环境污染和能源短缺等问题,受到了极大地关注。我国建筑物的制冷和制热消耗了大量的能源,使用电致变色智能窗[1](ECDs)可缓解这个问题。WO3的成本较低、着色效率高和化学稳定性良好,但是其开关速度不高和可逆性较差[2~4]。将贵金属粒子(如Au、Ag、Pt等)与WO3材料复合可提高其导电性,生成肖特基结界面使界面电荷转移与传输效率提高从而优化其变色性能[5,6]。Cui等[7]用介孔二氧化硅作为硬模板合成介孔氧化钨并用硼氢化物还原法将Pt负载在介孔氧化钨上,制备出的介孔结构Pt/WO3具有较高的电催化活性。Qin等[8]用水热法和光诱导沉积法制备的2D/2D WO3/Pt/g-C3N4肖特基欧姆结光催化剂,可用于制备氢气。优化后的肖特基欧姆结光催化剂,具有较高的光解水性能。Zhou等[9]将水热法和电沉积相结合制备的三维WO3/Pt复合薄膜,具有优异的光电催化性能。这些结果表明,WO3/Pt复合薄膜较多的高反应活性位点不仅有利于提高光电催化活性和光解水性能,还有利于优化其电致变色性能。Pang等[10]用真空沉积方法制备的WO3/Ag纳米粒子复合薄膜,具有更为优异的电致变色性能。Rahmanzade等[11]用溶胶凝胶合成法制备的Au-WO3复合薄膜,比单一的WO3薄膜的响应时间更短,电致变色性能更优,表明WO3复合贵金属能优化其电致变色性能。
水热法是一种简便、低成本方法,制备的薄膜表面积大且形貌独特。电沉积法和连续离子层沉积法,其成本低廉和易于操作。将上述方法结合制备具有三维结构的复合薄膜,可提高电解质的传输能力进而提高器件的电致变色性能。鉴于此,本文将水热法与电沉积法相结合制备WO3/Pt复合薄膜,研究这种薄膜阴极器件的电致变色性能。
1 实验方法
1.1 WO3 薄膜和WO3/Pt复合薄膜的制备
切割电导率约为0.1~7 S/cm的导电玻璃(FTO)使其截面为1.5 cm × 2.5 cm,然后将其放在超声波清洗机内依次用丙酮、异丙醇、甲醇、去离子水清洗10 min,用N2吹干后用万用表检测其导电面。
将100 mL去离子水置于烧杯中,加入3.3 g的Na2WO4·2H2O后搅拌至完全溶解,再加入70 mL浓度为2.1 mol/L的盐酸(质量分数37%)继续搅拌。形成淡黄色的悬浊液后再加入2.8 g的(NH4)2C2O4·H2O,持续搅拌至悬浊液变得透明。将3.5 mL的混合液放入聚四氟乙烯内衬中,然后将FTO导电面朝下放入内衬中,在150℃的烘箱中水热反应4 h,冷却至室温后用去离子水冲洗并用N2吹干,再在450℃的管式炉中退火1 h,得到WO3薄膜样品。
将1 mmol/L的 H2PtCl4加入0.5 mol/L的H2SO4溶液中作为沉积液,使用三电极体系进行电沉积。工作电极是性能最佳的WO3薄膜,对电极为Pt网,参比电极为Ag/AgCl电极。使用恒电位法沉积,电位为-0.4 V,分别电沉积40和80 s。沉积后的产物用去离子水冲洗并用N2吹干,再将其300℃的管式炉中退火30 min,得到WO3/Pt复合薄膜样品,分别记为WO3/Pt-40 s和WO3/Pt-80 s。
1.2 NiO薄膜的制备
将1000 mL去离子水置于烧杯中,加入0.5816 g六水合硝酸镍(Ni(NO3)2·6H2O)后搅拌至完全溶解,作为沉积液。使用三电极体系进行电沉积。工作电极为FTO,对电极为Pt网,参比电极为饱和硫酸亚汞电极。每次量取100 mL沉积液,用恒电流法进行电沉积,电流为-0.0002 A,电沉积时间为10 s。将沉积后得到的产物用去离子水冲洗并用N2烘干,再将其在300℃的管式炉中退火1 h,得到NiO薄膜样品。
1.3 器件的组装和性能表征
将1 mol/L高氯酸锂(LiClO4)溶解于碳酸丙烯酯(C4H6O3,PC)溶液中,用作电解液。选取电致变色性能最好的WO3/Pt复合薄膜和NiO薄膜分别作为变色阴极和阳极,用聚四氟乙烯垫片将两个电极连接,中间用聚四氟乙烯垫片间隔,用环氧树脂封装且预留小口,再将上述电解液填充至阴极和阳极中间并用环氧树脂封装小口,制作出WO3/Pt-NiO电致变色器件(图1)。
图1
图1
电致变色器件的结构和原理
Fig.1
Structure and schematic diagram of electrochromic device
1.4 表征与测试
用Ultima IV型X射线衍射仪(XRD)测试薄膜晶体XRD谱。用K-Alpha+型X射线光电子能谱(XPS)仪测定薄膜的成分。用附有能谱仪(EDS)的S4800型扫描电子显微镜(SEM)表征薄膜的形貌和结构,并测试WO3/Pt复合薄膜的EDS能谱。使用CHI660E型电化学工作站测试薄膜和器件的变色性能。用常规三电极体系测试薄膜的电致变色性能,以铂网为对电极,Ag/AgCl为参比电极,制备的薄膜为工作电极,将1 mol/L高氯酸锂(LiClO4)溶解于碳酸丙烯酯(C4H6O3,PC)溶液中得到的溶液为电解液。用两电极体系测试器件的变色性能,器件的阴极连接电化学工作站的工作电极,器件的阳极同时连接对电极和参比电极。同时用PLS-SXE30型氙灯光源和E820型光纤光谱仪辅助电化学工作站检测薄膜和器件的光学性能。
对薄膜样品进行循环伏安测试(CV),样品的电势在-1 V与1 V之间,扫描速率为50 mV/s。测试WO3薄膜和WO3/Pt复合薄膜的计时电量(CC)曲线,电位在-1.0 V和1.0 V之间切换,时间间隔为50 s。为了研究WO3/Pt复合薄膜的响应时间,对WO3/Pt复合薄膜施加1.5 V电压,用光纤光谱仪记录WO3/Pt复合薄膜在630 nm处透过率的变化和响应时间。为了研究WO3-NiO器件和WO3/Pt-NiO器件的响应时间,对WO3-NiO器件和WO3/Pt-NiO器件施加1.5 V电压,同时使用光纤光谱仪记录WO3/Pt复合薄膜在630 nm处的透过率变化,得到透过率与响应时间变化曲线。
2 结果和讨论
2.1 WO3/Pt复合薄膜和NiO薄膜的组成和微结构
图2给出了WO3/Pt-40 s复合薄膜和NiO薄膜的XRD谱和NiO薄膜的XPS全谱。在图2a中,WO3/Pt-40 s复合薄膜在2θ为23.2°、26.9°、33.6°、38.1°、42.8°、50.1°、55.3°处(JCPDS卡号41-0905)的衍射峰分别对应(100)、(001)、(110)、(200)、(201)以及(221)晶面,属于六方相,表明制备出的是六方相WO3薄膜。谱中没有出现Pt粒子的衍射峰,可能是电沉积时间较短(40 s)Pt粒子的含量较低,Pt粒子没有达到XRD谱仪的检测下限。在NiO的XRD谱中除了FTO的衍射峰,没有出现NiO的衍射峰。为了进一步确定NiO薄膜的元素组成和价态,对其进行了XPS测试。从NiO薄膜的XPS全谱中可见,NiO的Ni 2p3/2主峰分别位于853.9 eV和855.7 eV,其中C元素是空气产生的干扰。XPS的结果表明,已经成功地制备出NiO薄膜。
图2
图2
WO3/Pt-40 s和NiO薄膜的XRD谱和NiO薄膜的XPS全谱
Fig.2
XRD patterns of WO3/Pt-40 s and NiO film (a) and XPS full spectrum of NiO thin film (b)
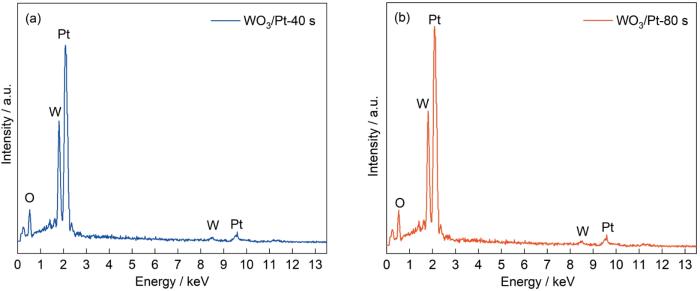
图3a~d给出了WO3薄膜、沉积不同时长(40、80 s)Pt的WO3/Pt复合薄膜和NiO薄膜的SEM照片。从图3a可以看出,WO3薄膜样品表面呈现出形状规则的纳米棒状结构,膜的厚度为1.37~1.61 μm。从图3b、c可见,Pt粒子附着在WO3纳米棒表面,且Pt粒子的量随着电沉积时间的增加而增多,Pt粒子的大小随着电沉积时间的增加而逐渐变大,膜的厚度均为1.37~1.61 μm。这表明,已经成功地制备出WO3/Pt复合薄膜样品,且复合Pt粒子没有显著影响WO3薄膜的形貌和厚度。从图3d可以看出,NiO薄膜具有大小约为50~150 nm的团簇互联结构。薄膜的团簇互联结构较致密,表面又较多的缝隙,截面较为平整。NiO薄膜的厚度为131 nm。SEM照片表明,Pt纳米粒子的沉积没有影响或改变WO3纳米棒薄膜的表面形貌。从图4可以看出,WO3/Pt复合薄膜中只有W、O、Pt三种元素,且随着沉积时间的增加Pt元素的含量提高。XRD谱、SEM照片及EDS能谱测试结果表明,已经成功地制备出WO3/Pt复合薄膜。
图3
图3
WO3、WO3/Pt-40 s、WO3/Pt-80 s和NiO膜的SEM形貌
Fig.3
SEM images of WO3 (a), WO3/Pt-40 s (b), WO3/Pt-80 s (c) and NiO (d) films
图4
图4
WO3/Pt-40 s和WO3/Pt-80 s复合薄膜局部的EDS能谱
Fig.4
EDS energy spectrum of WO3/Pt-40 s (a) and WO3/Pt-80 s (b) of composite films sample
2.2 WO3/Pt复合薄膜的电致变色性能
为了确定WO3薄膜和电沉积不同时长(40、80 s)Pt的WO3/Pt复合薄膜在变色过程中离子的提取/插入过程,对薄膜样品进行了循环伏安测试(CV),结果在图5a中给出。可以看出,WO3薄膜和沉积不同时长Pt的WO3/Pt复合薄膜样品的循环伏安曲线闭合面积大小的排序为:WO3/Pt-40 s > WO3/Pt-80 s >WO3。这表明,复合Pt纳米粒子后WO3/Pt复合薄膜样品的循环伏安曲线闭合面积明显增大,即复合Pt纳米粒子使薄膜容纳Li+的能力明显提高。还可以看出,与其他样品相比,WO3/Pt-40 s复合薄膜样品的曲线闭合面积更大,表明WO3/Pt-40 s复合薄膜样品的Li+离子容纳能力更大和电化学活性更高。图5b给出了WO3薄膜和WO3/Pt复合薄膜的计时电量(CC)曲线。对薄膜样品可逆性(Reversibility)的计算结果表明,提取(Qdi)与插入(Qi)电荷密度的比值越高则样品的可逆性越好,其数值列于表1。从图5b和表1可见,WO3/Pt复合薄膜的可逆性优于WO3薄膜,其中WO3/Pt-40 s复合薄膜具有最高的电荷提取/插入性能,提取和插入电荷密度分别为34.92 mC/cm2 和38.74 mC/cm2,可逆性为90.12%。图5c给出了WO3薄膜和WO3/Pt复合薄膜在着色和褪色状态下的透射光谱(400~800 nm)。Tb和Tc分别为褪色态和着色态在630 nm处的透射率,WO3薄膜和WO3/Pt复合薄膜的Tb和Tc与光调制范围(∆T)参数也列于表1。从图5c和表1可见,WO3/Pt复合薄膜比WO3薄膜的光调制范围大,其中WO3/Pt-40 s复合薄膜在630 nm处的光调制范围为55.02%。WO3/Pt-40 s的光调制范围,分别是WO3和WO3/Pt-80 s的1.17倍和1.05倍。
图5
图5
WO3、WO3/Pt-40 s和WO3/Pt-80 s薄膜的循环伏安曲线、注入和抽取电荷变化曲线、透射光谱、光学透过率响应、着色效率和奈奎斯特曲线
Fig.5
Cyclic voltammetric curve (a), injection and extraction charge change curve (b), transmission spectra (c), transmittance and response time curve (d), coloration efficiency (e) and Nyquist curve (f) of WO3、WO3/ Pt-40 s、WO3/Pt-80 s thin film samples
表1 WO3和WO3/Pt薄膜的电致变色性能
Table 1
Qi or Qdi mC·cm-2 | Reversibility % | tc or tb s | Tb/Tc % | ∆T % | CE cm2·C-1 | |
|---|---|---|---|---|---|---|
| WO3 | 31.26/26.57 | 85.01 | 31.22/21.63 | 71.72/24.63 | 47.09 | 14.26 |
| WO3/Pt-40 s | 38.74/34.92 | 90.12 | 27.47/16.72 | 70.53/15.51 | 55.02 | 26.12 |
| WO3/Pt-80 s | 34.49/30.35 | 87.98 | 28.13/18.84 | 71.36/18.72 | 52.64 | 17.33 |
和
式中ΔOD为单位注入电荷与光学变化关系,Tb和Tc分别为薄膜在褪色态和着色态下的透过率,CE为着色效率,Q为电荷密度。
薄膜的着色效率数据列于表1。从表1可以看出,WO3/Pt复合薄膜的着色效率高于WO3薄膜.这表明,WO3/Pt复合薄膜在变色过程中发生的氧化还原反应速率更高,WO3/Pt-40 s复合薄膜的着色效率最高(为26.12 cm2/C)。WO3/Pt-40 s的着色效率分别是WO3和WO3/Pt-80 s的1.83倍和1.5倍。图5f给出了WO3薄膜和WO3/Pt复合薄膜的电化学阻抗谱(EIS)。EIS曲线的圆弧半径反映被测样品电极内部的电子转移效率。圆弧的半径越小且直线的斜率越大,表明电极与电解液界面间的电荷转移电阻越小,即被测样品电极内部电子传输效率越高[12]。从图5f可见,与WO3薄膜相比,WO3/Pt复合薄膜的EIS曲线圆弧的半径更小且直线的斜率更大,其中WO3/Pt-40 s复合薄膜的EIS曲线圆弧的半径最小且直线的斜率最大。这表明,WO3/Pt复合薄膜的电子转移阻抗比WO3薄膜的小,WO3/Pt-40 s复合薄膜的电子阻抗最小,即WO3/Pt-40 s复合薄膜的电子转移效率更高,具有更优异的电致变色性能。
2.3 电致变色器件的电致变色性能
图6a给出了WO3-NiO器件和WO3/Pt-NiO器件的计时电量图,反映在时间间隔为40 s时内抽出/注入的电荷量的变化。其结果列于表2,可逆性值大小的排序为WO3-NiO器件(83.96%) < WO3/Pt-NiO器件(87.98%)。图6b给出了WO3-NiO器件和WO3/Pt-NiO器件着色和褪色状态下的透射光谱(400 nm至800 nm)。WO3薄膜和WO3/Pt复合薄膜的Tb、Tc和光调制范围(∆T)参数也列于表2。从图6b和表2可以看出,WO3/Pt-NiO器件的光调制范围(47.00%)是WO3-NiO器件的1.09倍。Zhou等[18]制备的WO3/Ag-Ag电致变色器件其光调制范围位21.85%,而本文组装的WO3/Pt-NiO器件的光调制范围约是其2.15倍,表明其电致变色性能良好。图6c给出了WO3-NiO器件和WO3/Pt-NiO器件的其透过率与响应时间变化曲线。可以看出,与WO3-NiO器件相比,WO3/Pt-NiO器件的响应时间更短,其着色和褪色响应时间分别为29.62 s和18.84 s (表2)。图6d给出了WO3-NiO器件和WO3/Pt-NiO器件的着色效率图,用于评价电致变色性能。从图6d和表2可以看出,WO3/Pt-NiO器件的着色效率优于WO3-NiO器件。这表明,WO3/Pt-NiO器件在变色过程中发生的氧化还原反应速率和着色效率(21.33 cm2/C)远高于WO3-NiO器件(12.21 cm2/C)。
图6
图6
WO3-NiO器件和WO3/Pt-NiO器件的注入和抽取电荷变化曲线、透射光谱、光学透过率响应、和着色效率曲线
Fig.6
Injection and extraction charge change curve (a), transmission spectra (b), transmittance and response time curve (c),and coloration efficiency (d) of WO3-NiO、WO3/Pt-NiO devices
表2 WO3-NiO和WO3/Pt-NiO电致变色器件的电致变色性能
Table 2
Qi or Qdi mC·cm-2 | Reversibility % | tc or tb s | Tb/Tc % | ∆T % | CE cm2·C-1 | |
|---|---|---|---|---|---|---|
| WO3-NiO | 26.26/22.05 | 83.96 | 33.58/23.17 | 65.72/22.63 | 43.09 | 12.21 |
| WO3/Pt-NiO | 33.74/29.69 | 87.98 | 29.62/18.84 | 64.53/17.51 | 47.00 | 21.33 |
3 结论
(1) 将水热法和电沉积相结合可制备WO3/Pt变色阴极和NiO阳极,组装出的WO3/Pt-NiO电致变色器件性能优异。
(2) 与WO3薄膜相比,WO3/Pt复合薄膜具有更高的可逆性、更广泛的光调制、更短的响应时间和更高的着色效率。WO3/Pt-NiO电致变色器件具有优秀的电致变色性能,其着/褪色响应时间为29.62 s/18.84 s,在630 nm处的最大光调制范围为47.00%。
参考文献
Emerging thermal-responsive materials and integrated techniques targeting the energy-efficient smart window application
[J].
Self-switching photoelectrochromic device with low cost, plasmonic and conducting Ag nanowires decorated V2O5 and PbS quantum dots
[J].
Enhanced photoelectrocatalytic performance of Zn-doped WO3 photocatalysts for nitrite ions degradation under visible light
[J].WO(3) and Zn-doped WO(3) thin films were prepared on indium-tin oxide glass by a dip-coating. The composite films were characterized by UV-Vis absorption spectra, X-ray diffraction and scanning electron microscope. The effect of preparation conditions (concentration of Zn, annealing temperature, number of layers) on the photocurrent was studied. It was found that the photocurrent under visible light displayed the highest value for 2% Zn-WO(3) films annealed at 400 degrees C. The photocatalytic activity of the Zn-doped WO(3) was evaluated in terms of decay rate of nitrite ions under visible light. The influence of applied potential, initial pH and nitrite concentration on the reaction rate was studied. The experiments demonstrated that NO(2)(-) could be efficiently degraded on the doped photoanode that showed a higher activity than the undoped WO(3) especially under high anodic potential (>0.7 V). The rate of degradation was enhanced in aqueous NaCl solutions. Furthermore, it was demonstrated that the photodegradation mechanism of NO(2)(-) proceeded mainly indirectly via OH radicals. The possible reason of enhancement of reaction rate was also discussed.
Ion trapping and detrapping in amorphous tungsten oxide thin films observed by real-time electro-optical monitoring
[J].
Nanoarchitectonics of a Au nanoprism array on WO3 film for synergistic optoelectronic response
[J].
The injection of Ag nanoparticles on surface of WO3 thin film: enhanced electrochromic coloration efficiency and switching response
[J].
Electrocatalytic activity and CO tolerance properties of mesostructured Pt/WO3 composite as an anode catalyst for PEMFCs
[J].
Rationally constructing of a novel 2D/2D WO3/Pt/g-C3N4 Schottky-Ohmic junction towards efficient visible-light-driven photocatalytic hydrogen evolution and mechanism insight
[J].
Preparation and photoelectrocatalytic properties of WO3/Pt composite film
[J].
WO3/Pt复合薄膜的制备及其光电催化性能
[J].
Size-controlled Ag nanoparticle modified WO3 composite films for adjustment of electrochromic properties
[J].
Improving electrochromic properties of WO3 thin film with gold nanoparticle additive
[J].
Photoelectrochemical study on charge transfer properties of ZnO nanowires promoted by carbon nanotubes
[J].
Improved electrochromical properties of sol-gel WO3 thin films by doping gold nanocrystals
[J].
Enhanced optical modulation due to SPR in gold nanoparticles embedded WO3 thin films
[J].
Enhanced electrochromic coloration in Ag nanoparticle decorated WO3 thin films
[J].
Graphene/silicon nanowire Schottky junction for enhanced light harvesting
[J].
Novel p-type Ag-WO3 nano-composite for low-cost electronics, photocatalysis, and sensing: synthesis, characterization, and application
[J].
Electrochromic modulation of near-infrared light by WO3 films deposited on silver nanowire substrates
[J].