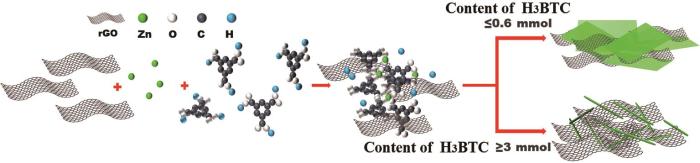
石墨烯是一种具有独特二维结构的碳同素异形体材料,具有优良的机械性能、较高的电子迁移率(2 × 105 cm2/(v·s))和电导率(106 S/m)以及较大的比表面(2630 m2/g)[13~17],可用做MOFs载体[18~20]。用做MOFs载体时,石墨烯的π-π共轭结构能促进电子的自由移动,使复合材料的导电性能及电化学性能提高[21]。Thi等[22]用水热法制备的Zn-MOF-rGO20复合材料电极在1 A·g-1的电流密度下比电容为205 C·g-1。同时,由Zn-MOF-rGO20电极组成的对称器件其比电容为82.5 F·g-1。Nguyen等[23]用水热法制备的rGO/Zn-MOF@PANI复合电极材料,电流密度为0.1 A·g-1时比电容为372 F·g-1。
目前关于Zn-BTC在超级电容器中应用的报道较少。本文用超声震荡法制备不同形貌的Zn-BTC/石墨烯复合材料,研究其作为超级电容器对称器件的电化学性能。
1 实验方法
1.1 实验用材料
分析纯化学试剂:ZnO、H2SO4、NaNO3、KMnO4、DMF、PTFE、乙炔炭黑以及阿拉丁试剂;300目的鳞片石墨和实验室自制去离子水。
1.2 石墨烯的制备
用改进的Hummers方法制备氧化石墨烯(Graphene oxide,GO)[24]。先在烧杯中加入1 g鳞片石墨(纯度99.9%)和0.5 g硝酸钠,然后加入70 mL浓H2SO4,在冰浴条件下搅拌2 h;向混合溶液中缓慢加入18 g的KMnO4,在冰浴条件下反应2 h;然后撤去冰浴,将混合溶液转移到35℃水浴条件下继续反应1 h;向上述反应体系中缓慢加入230 mL去离子水,控制反应温度为98℃反应30 min;最后加入20 mL双氧水移除未反应的KMnO4,此时溶液呈亮黄色,反应1 h。用离心的方式用去离子水彻底洗涤得到的反应产物以去除其中未反应的杂质离子,使溶液呈中性。最后将得到的产物在60℃干燥24 h得到GO样品。将制备的GO样品在350℃和5%氢氩混合气氛中焙烧2 h,得到石墨烯(Graphene,rGO)。
1.3 一维和二维Zn-BTC材料的制备
参考文献[25]的工作制备Zn-BTC材料:将不同质量(630 mg和126 mg)的1,3,5-苯三甲酸与30 mL无水乙醇在烧杯中混合后进行超声震荡使其完全溶解,再将243 mg (3 mmol)的纳米氧化锌和30 mL去离子水加入烧杯并在室温下超声混合8 h。将溶液过滤后得到的产物在110℃干燥12 h,制得一维Zn-BTC(Zn-BTC-1D)和二维Zn-BTC(Zn-BTC-2D)材料。
1.4 石墨烯负载一维、二维Zn-BTC复合材料的制备
在上述制备Zn-BTC-1D和Zn-BTC-2D材料的过程中按照每1 mmol Zn2+:30 mg GO的比例添加GO,将超声8 h后溶液过滤后得到的产物干燥(110℃)后在300℃和5%氢氩混合气氛中焙烧2 h,分别得到Zn-BTC/rGO-1D和Zn-BTC/rGO-2D两种复合材料。复合材料的制备过程如图1所示。
图1
图1
制备Zn-BTC/rGO复合材料的示意图
Fig.1
Schematic illustration of the synthesis process of Zn-BTC/rGO nanocomposite
1.5 性能表征
用XRD谱(PANalytical X'pert with CuKαλ =0.154178 nm)表征样品的物相;用场发射扫描电子显微镜(FESEM, ZEISS-ΣIGMA HD)观察样品的形貌;用JW-BK112型比表面积和微孔分析仪分析样品的比表面积,先将样品在200℃真空脱附处理2 h,以N2为吸附质在-196℃测试。使用Brunauer-Emmett-Teller (BET)模型计算样品的比表面积。用拉曼光谱仪(NEXUS 670)观察样品的化学键。用同步热分析仪(NETZSCH,STA449F3)在氮气气氛中对样品进行综合热分析,初始温度45℃,终止温度为600℃,升温速率为10℃/min。
在80 mg活性物质(rGO,Zn-BTC/rGO-1D,Zn-BTC/rGO-2D,Zn-BTC-1D)、10 mg粘结剂(聚四氟乙烯(PTFE))和10 mg导电剂(炭黑)中加入适量的N-甲基吡咯烷酮(NMP),充分研磨后得到分散均匀的浆料。把浆料均匀地涂敷在泡沫镍集流体(涂敷面积约为1 cm × 1 cm,质量约为2 mg)上,将其在120℃真空干燥12 h后得到工作电极。
使用CHI 660E电化学工作站进行电化学表征,采用Hg/HgO为参比电极、铂片电极为对电极、活性物质为工作电极,电解液为3 mol/L的 KOH。采用循环伏安法(CV)、电化学阻抗法(EIS)、恒流充放电法(GCD)和循环稳定性试验测试电化学性能。EIS测量频率范围为0.001~100 kHz,振幅为5.0 mV,参考开路电位。
比容量为
用活性物质极片组装成两电极Swagelok型对称超级电容器,水系电解液为3 mol/L KOH。用隔膜将其隔开。对称超级电容器的器件的比容量、能量密度和功率密度分别为
式中C为电极材料比容量,C·g-1;Ccell为超级电容器器件比容量,F·g-1;I为恒定放电电流,A;Δt为放电时间,s;V为电势,V;m为活性物质质量,g;E为能量密度,Wh·kg-1;P为功率密度,W·kg-1。
2 结果和讨论
2.1 微观结构
图2a~d给出了rGO,Zn-BTC-1D,Zn-BTC/rGO-1D和Zn-BTC/rGO-2D样品的SEM照片。可以看出,rGO样品呈卷曲的薄片状,纳米片表面有明显的褶皱(图1a)。从图1b可见,Zn-BTC-1D材料为棒状的一维结构,直径约为4~6 μm,长度约为8~20 μm,样品表面有明显的裂纹和缝隙。由Zn-BTC/rGO-1D样品的SEM照片(图2c)可见,rGO与Zn-BTC-1D正负电荷之间的相互作用使Zn-BTC-1D均匀地锚定在rGO纳米片表面,阻止了rGO纳米片层堆叠团聚。在Zn-BTC/rGO-1D样品中,Zn-BTC纳米棒的粒径小于其在单纯MOFs中的粒径(图2b),直径为0.3~1.2 µm,长度为1.5~7.0 µm。rGO可能限制了反应过程中Zn2+和有机配体的反应生长,减小了纳米复合材料中Zn-BTC-1D的粒径。由图2d可观察到,Zn-BTC-2D样品具有明显的二维结构,Zn-BTC纳米片堆叠在rGO表面。
图2
图2
rGO,Zn-BTC-1D,Zn-BTC/rGO-1D和Zn-BTC/rGO-2D的SEM照片
Fig.2
SEM images of rGO (a), Zn-BTC-1D (b), Zn-BTC/rGO-1D (c) and Zn-BTC/rGO-2D (d)
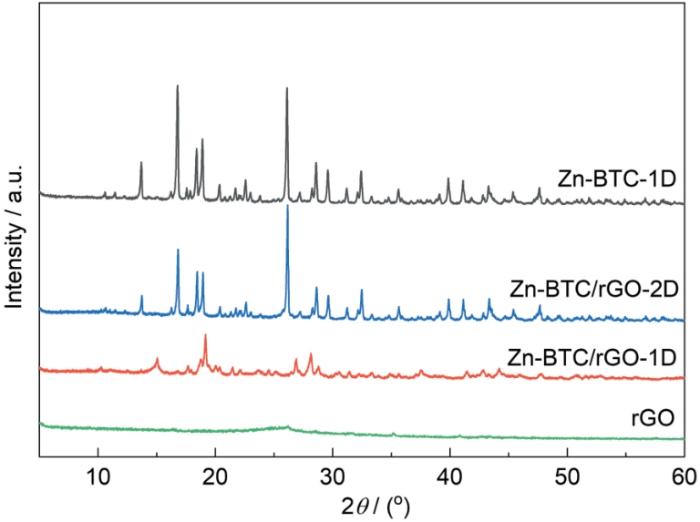
图3
图3
rGO、Zn-BTC-1D、Zn-BTC/rGO-1D和Zn-BTC/rGO-2D样品的XRD谱
Fig.3
XRD patterns of rGO, Zn-BTC-1D, Zn-BTC/rGO-1D and Zn-BTC/rGO-2D samples
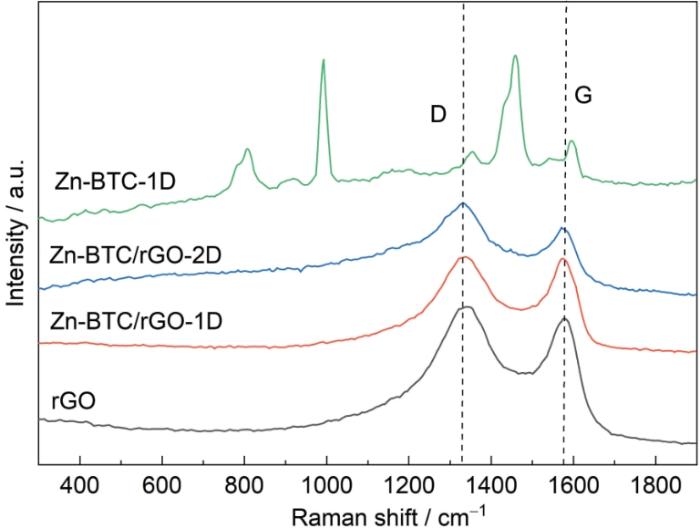
图4
图4
rGO,Zn-BTC/rGO-1D,Zn-BTC/rGO-2D,Zn-BTC-1D样品的Raman谱
Fig.4
Raman patterns of rGO, Zn-BTC/rGO-1D, Zn-BTC/rGO-2D and Zn-BTC-1D samples
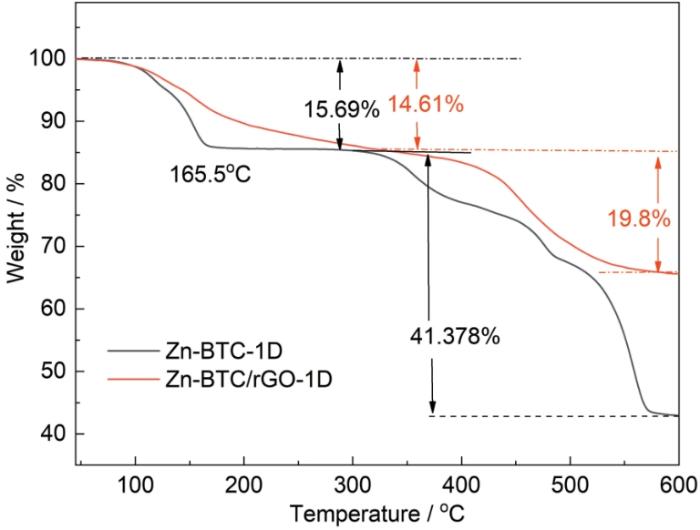
图5给出了Zn-BTC/rGO-1D和Zn-BTC-1D的TG曲线。可以看出,Zn-BTC-1D的TG曲线在升温过程中出现两个质量损失阶梯。温度升高到165℃,Zn-BTC-1D出现约10%的质量损失,是样品表面的吸附水和孔道内残留的有机溶剂分子挥发所致。从335℃开始有机骨架开始分解,温度高于450℃时Zn-BTC-1D发生剧烈的质量损失,对应材料内部的结晶有机材料分解。在600℃反应结束,Zn-BTC-1D和Zn-BTC/rGO-1D样品残留的质量分别为42.9%和65.6%。由此可以推算,Zn-BTC/rGO-1D样品中Zn-BTC的质量分数为60.3%。
图5
图5
Zn-BTC-1D和Zn-BTC/rGO-1D样品的TG曲线
Fig.5
TG curves of Zn-BTC-1D and Zn-BTC/rGO-1D samples
2.2 电化学性能
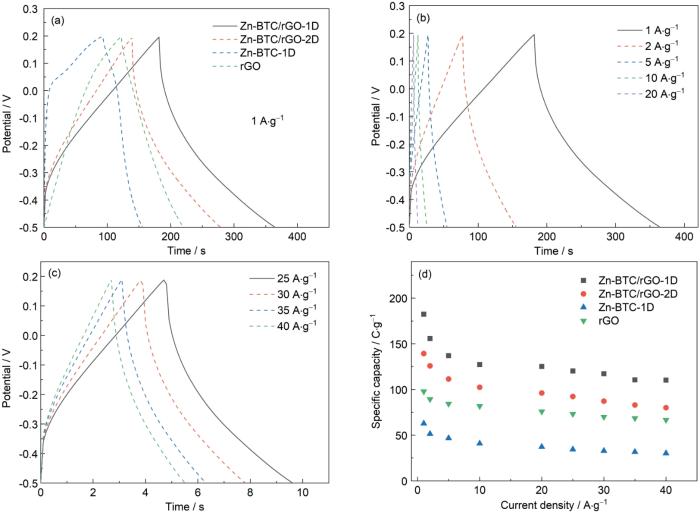
图6a给出了rGO、Zn-BTC-1D、Zn-BTC/rGO-2D和Zn-BTC/rGO-1D电极在电流密度为1 A·g-1条件下的恒流充放电(GCD)曲线。可以看出,Zn-BTC-1D样品的GCD曲线呈现出明显的非对称性,当充电电压在0~0.2 V时出现平台。另外三种样品的曲线均呈对称的线性三角形形状,表明其优异的电化学可逆性和良好的库伦效率。rGO的引入,使不同形貌的Zn-BTC的比表面积和导电性能提高。计算结果表明,rGO、Zn-BTC-1D、Zn-BTC/rGO-1D、Zn-BTC/rGO-2D电极在1 A·g-1的电流密度条件下的比电容分别为97.9、62.8、182.4和139.3 C·g-1。
图6
图6
使用三电极在3 mol/L KOH电解质中Zn-BTC/rGO-1D电极样品的GCD曲线和比容量
Fig.6
GCD curves of different samples at current density of 1 A·g-1 (a), GCD curves of Zn-BTC/rGO-1D electrode at different current densities from 1 to 40 A·g-1 (b, c), specific capacity at various current densities for all electrodes at the current density ranging from 1 to 40 A·g-1 (d) in 3 mol/L KOH electrolyte using three-electrode
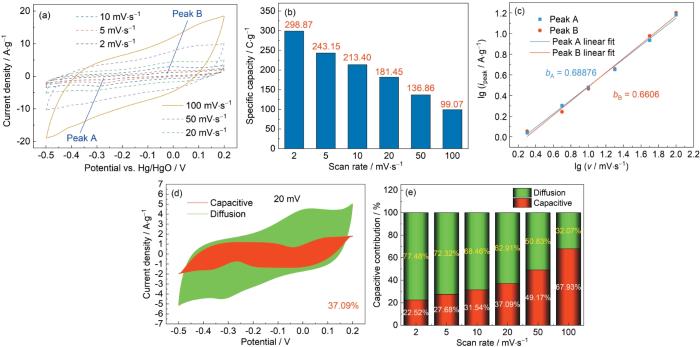
其中k1和k2可根据v1/2与i/v1/2的拟合线性曲线确定。电容行为和扩散控制过程对容量贡献,分别与k1和k2对应。图7d、e给出了拟合计算结果。随着扫描速率从2 mV·s-1提高到100 mV·s-1,电容行为在总比容量中的比例从22.52%提高到67.93%。这表明,随着扫描速率的提高离子/电子的转移和扩散过程变得越来越受约束,从而更多地依赖更快的过程(扩散控制过程)产生的容量。
图7
图7
使用三电极体系在3 mol/L KOH电解质中Zn-BTC/rGO-1D在不同扫描速率(2~100 mV·s-1)时的CV曲线和比容量、CV曲线中峰A和峰B的lg(i)和lg(v)之间的线性关系、在20 mV·s-1下将储存贡献与电容行为和扩散控制过程分开以及在不同扫描速率下电容行为和扩散控制过程的贡献比
Fig.7
Using three-electrode system in 3 mol/L KOH electrolyte CV curves (a) and specific capacity of Zn-BTC/rGO-1D electrode at various scan rates (2~100 mV·s-1) (b), linear relationships between lg(i) and lg(v) for peak A and peak B in CV curves (c), separation of storage contribution from the capacitive behavior and diffusion-controlled processes at 20 mV·s-1(d) and the contribution ratio of capacitive behavior and diffusion-controlled process at different scan rates (e)
图8a给出了四种复合电极样品的Nyquist阻抗图,高频区阻抗曲线与实轴的截距表示等效串联电阻Rs,包括电极材料的内部电阻、电解液的离子电阻和集流体之间的接触电阻。可以看出,Zn-BTC/rGO-1D的曲线在高频区的半圆直径最小,低频区尾部曲线接近于垂直。这表明,Zn-BTC/rGO-1D超级电容器有较快的电荷转移、较高的离子扩散速度、最低的等效串联电阻,以及接近理想EDLC的电容行为。图8b给出了四种电极材料的循环性能。可以看出,在电流密度为1 A·g-1的条件下循环1000次后rGO电容器的容量保持率为96.9%,而Zn-BTC/rGO-1D、Zn-BTC/rGO-2D、及Zn-BTC-1D三种超级电容器的容量保持率分别为94.1%、93.0%和68.8%。这表明,本文合成的Zn-BTC/rGO-1D复合材料的循环稳定性随着rGO添加量的增加而提高,但是相应的比电容减小。
图8
图8
rGO、Zn-BTC/rGO-1D、Zn-BTC/rGO-2D和Zn-BTC-1D电极的Nyquist图和电流密度为1 A·g-1下1000次充放电循环的比容量和容量保持率
Fig.8
Nyquist plot (a) and specific capacitance and capacitance retention at 1 A·g-1 after 1000 cycles (b) of rGO, Zn-BTC/rGO-1D, Zn-BTC/rGO-2D and Zn-BTC-1D electrodes
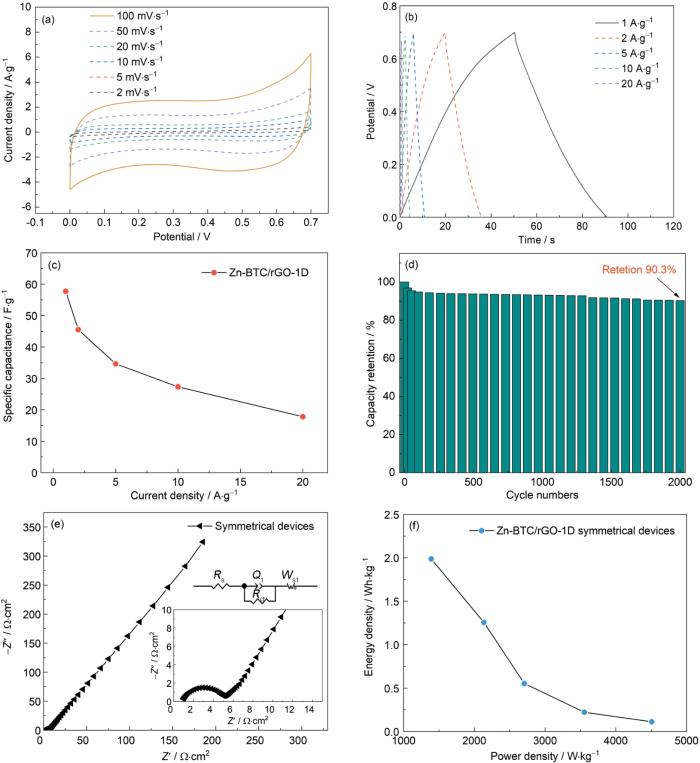
为了进一步评估合成Zn-BTC/rGO-1D材料在实际应用中的性能,将其组装成对称超级电容器器件(SSD),电解质溶液为3 mol/L的KOH,电压窗口为0~0.7 V。在不同扫描速率下(2~100 mV·s-1) Zn-BTC/rGO-1D样品的CV曲线均呈现相似的矩形,在CV测试过程中主要以EDLC提供电容性能,且具备良好的可逆性,结果如图9a所示。该SSD在不同电流密度下(1~20 A·g-1)GCD测试曲线如图9b所示,可见其类似对称三角形的充放电曲线,进一步验证了充放电过程有良好的可逆充放电性能。图9c给出了不同电流密度下SSD的比容量。根据
图9
图9
Zn-BTC/rGO-1D组装对称超级电容器器件的CV曲线、充放电曲线、比容量、循环稳定性、Nyquist图以及能量密度与功率密度的关系
Fig.9
CV curves at different scanning rates ranging from 2 mV·s-1 to 100 mV·s-1 (a); GCD curves at different current densities ranging from 1 A·g-1 to 20 A·g-1 (b); specific capacity at different current densities ranging from 1 A·g-1 to 20 A·g-1 (c); cycling performance after 2000 cycles (d); Nyquist plot (e) and (f) Ragon plot for symmetrical supercapacitor device assembled with Zn-BTC/rGO-1D
3 结论
(1) 用超声震荡法调节有机配体与金属离子的配比可制备出不同形貌结构可用作为超级电容器的电极的Zn-BTC复合石墨烯纳米复合材料。添加石墨烯载体可提高电极材料的导电性及电化学性能,Zn-BTC/rGO-1D电极的比电容可达182.4 C·g-1 (电流密度为1 A·g-1)。
(2) 使用这种复合材料电极组装的对称超级电容器器件,其功率密度1390 W·kg-1时能量密度最大为1.99 Wh·kg-1,在1 A·g-1电流密度下初始比容量为57.7 F·g-1,2000次充放电循环后比容量的保持率为90.3%。
参考文献
Review on supercapacitors: technologies and materials
[J].
A comprehensive review on novel quaternary metal oxide and sulphide electrode materials for supercapacitor: origin, fundamentals, present perspectives and future aspects
[J].
Three-dimensional nickel oxide@carbon hollow hybrid networks with enhanced performance for electrochemical energy storage
[J].
A review on the field patents and recent developments over the application of metal organic frameworks (MOFs) in supercapacitors
[J].
Conductive metal-organic frameworks for supercapacitors
[J].
Metal-organic frameworks (MOFs)‐based mixed matrix membranes (MMMs) for gas separation: a review on advanced materials in harsh environmental applications
[J].
Bimetal MOFs synthesized by one-step synthesize method and its catalytic performance for low-temperature selective catalytic reduction
[J].
一步合成法制备双金属有机骨架材料及其低温选择性脱硝催化性能
[J].
Cu-Fe-NH2 based metal-organic framework nanosheets via drop-casting for highly efficient oxygen evolution catalysts durable at ultrahigh currents
[J].
Bimetallic Cu/Fe MOF-based nanosheet film via binder-free drop-casting route: a highly efficient urea-electrolysis catalyst
[J].
Bimetal‐organic framework derived CoFe2O4/C porous hybrid nanorod arrays as high‐performance electrocatalysts for oxygen evolution reaction
[J].
Improving MOF stability: approaches and applications
[J].Metal-organic frameworks (MOFs) have been recognized as one of the most important classes of porous materials due to their unique attributes and chemical versatility. Unfortunately, some MOFs suffer from the drawback of relatively poor stability, which would limit their practical applications. In the recent past, great efforts have been invested in developing strategies to improve the stability of MOFs. In general, stable MOFs possess potential toward a broader range of applications. In this review, we summarize recent advances in the design and synthesis of stable MOFs and MOF-based materials synthesis and/or post-synthetic structural processing. Also, the relationships between the stability and functional applications of MOFs are highlighted, and finally, the subsisting challenges and the directions that future research in this field may take have been indicated.This journal is © The Royal Society of Chemistry 2019.
MOFs as proton conductors-challenges and opportunities
[J].Proton conducting materials have garnered immense attention for their role as electrolytes in fuel cells. Metal Organic Frameworks (MOFs) and coordination polymers have recently been investigated as possible candidates for proton-conducting applications. Their crystallinity, chemically functionalizable pores and options for systematic structural variation are some of the factors that allow for the targeted design of better proton conductors operating over a wide variety of temperatures and/or humidity conditions. This review will examine selected examples from this nascent field, and will focus on the design and synthesis of proton conducting MOFs, their properties and conditions under which they operate.
Low-temperature SCR of NO with NH3 over CeO2 supported on modified activated carbon fibers
[J].
Effect of different oxidizing agent treatments on the surface properties of activated carbons
[J].
Low-temperature SCR of NO x with NH3 over carbon-ceramic supported catalysts
[J].
Preparation, decoration and characterization of graphene sheets for methyl green adsorption
[J].
One-pot microwave-assisted combustion synthesis of graphene oxide-TiO2 hybrids for photodegradation of methyl orange
[J].
Graphene-based electrochemical supercapacitors
[J].
Ultrathin planar graphene supercapacitors
[J].With the advent of atomically thin and flat layers of conducting materials such as graphene, new designs for thin film energy storage devices with good performance have become possible. Here, we report an "in-plane" fabrication approach for ultrathin supercapacitors based on electrodes comprised of pristine graphene and multilayer reduced graphene oxide. The in-plane design is straightforward to implement and exploits efficiently the surface of each graphene layer for energy storage. The open architecture and the effect of graphene edges enable even the thinnest of devices, made from as grown 1-2 graphene layers, to reach specific capacities up to 80 μFcm(-2), while much higher (394 μFcm(-2)) specific capacities are observed multilayer reduced graphene oxide electrodes. The performances of devices with pristine as well as thicker graphene-based structures are examined using a combination of experiments and model calculations. The demonstrated all solid-state supercapacitors provide a prototype for a broad range of thin-film based energy storage devices.
Highly concentrated, stable nitrogen-doped graphene for supercapacitors: simultaneous doping and reduction
[J].
Understanding integrated graphene-MOF nanostructures as binder- and additive-free high-performance supercapacitors at commercial scale mass loading
[J].
Electrochemical performance of zinc-based metal-organic framework with reduced graphene oxide nanocomposite electrodes for supercapacitors
[J].
Electrochemical performance of composites made of rGO with Zn-MOF and PANI as electrodes for supercapacitors
[J].
Mn-Ce oxide nanoparticles supported on nitrogen-doped graphene for low-temperature catalytic reduction of NO x : de-nitration characteristics and kinetics
[J].
A bottom-up sonication-assisted synthesis of Zn-BTC MOF nanosheets and the ppb-level acetone detection of their derived ZnO nanosheets
[J].
Highly selective separation of rare earth elements by Zn-BTC metal-organic framework/nanoporous graphene via in situ green synthesis
[J].
Raman spectroscopy for carbon nanotube applications
[J].
2-aminoanthraquinone anchored on N-doped reduced graphene oxide for symmetric supercapacitor with boosting energy density
[J].
Nickel foam-based manganese dioxide-carbon nanotube composite electrodes for electrochemical supercapacitors
[J].
Template-free synthesis and supercapacitance performance of a hierachically porous oxygen-enriched carbon material
[J].
Synthesis and application of naphthalene diimide as an organic molecular electrode for asymmetric supercapacitors with high energy storage
[J].