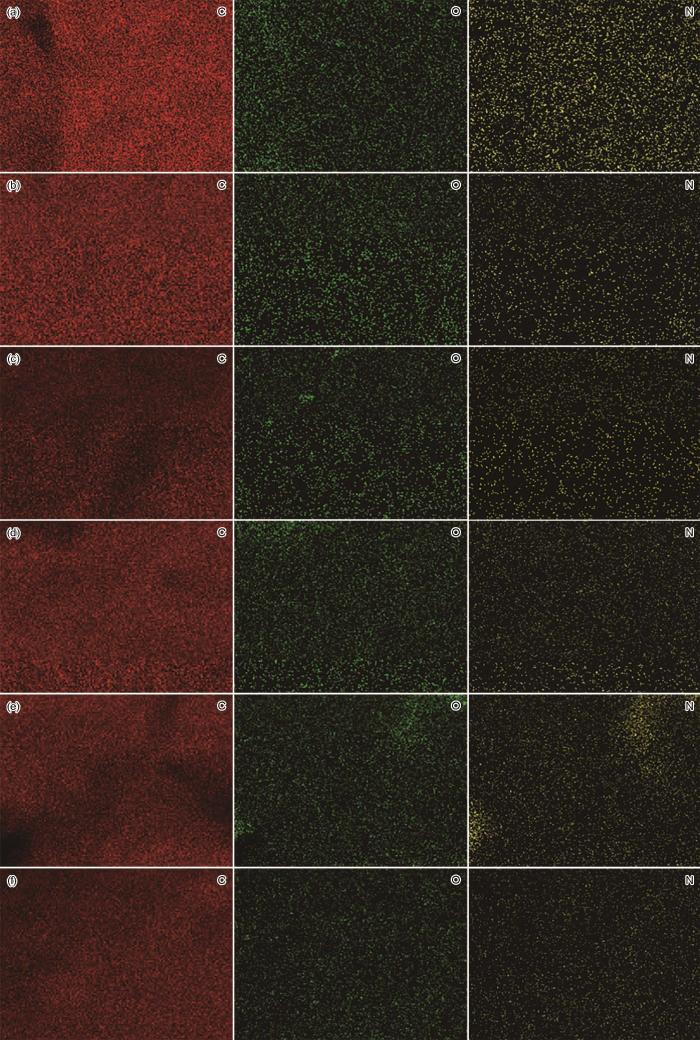
2.6 DA-PEI共沉积改性膜面元素的含量和分布
表2 原膜和改性膜表面元素的含量
Table 2
| C | O | N | |
|---|---|---|---|
| M0 | 95.0 | 4.8 | 0 |
| M1 | 94.7 | 5.0 | 0.2 |
| M2 | 93.7 | 6.0 | 0.3 |
| M3 | 92.9 | 6.4 | 0.6 |
| M4 | 90.7 | 7.4 | 0.8 |
| M5 | 94.3 | 5.4 | 0.3 |
图9
图9
原膜和改性膜的EDS元素面扫描图
Fig.9
EDS elemental surface scans of the original and modified membranes (a) M0, (b) M1, (c) M2, (d) M3, (e) M4 and (f) M5
2.7 DA-PEI共沉积改性膜表面的化学组成
图10
图10
原膜和不同DA与PEI浓度比的改性膜的红外光谱
Fig.10
Original membrane and the modified membranes with different concentration ratios of DA and PEI
3 结论
(1) 在DA-PEI表面共沉积改性阳膜,使用FeCl3氧化剂可加速改性层的生成。
(2) 随着PEI浓度的提高,改性膜表面逐渐形成聚集体形貌;改性膜的氧化百分比先降低后提高;改性膜的含水率、离子交换容量、选择透过性和膜电阻均呈先增加后降低趋势。
(3) DA与PEI浓度比为1∶1的改性膜选择透过性高达97.8%、抗氧化能力较高、膜电阻略比原膜的电阻高。
参考文献
Preparation of NASICON-structured NaTi2(PO4)3 material and device assembly and reduction/recovery of trace reducible metal ions in water
[D].
NASICON型NaTi2(PO4)3材料的制备、器件组装及其对水体中微量可还原金属离子的还原回收
[D].
A hierarchical model for novel schemes of electrodialysis desalination
[J].A new hierarchical model for the electrodialysis (ED) process is presented. The model has been implemented into gPROMs Modelbuilder (PSE), allowing the development of a distributed-parameters simulation tool that combines the effectiveness of a semi-empirical modelling approach to the flexibility of a layered arrangement of modelling scales. Thanks to its structure, the tool makes possible the simulation of many different and complex layouts, requiring only membrane properties as input parameters (e.g. membrane resistance or salt and water permeability). The model has been validated against original experimental data obtained from a lab scale ED test rig. Simulation results concerning a 4-stage treatment of seawater and dynamic batch operations of brackish water desalination are presented, showing how the model can be effectively used for predictive purposes and for providing useful insights on design and optimisation.
Electrodeposition of cobalt in double-membrane three-compartment electrolytic reactor
[J].
Ion transport for electrodeposition of cobalt in double-membrane three-compartment electrolytic cell
[J].
双膜三室电解槽中电沉积钴的离子传输
[J].
Surface modifications for antifouling membranes
[J].
Enhancement of lithium extraction from low grade brines by highly hydrophilic blend membranes using MnO2 ion sieve as adsorbents
[J].
Progress in the modification of ion-exchange membranes
[J].
离子交换膜改性的研究进展
[J].
Monovalent cation selective membranes: state and development perspective
[J].
单价选择性阳离子交换膜的研究进展
[J].
Surface modification of ion-exchange membranes: methods, characteristics, and performan-ce
[J].
Ultrathin and stable active layer of dense composite membrane enabled by poly(dopamine)
[J].We demonstrate that dopamine is able to self-polymerize and adhere firmly onto the substrate, which can create a hierarchical structure comprising an ultrathin active layer and a porous support layer. Such an approach opens a novel way to fabricating highly efficient and stable composite materials including composite membranes. More specifically, in this study the composite membranes are fabricated by simply dipping microporous substrate in aqueous dopamine solution under mild conditions. Nanoindentation measurement reveals the tight adhesion of dopamine onto microporous substrate, which is ascribed to numerous pi-pi and hydrogen-bonding interactions. The chemical composition of the active layer is analyzed by XPS, which demonstrates the self-polymerization of dopamine. The water contact angle of the dopamine coated membranes is reduced remarkably compared with that of the uncoated counterpart. Stylus profiler measurements display that the poly(dopamine) thickness increases as the coating time increases. FESEM images of the membranes' cross section show that an active layer (<100 nm) is deposited on the porous polysulfone (PS) substrate. Positron annihilation spectroscopy (PAS) is introduced to probe the fractional free volume properties throughout the cross section of the composite membranes and reveal that after dopamine double-coating the active layer becomes thicker and more compact. Moreover, pH and concentration of the dopamine solution exert notable influence on the fractional free volume of the composite membranes. The as-prepared membranes are tentatively employed for pervaporative desulfurization and exhibits satisfying separation performance as well as durability. This facile, versatile, and efficient approach enables a promising prospect for the wide applications of such novel kinds of ultrathin composite materials.
Mussel-inspired surface chemistry for multifunctional coatings
[J].We report a method to form multifunctional polymer coatings through simple dip-coating of objects in an aqueous solution of dopamine. Inspired by the composition of adhesive proteins in mussels, we used dopamine self-polymerization to form thin, surface-adherent polydopamine films onto a wide range of inorganic and organic materials, including noble metals, oxides, polymers, semiconductors, and ceramics. Secondary reactions can be used to create a variety of ad-layers, including self-assembled monolayers through deposition of long-chain molecular building blocks, metal films by electroless metallization, and bioinert and bioactive surfaces via grafting of macromolecules.
Dopamine-melanin film deposition depends on the used oxidant and buffer solution
[J].The deposition of "polydopamine" films, from an aqueous solution containing dopamine or other catecholamines, constitutes a new and versatile way to functionalize solid-liquid interfaces. Indeed such films can be deposited on almost all kinds of materials. Their deposition kinetics does not depend markedly on the surface chemistry of the substrate, and the films can reach thickness of a few tens of nanometers in a single reaction step. Up to now, even if a lot is known about the oxidation mechanism of dopamine in solution, only little information is available to describe the deposition mechanism on surfaces either by oxidation in solution or by electrodeposition. The deposition kinetics of melanin was only investigated from dopamine solutions using oxygen or ammonium persulfate as an oxidant and from a tris(hydroxymethyl) aminomethane (Tris) containing buffer solutions at pH 8.5. Many other oxidants could be used, and the buffer agent containing a primary amine group may influence the deposition process. Herein we show that the deposition kinetics of melanin from dopamine containing buffers at pH 8.5 can be markedly modified using Cu(2+) instead of O2 as an oxidant: the deposition kinetics remains linear up to thicknesses of more than 70 nm, whereas the film growth stops at 45 ± 5 nm in the presence of 02. In addition, the films prepared from Cu(2+) containing solutions display an absorption spectrum with defined peaks at 320 and 370 nm, which are absent in the spectra of films prepared in oxygenated solutions. The replacement of Tris buffer by phosphate buffer also has a marked effect on the melanin deposition kinetics.
Surface and interface engineering for advanced nanofiltration membranes
[J].
Fe3+ ions induced rapid co-deposition of polydopamine-polyethyleneimine for monovalent selective cation exchange membrane fabrication
[J].
Rapid co-deposition of dopamine and polyethyleneimine triggered by CuSO4/H2O2 oxidation to fabricate nanofiltration membranes with high selectivity and antifouling ability
[J].
Oxidant-induced dopamine polymerization for multifunctional coatings
[J].
UV-triggered dopamine polymerization: control of polymerization, surface coating, and photopatterning
[J].
Mussel-inspired modification of a polymer membrane for ultra-high water permeability and oil-in-water emulsion separation
[J].
High flux electroneutral loose nanofiltration membranes based on rapid deposition of polydopamine/polyethyleneimine
[J].
Composite modification of anion exchange membrane by in-situ layer-by-layer assembly to improve antifouling performance
[J].
Preparation and characterization of CoPc(COOH)8-SA/mCS bipolar membranes
[J].
CoPc(COOH)8-SA/mCS双极膜的制备及表征
[J].
Method for determining ion exchange membrane resistance for electrodialysis systems
[J].
Preparation, characterization and application of cation exchange membranes based sulfonated PVDF
[D].
PVDF磺化阳离子交换膜的制备、表征与应用研究
[D].
CuSO4/H2O2- induced rapid deposition of polydopamine coatings with high uniformity and enhanced stability
[J].Mussel-inspired polydopamine (PDA) deposition offers a promising route to fabricate multifunctional coatings for various materials. However, PDA deposition is generally a time-consuming process, and PDA coatings are unstable in acidic and alkaline media, as well as in polar organic solvents. We report a strategy to realize the rapid deposition of PDA by using CuSO4/H2O2 as a trigger. Compared to the conventional processes, our strategy shows the fastest deposition rate reported to date, and the PDA coatings exhibit high uniformity and enhanced stability. Furthermore, the PDA-coated porous membranes have excellent hydrophilicity, anti-oxidant properties, and antibacterial performance. This work demonstrates a useful method for the environmentally friendly, cost-effective, and time-saving fabrication of PDA coatings.© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Fe3+ ions induced rapid electrodeposition of polydopamine-polyethyleneimine for monovalent selective membrane fabrication
[J].
Fe3+诱导聚多巴胺-聚乙烯亚胺电沉积制备单价选择性膜
[J].
Tunable, metal-loaded polydopamine nanoparticles analyzed by magnetometry
[J].
Fe(III)-coordination properties of neuromelanin components: 5, 6-dihydroxyindole and 5, 6-dihydroxyindole-2-carboxylic acid
[J].The Fe(III)-coordination chemistry of neuromelanin building-block compounds, 5,6-dihydroxyindole (DHI), 5,6-dihydroxyindole-2-carboxylic acid (DHICA), and 5,6-dihydroxy-N-methyl-indole (Me-DHI), and the neurotransmitter dopamine were explored in aqueous solution by anaerobic pH-dependent spectrophotometric titrations. The Fe(III)-binding constants and pH-dependent speciation parallel those of catechol in that mono, bis, and tris FeLx species are present at concentrations dependent on the pH. The bis FeL2 dihydroxyindole species are favorable for L = DHI and DHICA under neutral to mildly acidic conditions. DHI and DHICA are stronger Fe(III) chelates than catechol, dopamine, and Me-DHI at pH values from 3 to 10. Oxidation studies reveal that iron accelerates the air oxidation of DHI and DHICA.
Simultaneous improvement of the monovalent anion selectivity and antifouling properties of an anion exchange membrane in an electrodialysis process, using polyelectrolyte multilayer deposition
[J].
Study on the modification and properties of polyethylene anion exchange membrane
[D].
聚乙烯阴离子交换膜的改性及性能研究
[D].
Comparison of synthetic dopamine-eumelanin formed in the presence of oxygen and Cu2+ cations as oxidants
[J].
Preparation, characterization and application of organic-inorganic hybrid SiO2 cation exchange membranes
[D].
有机-无机杂化SiO2阳离子交换膜的制备、表征与应用研究
[D].
Surface characteristics of a self-polymerized dopamine coating deposited on hydrophobic polymer films
[J].This study aims to explore the fundamental surface characteristics of polydopamine (pDA)-coated hydrophobic polymer films. A poly(vinylidene fluoride) (PVDF) film was surface modified by dip coating in an aqueous solution of dopamine on the basis of its self-polymerization and strong adhesion feature. The self-polymerization and deposition rates of dopamine on film surfaces increased with increasing temperature as evaluated by both spectroscopic ellipsometry and scanning electronic microscopy (SEM). Changes in the surface morphologies of pDA-coated films as well as the size and shape of pDA particles in the solution were also investigated by SEM, atomic force microscopy (AFM), and transmission electron microscopy (TEM). The surface roughness and surface free energy of pDA-modified films were mainly affected by the reaction temperature and showed only a slight dependence on the reaction time and concentration of the dopamine solution. Additionally, three other typical hydrophobic polymer films of polytetrafluoroethylene (PTFE), poly(ethylene terephthalate) (PET), and polyimide (PI) were also modified by the same procedure. The lyophilicity (liquid affinity) and surface free energy of these polymer films were enhanced significantly after being coated with pDA, as were those of PVDF films. It is indicated that the deposition behavior of pDA is not strongly dependent on the nature of the substrates. This information provides us with not only a better understanding of biologically inspired surface chemistry for pDA coatings but also effective strategies for exploiting the properties of dopamine to create novel functional polymer materials.© 2011 American Chemical Society
Low-pressure electroneutral loose nanofiltration membranes with polyphenol-inspired coatings for effective dye/divalent salt separation
[J].Low-pressure electroneutral loose nanofiltration (NF) membranes were facilely fabricated by co-deposition of polyphenol-inspired epigallocatechin gallate (EGCg) and polyethyleneimine (PEI) on polyethersulfone (PES) ultrafiltration substrates. The effects of PEI with different molecular weights on co-deposition behavior, morphologies, surface properties, as well as separation performance of the resultant membranes were investigated in detail. The results showed that with the increase of PEI molecular weight from 600 to 10,000, the co-deposition amount of EGCg/PEI on membrane surface decreased, while the EGCg/PEI selective layer became denser. As a result, the EGCg/PEI 600 co-deposited membrane, which possessed an electroneutral surface with the best hydrophilicity, exhibited the optimal separation performance among the co-deposited membranes in the context of textile wastewater treatment. It had the highest pure water permeability (19.0 L/(m(2).h.bar)) and excellent rejections towards dyes with different charges (e.g. 99.0% for Congo Red) as well as the lowest rejections towards divalent salts (e.g. 4.1% for Na2SO4) at an operating pressure of 2 bar. Moreover, the membranes exhibited good organic solvent resistance, structural stability together with favorable performance in dealing with textile effluent, demonstrating their great potential in practical applications.
Study on intelligent modification of membrane surface based on membrane cleaning response
[D].
基于膜清洗响应的膜表面智能改性研究
[D].
Mussel-inspired coatings directed and accelerated by an electric field
[J].
Super-hydrophilic TiO2-based coating of anion exchange membranes with improved antifouling performance
[J].
Electrosynthesis of polydopamine-ethanolamine films for the development of immunosensing interfaces
[J].We report a straightforward and reproducible electrochemical approach to develop polydopamine-ethanolamine (ePDA-ETA) films to be used as immunosensing interfaces. ETA is strongly attached to polydopamine films during the potentiodynamic electropolymerization of dopamine. The great advantage of the electrochemical methods is to generate the oxidized species (quinones), which can readily react with ETA amine groups present in solution, with the subsequent incorporation of this molecule in the polymer. The presence of ETA and its effect on the electrosynthesis of polydopamine was accessed by cyclic voltammetry, ellipsometry, atomic force microscopy, FTIR and X-ray photoelectron spectroscopy. The adhesive and biocompatible films enable a facile protein linkage, are resilient to flow assays, and display intrinsic anti-fouling properties to block non-specific protein interactions, as monitored by real-time surface plasmon resonance, and confirmed by ellipsometry. Immunoglobulin G (IgG) and Anti-IgG were used in this work as model proteins for the affinity sensor. By using the one-step methodology (ePDA-ETA), the lower amount of immobilized biorecognition element, IgG, compared to that deposited on ePDA or on ETA post-modified film (ePDA/ETA), allied to the presence of ETA, improved the antibody-antigen affinity interaction. The great potential of the developed platform is its versatility to be used with any target biorecognition molecules, allowing both optical and electrochemical detection.
Nanofiltration membranes via co-deposition of polydopamine/polyethylenimine followed by cross-linking
[J].