2015年Rost等[5]以MgO,CoO,NiO,CuO和ZnO为原料用固相烧结法合成出单相岩盐结构的HEO (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O。Sarkar等[6,7]以Co(NO3)2·6H2O、Cu(NO3)2·2.5H2O、Mg(NO3)2·6H2O、Ni(NO3)2·6H2O、Zn(NO3)2·6H2O为原料用喷雾热解法(Nebulized spray pyrolysis,NSP)合成出岩盐型纳米级(Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)O HEO粉体,将其用作LIBs负极材料,可逆比容量(> 500 mAh·g-1)高于四元中熵氧化物,且循环稳定性和倍率性能优异(在200 mA·g-1的电流密度下循环500圈后容量保持率接近100%)。HEO具有优异电化学性能的原因是,熵稳结构使其晶体结构得以保留。Qiu等[8]将五种金属氧化物球磨后用固相烧结制备出(Mg0.2Co0.2Ni0.2-Cu0.2Zn0.2)O HEOs纳米粉体材料,用其制备的LIBs负极具有较高的初始放电比容量(1585 mAh·g-1),即使电流密度高达3000 mAh·g-1其可逆容量仍达到490 mAh·g-1。Qiu等提出,非活性MgO产生的“旁观者效应”是稳定HEO晶体结构、阻止纳米颗粒聚集、缓解体积膨胀的主要因素。Wang等[9]将CoO、NiO、CuO、ZnO和不同MgO含量的氧化物在1000℃固相烧结,研究了Mg含量对产物电化学性能的影响。结果表明,非活性的Mg离子能维持其晶体结构,减缓了电极材料的体积膨胀和粉化。Chen等[10]用固态合成法制备了不同粒径的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O电极,发现纳米尺寸的HEO-72 h负极具有优异的倍率性能和较高的可逆比容量(在2000 mA·g-1的电流密度下为358 mAh·g-1)。Guo等[11]用石墨烯对HEO进行表面改性制备出HEO@G,其在1000 mA·g-1电流密度下循环1000次后可逆比容量仍保持为460 mAh·g-1。Triolo等[12]用静电纺丝和溶剂热法制备出不同形貌的单相(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O HEOs,其中纳米纤维锂离子电池负极在电流密度为200 mA·g-1时具有480 mAh·g-1的高可逆比容量、优异的倍率性能和循环稳定性。
但是,(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O HEO作为LIBs负极材料在原子尺度上的多主元协同效应没有得到解释。最近,Wang等[13]进行价态分析和微观结构表征,揭示了HEO中各阳离子在转化反应中的协同作用:Zn和一部分Co具有电化学活性,从第一次锂化过程就开始为电池提供主要容量;Ni、Cu和部分被还原的Co不再参与之后的反应,而是以三维金属纳米网络的形式存在使电极的导电性提高;Mg2+具有电化学惰性,电极中的MgO使其晶体结构保持稳定。为了揭示岩盐型HEO在充放电过程中各金属阳离子的协同作用以及其含量的变化对电化学性能的影响,本文改变Zn的含量,用溶液燃烧法制备不同阳离子比例的粉体,研究Zn的含量对岩盐型HEO电化学性能的影响。
1 实验方法
1.1 材料的制备
按等摩尔量将1.0914 g的Co(NO3)2·6H2O、0.7033 g的Cu(NO3)2·3H2O、0.9615 g的Mg(NO3)2·6H2O、1.0905 g的Ni(NO3)2·6H2O和0.6067 g的Zn(NO3)2·6H2O溶于适量的蒸馏水中,加入0.7101 g甘氨酸后搅拌均匀,在90℃烘成凝胶状的高熵氧化物前驱体;透明电气炉以10℃·min-1的速率升温至950℃后放入样品,在空气气氛中煅烧30 min后空冷,将产物研磨得到(Co0.22Cu0.22Mg0.22Ni0.22Zn0.12)O粉体(记为Zn0.12)。分别改变Zn(NO3)2·6H2O的量为1.1153 g和1.7398 g,改变甘氨酸的量为0.7812 g和0.8688 g,在相同的条件下制得粉体 (Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)O(记为Zn0.2)和粉体(Co0.18Cu0.18Mg0.18Ni0.18Zn0.28)O (记为Zn0.28)样品。
1.2 性能表征
用Rigaku Mini-Flex 600X射线衍射仪测试各样品的XRD谱,扫速为5(°)/min,Cu靶,Ka辐射,测定管压为40 kV,管流为30 mA,步长0.02°,扫描范围为25°~80°;用ΣIGMA HV-01-043型扫描电子显微镜观察样品的形貌,用Bruker Nano Xflash Detector 5010型能谱仪分析样品的组成元素及其分布;用JW-BK200型比表面和孔径分析仪测试样品对N2的吸脱附等温曲线,测试条件:液氮为吸附介质,相对压力P/P0为0.0292~0.9931,使用Brunauer-Emmett-Teller(BET)模型计算比表面积,用 Barrett-Joyner-Halenda(BJH)法计算孔分布和孔容;用EscaLab 250 Xi型X射线光电子能谱仪测试材料表面的化学价态和组成,以C 1s (284.8 eV)为标准校正光谱的结合能;用RTS-4型四探针测试仪测试仪测量圆片样品((用刀片将制备工作电极的浆料均匀地涂在整洁的塑料基板上,随即将其在60℃真空干燥10 h,冷却后切成直径为12 mm、厚度为0.1 mm的圆片)的电导率,测试电流为4.28 μA。
本实验采用CR2025扣式半电池来探究HEO电极材料的电化学性能。将70 mg的HEOs粉体、20 mg的导电炭黑(Super P-45)与10 mg的海藻酸钠粘结剂混合后充分研磨,直至浆料成为不成股下流的浆料。用刀片将浆料均匀地涂覆在铜箔上,在60℃真空干燥10 h后冷却,最后将涂覆浆料的铜箔裁成直径为12 mm的电极片(各电极的面载量为1.2~1.7 mg·cm-2)。用压片机将电极片在8 MPa的压力下保持30 s压实,得到工作电极。在充满高纯氩气的Super (1220/750/900)型手套箱(H2O < 1 × 10-8,O2 < 1 × 10-8,体积分数)中组装成扣式电池(即CR2025扣式半电池)。对电极是直径为13.1 mm的纯锂片,隔膜是聚丙烯多孔膜,电解液是将l mol·L-1的六氟磷酸锂(LiPF6)溶于等体积的碳酸乙烯酯(EC)、碳酸甲乙酯(EMC)和碳酸二甲酯(DMC)中得到的混合溶液。用Newware BTS-5V 10 mA型充放电测试仪在室温下测试电池的充放电曲线、循环性能和倍率性能,测试条件为:电压0.01~3 V;GITT的测试条件为:0.01~3 V,电流密度为100 mA·g-1,弛豫时间为300 s。使用chi760e电化学工作站测试扣式电池的循环伏安(Cyclic voltammetry,CV)曲线和EIS。CV曲线的测试条件为:电压0.01~3 V,扫描速率分别为0.1 mV·s-1、0.2 mV·s-1、0.5 mV·s-1、0.8 mV·s-1和1.0 mV·s-1;EIS谱的测试条件为:频率范围为0.01 Hz~100000 Hz,扫速为0.5 mV·s-1,振幅为0.005 V。
2 结果和讨论
2.1 样品的结构
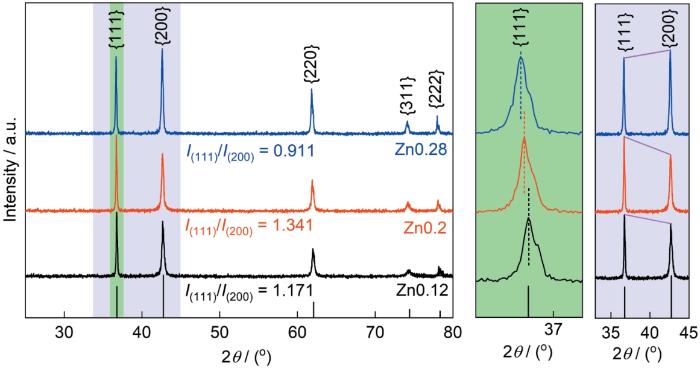
图1给出了样品Zn0.12、Zn0.2和Zn0.28的XRD谱。可以看出,三个样品均为单一岩盐结构,谱中36.7°、42.6°、61.8°、74.1°和78.0°处的衍射峰分别对应Fm-3m岩盐型标准卡片(JCPDS 70-2855)的(111)、(200)、(220)、(311)和(222)晶面[10]。与标准卡片相比,Zn0.12和Zn0.28样品的峰发生了不同角度的偏移。其原因是,Zn2+的半径与其他金属阳离子相比稍大,Zn量的增多使晶体的晶格常数增大。使用Jade软件计算出Zn0.12、Zn0.2和Zn0.28的晶格常数分别为0.42336、0.42375和0.42415 nm。晶格常数的增大使晶面间距随之增大。根据Bragg方程2dsinθ = nλ,晶面间距增大使衍射峰向小角度方向偏移。同时,(111)和(200)晶面的相对强度也发生了明显的变化。岩盐HEO中晶体的晶格畸变或无序程度是(111)和(200)峰的峰强比即I(111)/I(200)决定的,理想岩盐晶体中I(111)/I(200)的强度比约为0.67[14]。计算结果表明,三种样品的晶格畸变程度从小到大的排序为Zn0.28 < Zn0.12 < Zn0.2。这可能是Cu2+的Jahn-Teller效应[3]和不同元素的原子尺寸差造成的。由公式
图1
和
可计算出Zn0.12、Zn0.2和Zn0.28的原子尺寸差分别为0.345、0.343和0.341[15]。式中Ci 为第i种原子的原子百分数,ri 为相对应的原子半径,
图2
图2
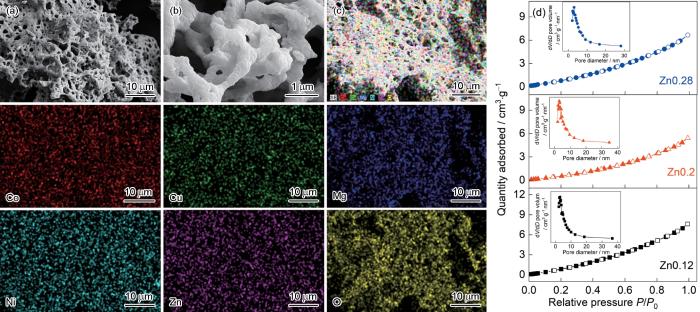
Zn0.28样品的SEM照片和EDS mapping图、三种样品的N2吸脱附等温曲线和BJH孔径分布
Fig.2
SEM (a, b) and EDS mapping pictures (c) of Zn0.28 sample and N2 adsorption/desorption isotherms and the BJH pore size distribution curves of three samples (d)
表1 样品的BET比表面积(SBET)、BJH吸附孔体积(VBJH)、平均孔径(Daver)和最可几孔径(Dmost)
Table 1
| Samples | SBET / m2·g-1 | VBJH / cm3·g-1 | Daver / nm | Dmost / nm |
|---|---|---|---|---|
| Zn0.12 | 8.842 | 0.016 | 7.733 | 3.106 |
| Zn0.2 | 4.299 | 0.011 | 5.305 | 2.813 |
| Zn0.28 | 5.474 | 0.014 | 7.495 | 3.082 |
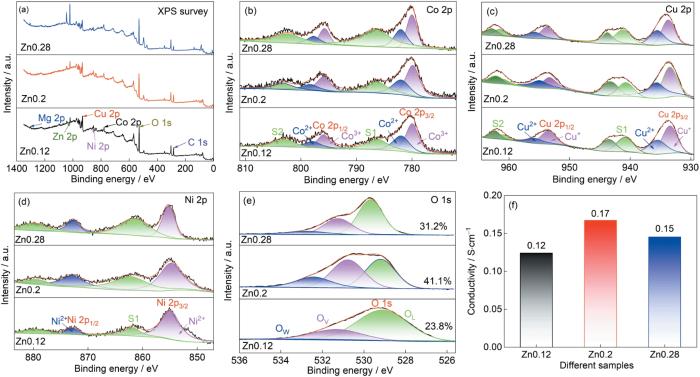
图3a中的XPS全谱证实,材料中有Co、Cu、Mg、Ni、Zn和O等元素。图3b给出了Co 2p的高分辨谱,谱中结合能为780.4 eV (Co 2p3/2)和795.9 eV (Co 2p1/2)处的两个特征峰对应Co3+,位于782.1 eV (Co 2p3/2)和797.9 eV (Co 2p1/2) 处的峰与Co2+相关[21],结合能位于786.5 eV和803.3 eV处的卫星峰证实存在Co2+和Co3+ [22]。图3c给出了Cu 2p光谱,结合能位于933.8 eV (Cu 2p3/2)和953.9 eV (Cu 2p1/2)处的双峰表明存在Cu+,而位于935.7 eV (Cu 2p3/2)和956.1 eV (Cu 2p1/2)的双峰是Cu2+的特征峰[23]。图3d中Ni 2p的光谱由Ni 2p3/2峰(855.6 eV)和Ni 2p1/2峰(873.1 eV)组成,在879.8 eV和861.5 eV处出现了相应的卫星峰,表明Ni元素以Ni2+的形式存在[24]。XPS测试结果表明Mg和Zn元素均以正二价的形式存在[25,26]。表2列出了根据峰面积计算出的Co2+/Co3+以及Cu+/Cu2+的比例。可以看出,Zn0.28样品中Co和Cu元素的平均价态均低于Zn0.2。参考文献[13]中关于岩盐型(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O作为LIBs负极材料的转化反应机理,Zn0.28样品的转化容量较高。根据公式
图3
图3
样品的XPS、Co、Cu、Ni、O的高分辨XPS谱和电导率
Fig.3
XPS survey spectra of the samples (a), High resolution XPS spectra of Co、Cu、Ni、O (b~e) and electrical conductivity (f)
表2 根据XPS分析结果计算出的Co和Cu元素的价态分布和平均化合价
Table 2
| Samples | Co2+ | Co3+ | Cu+ | Cu2+ | Co average | Cu average |
|---|---|---|---|---|---|---|
| Zn0.12 | 38.7% | 61.3% | 61.3% | 38.7% | 2.61 | 1.39 |
| Zn0.2 | 36.1% | 63.9% | 54.5% | 45.5% | 2.64 | 1.46 |
| Zn0.28 | 47.1% | 52.9% | 56.8% | 43.2% | 2.53 | 1.43 |
可计算三样品的理论转化容量,其中n为每摩尔活性物质发生转化反应所含的电子数(Zn0.12,n = 0.68;Zn0.2,n = 0.8;Zn0.28,n = 0.92),F为法拉第常数(F = 96485 C·mol-1),M为物质的相对分子质量。计算结果表明,Zn0.12、Zn0.2和Zn0.28的理论转化容量分别为263.99 mAh·g-1、305.6 mAh·g-1和345.9 mAh·g-1。
图3e中的O 1s光谱可拟合位于529.7、531.6和533.4 eV处的三个峰,分别代表晶格氧(OL)、氧空位(OV)和表面吸附氧(OW)[27]。可计算出Zn0.12、Zn0.2和Zn0.28样品表面的氧空位浓度分别为23.8%、41.1%和31.2%。丰富的氧空位不仅能提高材料的导电性和极大地提高电子转移动力学,还能提供更多的活性位点、缩短离子和电子的扩散路径,进而提高材料的电化学性能[28,29]。但是,过多的氧空位也会加速电极材料结构的退化并由此降低电池的性能[30]。图3f表明,Zn0.2的电导率(0.17 S·cm-1)最高,因为较大的晶格畸变和较多的氧空位使Zn0.2样品的导电性最好[31]。
2.2 电化学性能
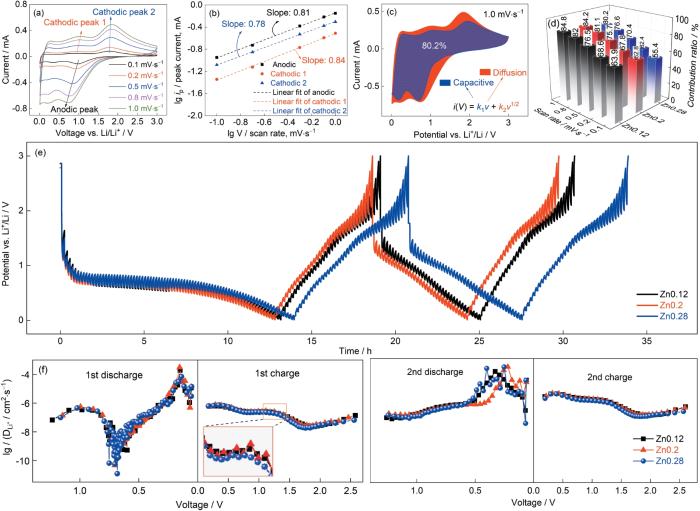
图4a~c给出了Zn0.12、Zn 0.2和Zn0.28电极的CV曲线。可以看出,三电极的氧化还原反应相似。Zn0.28电极第一次还原时,在0.43 V和0.15 V处出现的还原峰可归因于Co,Cu,Ni,Zn几种金属阳离子的还原反应和生成了Zn-Li合金[32],并伴随着固体电解质中间相(Solid-electrolyte interphase,SEI膜)以及Li2O基体的生成[33]。放电后保留的非活性MgO引起的“旁观者效应”,能缓冲电极的体积变化和抑制活性纳米颗粒的团聚,有利于保持循环稳定性[34]。在氧化反应中,以0.78 V和1.77 V为中心的峰对应Zn-Li合金的脱合金化、部分金属元素的再氧化和Li2O的分解[13]。第2、3圈的还原峰和氧化峰重叠得很好,表明在锂化/脱锂过程中电极的稳定性和可逆性良好[35]。图4d~f给出了三电极在电流密度为200 mA·g-1时的充放电曲线。充放电曲线的形状相似,表明所制备电极的充放电行为相似,Zn0.28电极在电压为0.43 V时出现了典型的电压平台,与CV的结果一致。Zn0.12、Zn0.2和Zn0.28的初始放电比容量分别为686.97 mAh·g-1、629.74 mAh·g-1和777.06 mAh·g-1,表明Zn0.28电极的初始放电比容量和库仑效率最高。
图4
图4
Zn0.12、Zn0.2和Zn0.28电极在0.1 mV·s-1扫速下的循环伏安曲线、充放电曲线、不同电流密度下的循环性能和倍率性能
Fig.4
CV plots at 0.1 mV·s-1 sweep rate (a~c) and charge/discharge profiles (d~f) of Zn0.12、Zn0.2 and Zn0.28 electrodes; cycling performance and coulomb efficiency at a specific current of 200 mA·g-1 (g), rate performance (h) and long cycle performance at 1000 mA·g-1 (i)
图4g给出了三电极在电流密度200 mA·g-1时的循环性能。可以看出,随着循环的进行所有电极的容量都呈现先降低后升高的趋势。前几个循环的容量降低是SEI和不可逆Li2O的形成所致,在随后的循环中电极材料的活化使容量有所提高[12]。值得注意的是,文献[13]的研究结果表明:(Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)O负极材料中的Ni、Cu和部分还原的Co在首次放电后不再参与反应,而是以3D合金网络的形式存在而使电极的导电性提高[13]。电极材料的活化,例如微观结构的重排和晶粒尺寸的减小,都有利于电子传导和Li+扩散,从而使容量提高甚至超过理论比容量[12]。一方面,电负性较强的元素(Cu、Ni和部分Co)在第一次循环中形成的3D导电金属网络能加速电子传输,甚至催化SEI界面的可逆形成和分解以提供额外的容量[36]。SEI界面的可逆形成和分解,可通过原位EIS加以确认(图7a~d)。另一方面,晶粒细化能缩短电子离子的传输路径和加速Li+的扩散,而界面电荷的存储也能产生一部分额外容量[37]。随着循环的进行Zn0.28表现出最佳的循环性能,电流密度为200 mA·g-1时150次循环后的容量最高(341.3 mAh·g-1),而Zn0.12和Zn0.2的容量只有278.6和321.5 mAh·g-1。其原因是,Zn0.28样品中Zn的含量高于其他两种样品。循环过程中容量的主要来源,是Zn和部分Co的氧化还原反应以及Zn-Li合金的生成和去合金化[13]。
图5
图5
三电极在200 mA·g-1下循环前和循环150次后的SEM照片
Fig.5
SEM images of as-synthesized electrodes before and after 150 cycles at 200 mA·g-1 (a, d) Zn0.12, (b, e) Zn 0.2, (c, f) Zn0.28
图6
图6
Zn0.28电极在不同扫速下的CV曲线、lg(ip)与lg(v)的关系曲线、1 mV·s-1时的赝电容(蓝色区域)和扩散控制(红色区域)贡献的占比、三电极在不同扫描速率下的赝电容贡献率汇总图、恒电流间歇滴定技术(GITT)测试过程中的充放电曲线以及充放电过程中锂离子的扩散系数
Fig.6
CV curves under various scan rates of Zn0.28 electrode (a), lg(ip) vs. lg(v) curves (b), capacitance (blue area) and diffusion contribution (red area) at a scan rate of 1 mV·s-1 (c), and capacitive contribution ratio at different scan rates (d); GITT curves (e) and lithium-ion diffusion coefficients calculated from GITT curves of electrodes during the charge/discharge process (f)
图7
图7
Zn0.28电极首次(a, b)和第三次(c, d)循环过程中的原位阻抗;三电极的初始阻抗(e)及对应低频区的ω-1/2与Z′的关系图(i);电极在循环前、3和100次后的电化学阻抗谱和等效电路:Zn0.12 (f), Zn 0.2 (g),Zn0.28 (h)以及相对应低频区的ω-1/2与Z′的关系图(j~l)
Fig.7
In-situ EIS during the first (a, b) and third (c, d) cycles of Zn0.28 electrode. EIS measurements of the three electrodes before cycling (e) and corresponding plots of Z' against ω-1/2 in the low-frequency region (i); EIS spectra together with the equivalent circuit before cycling, after 3 and 150 cycles: Zn0.12 (f), Zn 0.2 (g), Zn0.28 (h) and the corresponding plots of Z′ against ω-1/2 in the low-frequency region (j~l)
图4h给出了样品的倍率性能。可以看出,随着电流密度从100 mA·g-1提高到3000 mA·g-1,样品Zn0.12、Zn0.2和Zn0.28的平均放电比容量分别降低到74.1、103.9和140.2 mAh·g-1,容量保持率分别为24.1%、37.1%和39.8%。当电流密度恢复到100 mA·g-1时容量随之恢复,意味着电极具有较高的可逆性。可以看出,Zn0.28样品的倍率性能最好,在3000 mA·g-1的电流密度下的容量保持率最高。
图4i给出了电流密度为1000 mA·g-1时电极的循环性能。可以看出,在整个循环过程中Zn0.28样品的可逆比容量最高。计算结果表明:随着Zn的摩尔量由(Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)O提高了8%,电流密度为200 mA·g-1和1000 mA·g-1时(Co0.18Cu0.18Mg0.18-Ni0.18Zn0.28)O电极的比容量分别提高了6%和17%。
2.3 动力学分析
图6a给出了电极在不同扫描速率下的CV曲线。可以看出,所有CV曲线的形状都比较相似。随着扫速的提高阳极峰和阴极峰的峰值电流都增大。同时,峰值电流和扫描速率之间的关系为
根据公式
可将一定扫描速率下的总容量分成两部分以计算赝电容贡献率。式中k1和k2为特定电压下的常数,k1v为赝电容控制的贡献,k2v1/2为扩散控制的贡献。图6c中CV曲线的蓝色区域表示在1.0 mV·s-1的扫描速率下赝电容控制的贡献率,总容量的80.2%来自赝电容的贡献。从图6d可以看出,赝电容贡献率随着扫描速率的提高而提高。随着扫描速率从0.1 mV·s-1提高到1.0 mV·s-1,赝电容贡献率也从55.4%提高到80.2%。值得注意的是,在不同扫速下Zn0.28的赝电容贡献率始终最低,而Zn0.12的赝电容贡献率始终最高。赝电容贡献较高的材料其充放电的能力较高,而介孔结构能提高赝电容贡献率[41]。Zn0.12的高赝电容贡献,可能源于其较大的比表面积和孔径缩短了锂离子扩散的距离和提供了较多的电化学活性位点[42]。
计算出的D
图7a~d给出了三电极前三圈的原位阻抗。图7e给出了Zn0.12、Zn0.2和Zn0.28电极循环前的Nyquist图,插图为等效电路图,由中高频区域的半圆和低频区域的斜率线构成,半圆直径与电荷转移电阻(Rct)呈正相关,斜线的斜率σ与扩散过程中的Warburg阻抗(Zw )的值相关[43]。可以看出,在首次放电过程中三电极的Rct都较大,充电过程中Rct逐渐减小表明电荷转移的阻力变小[25]。在充放电过程中低频区的斜率σ均呈现先增大后减小的趋势,σ越大则Zw 越大,相应的D
表3 所制备的电极循环前和循环3、100圈后的等效电路图参数和锂离子扩散系数的计算值
Table 3
| Samples | Rs / Ω | Rct / Ω | D | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pristine | 3rd | 100th | Pristine | 3rd | 100th | Pristine | 3rd | 100th | |
| Zn0.12 | 4.4 | 5.2 | 6.1 | 338.0 | 26.8 | 31.8 | 14.9 | 2.1 | 14.8 |
| Zn0.2 | 8.3 | 6.0 | 8.6 | 248.0 | 42.0 | 14.9 | 10.4 | 2.5 | 20.4 |
| Zn0.28 | 5.6 | 4.3 | 6.4 | 283.7 | 8.1 | 26.9 | 17.3 | 2.5 | 10.8 |
根据公式
计算D
根据动力学分析结果,Zn0.2样品较高的晶格畸变和氧空位浓度,使其电导率较高。这些因素的共同作用使Zn0.2电极具有比Zn0.28电极更低的阻抗、更高的赝电容贡献率和D
3 结论
用溶液燃烧法在950℃制备的不同Zn含量的单相岩盐结构HEO Zn0.12、Zn0.2和Zn0.28,随着Zn含量的提高其电化学性能随之提高。虽然Zn0.2样品的反应动力学较高,但是Zn0.28的可逆比容量和倍率性能以及大电流下的循环稳定性更高。这些结果证实了岩盐结构HEO作为LIBs负极材料的转化机理。电化学活性较高的Zn元素在氧化还原反应中完全转化,Zn含量最高的Zn0.28样品其可逆比容量最高。Zn0.28样品出现了适当的晶格畸变和氧空位,为反应提供更多的活性位点和缩短了离子和电子的扩散路径,有利于提高倍率性能和使其在大电流下具有优异的循环稳定性。
参考文献
Challenges and opportunities towards fast-charging battery materials
[J].Extreme fast charging, with a goal of 15 minutes recharge time, is poised to accelerate mass market adoption of electric vehicles, curb greenhouse gas emissions and, in turn, provide nations with greater energy security. However, the realization of such a goal requires research and development across multiple levels, with battery technology being a key technical barrier. The present-day high-energy lithium-ion batteries with graphite anodes and transition metal oxide cathodes in liquid electrolytes are unable to achieve the fast-charging goal without negatively affecting electrochemical performance and safety. Here we discuss the challenges and future research directions towards fast charging at the level of battery materials from mass transport, charge transfer and thermal management perspectives. Moreover, we highlight advanced characterization techniques to fundamentally understand the failure mechanisms of batteries during fast charging, which in turn would inform more rational battery designs.
Improvement of rate capability by graphite foam anode for Li secondary batteries
[J].
High-entropy ceramics: Present status, challenges, and a look forward
[J].
Research progress in preparation and application of high-entropy oxides
[J].
高熵氧化物的制备及应用研究进展
[J].高熵氧化物作为近几年发展起来的新型氧化物体系,打破了传统掺杂氧化物的设计理念,由五种及以上氧化物以等摩尔或近等摩尔构成,因其具有简单的结构和优异的性能等受到国内外研究人员的广泛关注。高熵氧化物由于多主元且主元之间混乱排列,易形成岩盐型、氟化钙型、尖晶石型或钙钛矿等固溶体结构,从而表现出优异的性能,尤其在能源存储材料和磁性材料方面有十分广阔的应用前景,但目前对高熵氧化物应用研究较少。本工作介绍了国内外高熵氧化物的制备方法,主要包括固相法、热解法、共沉淀法、水热合成法和液相燃烧合成法等,比较了各方法的优缺点和发展前景;归纳了高熵氧化物作为锂离子电极材料、巨介电材料、磁性材料和催化材料等方面的应用;指出了高熵氧化物目前研究存在的问题,讨论了解决措施,展望了高熵氧化物未来的发展趋势。
Entropy-stabilized oxides
[J].Rost, Christina M.; Sachet, Edward; Borman, Trent; Moballegh, Ali; Dickey, Elizabeth C.; Hou, Dong; Jones, Jacob L.; Maria, Jon-Paul N Carolina State Univ, Dept Mat Sci & Engn, Raleigh, NC 27695 USA. Curtarolo, Stefano Duke Univ, Dept Mech Engn & Mat Sci, Ctr Mat Genom, Durham, NC 27708 USA.
Nanocrystalline multicomponent entropy stabilised transition metal oxides
[J].
High entropy oxides for reversible energy storage
[J].In recent years, the concept of entropy stabilization of crystal structures in oxide systems has led to an increased research activity in the field of "high entropy oxides". These compounds comprise the incorporation of multiple metal cations into single-phase crystal structures and interactions among the various metal cations leading to interesting novel and unexpected properties. Here, we report on the reversible lithium storage properties of the high entropy oxides, the underlying mechanisms governing these properties, and the influence of entropy stabilization on the electrochemical behavior. It is found that the stabilization effect of entropy brings significant benefits for the storage capacity retention of high entropy oxides and greatly improves the cycling stability. Additionally, it is observed that the electrochemical behavior of the high entropy oxides depends on each of the metal cations present, thus providing the opportunity to tailor the electrochemical properties by simply changing the elemental composition.
A high entropy oxide (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2O) with superior lithium storage performance
[J].
Operando synchrotron transmission X-ray microscopy study on (Mg, Co, Ni, Cu, Zn)O high-entropy oxide anodes for lithium-ion batteries
[J].
Tunable pseudocapacitive contribution by dimension control in nanocrystalline-constructed (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O solid solutions to achieve superior lithium-storage properties
[J].
Design and fabrication of high-entropy oxide anchored on graphene for boosting kinetic performance and energy storage
[J].
Evaluation of entropy‐stabilized (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O oxides produced via solvothermal method or electrospinning as anodes in lithium‐ion batteries
[J].
Synergy of cations in high entropy oxide lithium ion battery anode
[J].High entropy oxides (HEOs) with chemically disordered multi-cation structure attract intensive interest as negative electrode materials for battery applications. The outstanding electrochemical performance has been attributed to the high-entropy stabilization and the so-called 'cocktail effect'. However, the configurational entropy of the HEO, which is thermodynamically only metastable at room-temperature, is insufficient to drive the structural reversibility during conversion-type battery reaction, and the 'cocktail effect' has not been explained thus far. This work unveils the multi-cations synergy of the HEO MgCoNiCuZnO at atomic and nanoscale during electrochemical reaction and explains the 'cocktail effect'. The more electronegative elements form an electrochemically inert 3-dimensional metallic nano-network enabling electron transport. The electrochemical inactive cation stabilizes an oxide nanophase, which is semi-coherent with the metallic phase and accommodates Li ions. This self-assembled nanostructure enables stable cycling of micron-sized particles, which bypasses the need for nanoscale pre-modification required for conventional metal oxides in battery applications. This demonstrates elemental diversity is the key for optimizing multi-cation electrode materials.© 2023. The Author(s).
Controlled Jahn-Teller distortion in (MgCoNiCuZn)O-based high entropy oxides
[J].
Research progress in preparation and application of high-entropy-alloy powders
[J].
高熵合金粉体制备及应用研究进展
[J].
Coupling lattice instabilities across the interface in ultrathin oxide heterostructures
[J].
Entropy engineering design of high-performing lithiated oxide cathodes for proton-conducting solid oxide fuel cells
[J]. J.
Solution combustion synthesis and magnetic property of rock-salt (Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)O high-entropy oxide nanocrystalline powder
[J].
Scalable synthesis of hierarchical hollow Li4Ti5O12 microspheres assembled by zigzag-like nanosheets for high rate lithium-ion batteries
[J].
MOF-derived porous ZnO/ZnFe2O4/C octahedra with hollow interiors for high-rate lithium-ion batteries
[J].
Application of a Cu-Co alloy dendrite on glucose and hydrogen peroxide sensors
[J].
Spinel-structured high entropy oxide (FeCoNiCrMn)3O4 as anode towards superior lithium storage performance
[J].
Role of size, alio-/multi-valency and non-stoichiometry in the synthesis of phase-pure high entropy oxide (Co, Cu, Mg, Na, Ni, Zn)O
[J].
Inactive Al3+-doped La(CoCrFe-MnNiAl x )1/(5+ x)O3 high-entropy perovskite oxides as high performance supercapacitor electrodes
[J].
Zn0.5Co0.5Mn0.5Fe0.5Al0.5Mg0.5O4 high-entropy oxide with high capacity and ultra-long life for Li-ion battery anodes
[J].
Ultrafast synthesis of high entropy oxide nanoparticles by flame spray pyrolysis
[J].
A high-entropy spinel ceramic oxide as the cathode for proton-conducting solid oxide fuel cells
[J].
Oxygen vacancies boosting lithium-ion diffusion kinetics of lithium germanate for high-performance lithium storage
[J].
High-entropy oxides as advanced anode materials for long-life lithium-ion batteries
[J].
The oxygen vacancy in Li-ion battery cathode materials
[J].
Elucidating the strain-vacancy-activity relationship on structurally deformed Co@CoO nanosheets for aqueous phase reforming of formaldehyde
[J].
Charge-discharge mechanism of high-entropy Co-free spinel oxide toward Li+ storage examined using operando quick-scanning X-ray absorption spectroscopy
[J].
Kinetically accelerated lithium storage in high-entropy (LiMgCoNiCuZn)O enabled by oxygen vacancies
[J].
A high entropy oxide (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O with superior lithium storage performance
[J].
Preparation and lithium storage performance of pseudocapacitance-controlled chalcogenide high-entropy oxide La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3 anode materials
[J].
赝电容控制型钙钛矿高熵氧化物La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3材料的制备及储锂性能
[J].
Extra storage capacity in transition metal oxide lithium-ion batteries revealed by in situ magnetometry
[J].
High entropy spinel-structure oxide for electrochemical application
[J].
Novel P2-type layered medium-entropy ceramics oxide as cathode material for sodium-ion batteries
[J].
High-entropy chemistry stabilizing spinel oxide (CoNiZnXMnLi)3O4 (X = Fe, Cr) for high-performance anode of Li-ion batteries
[J].
Peapod-like Li3VO4/N-doped carbon nanowires with pseudocapacitive properties as advanced materials for high-energy lithium-ion capacitors
[J].
Preparation and High-performance Lithium-ion Storage of Cobalt-free Perovskite High-entropy Oxide Anode Materials
[J].
高性能无钴化钙钛矿型高熵氧化物负极材料的制备及储锂性能研究
[J].高熵氧化物由于独特的高熵效应、多主元协同效应和可定制的结构, 作为能量存储材料受到广泛地关注. 本研究采用金属硝酸盐为金属源, 甘氨酸为燃料, 通过溶液燃烧法成功合成了一系列无钴的钙钛矿型高熵氧化物La(Cr<sub>0.2</sub>Fe<sub>0.2</sub>Mn<sub>0.2</sub>Ni<sub>0.2</sub>M<sub>0.2</sub>)O<sub>3</sub> (M=Cu, Mg, Zn)锂离子电池负极材料, 研究了粉体的微观结构和电化学性能. 结果表明: 所制备的钙钛矿型高熵氧化物La(Cr<sub>0.2</sub>Fe<sub>0.2</sub>Mn<sub>0.2</sub>Ni<sub>0.2</sub>M<sub>0.2</sub>)O<sub>3</sub>均为单相钙钛矿结构, 形貌为多孔网状且各组成元素分布均匀, 其中引入非活性元素Mg或活性元素Cu的高熵氧化物电化学性能相近; 无钴化La(Cr<sub>0.2</sub>Fe<sub>0.2</sub>Mn<sub>0.2</sub>Ni<sub>0.2</sub>Zn<sub>0.2</sub>)O<sub>3</sub>电极具有最高的比容量(200 mA•g<sup>−1</sup>电流密度下循环250圈后可逆比容量为1014 mAh•g<sup>−1</sup>)、优异的循环稳定性(1000 mA•g<sup>−1</sup>电流密度下循环1000圈后可逆比容量为450 mAh•g<sup>−1</sup>且几乎没有容量衰减)以及卓越的倍率性能(100 mA•g<sup>−1</sup>电流密度下可逆比容量为396 mAh•g<sup>−1</sup>, 3000 mA•g<sup>−1</sup>电流密度下可逆比容量为198 mAh•g<sup>−1</sup>, 容量保持率为47.9%). 电化学性能提升主要归因于活性元素Zn的加入可以在还原过程中形成Li-Zn合金使得电极比容量明显增加; 同时较高的比表面积、介孔结构以及丰富的表面氧空位使其具有较高的电导率(0.14 S•cm<sup>−1</sup>)、锂离子扩散系数(2.1×10<sup>−12</sup> cm<sup>2</sup>•s<sup>−1</sup>)和较大的赝电容贡献率, 从而显著提升了材料的比容量和倍率性能. 因此, 引入能够与锂发生合金化反应的元素(如Zn)可以极大地提高电化学性能, 有利于为设计成本低廉、性能优异的无钴化高熵材料提供新的设计理念和思路.
Preparation and lithium storage performance of K+-doped spinel (Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)3O4 high-entropy oxide anode materials
[J].
K+掺杂尖晶石型(Co0.2Cr0.2Fe0.2Mn0.2-Ni0.2)3O4高熵氧化物负极材料制备与储锂性能研究
[J].通过溶液燃烧法成功合成了一系列非活性K<sup>+</sup>掺杂的尖晶石型 (K <sub>x</sub> CoCrFeMnNi)<sub>3/(5+</sub><sub>x</sub><sub>)</sub>O<sub>4</sub>(x=0,0.5,1,1.5)高熵氧化物锂离子电池负极材料,系统研究了K<sup>+</sup>掺杂对结构和储锂性能的影响。结果表明:随着K<sup>+</sup>掺杂量的增加,均可制备出具有单一尖晶石结构的纳米晶粉体材料,其中等摩尔K<sup>+</sup>掺杂的 (K<sub>1/6</sub>Co<sub>1/6</sub>Cr<sub>1/6</sub>Fe<sub>1/6</sub>Mn<sub>1/6</sub>Ni<sub>1/6</sub>)<sub>3</sub>O<sub>4</sub>高熵氧化物负极材料具有最高的比容量、优异的循环稳定性和倍率性能。(K<sub>1/6</sub>Co<sub>1/6</sub>Cr<sub>1/6</sub>Fe<sub>1/6</sub>Mn<sub>1/6</sub>Ni<sub>1/6</sub>)<sub>3</sub>O<sub>4</sub>电极在200 mA·g<sup>-1</sup>电流密度下,首次放电比容量为1295 mA·h·g<sup>-1</sup>(首次库仑效率78%);随着循环的进行,可逆比容量先降低后增加,循环150次可逆比容量增加至1505 mA·h·g<sup>-1</sup>;即使在1000 mA·g<sup>-1</sup>大电流密度下循环500次后仍具有1402 mA·h·g<sup>-1</sup>的可逆比容量(均高于理论比容量898 mA·h·g<sup>-1</sup>)。低价非活性K<sup>+</sup>的掺杂由于电荷补偿效应使晶格常数降低,但高构型熵稳定的晶体结构提高了循环稳定性;丰富的表面氧空位、较小的晶粒尺寸和介孔结构,增加了赝电容贡献率和电子/离子传输能力,从而显著提升了材料的比容量和倍率性能。
Oxygen vacancy-expedited ion diffusivity in transition-metal oxides for high-performance lithium-ion batteries
[J].
Synergetic effect of lattice distortion and oxygen vacancies on high-rate lithium-ion storage in high-entropy perovskite oxide
[J].
Enhanced Li-ion diffusion and cycling stability of Ni-free high-entropy spinel oxide anodes with high-concentration oxygen vacancies
[J].