奥氏体不锈钢具有良好的塑性、韧性、强度和耐蚀性,得到了广泛的应用。但是,奥氏体不锈钢组成中的合金元素Ni价格极其昂贵[1]。研究发现,可添加N等奥氏体稳定元素部分替代Ni[2]。N在奥氏体钢中的固溶度不到1%(质量分数),但是对其强度和耐蚀性的影响却极为显著。奥氏体钢中的N通过钉扎位错、固溶强化和细晶强化等机制使其强度提高[3,4]。在含Cl-、H+等的腐蚀性溶液中不锈钢容易发生局部腐蚀,其耐点蚀当量(Pitting resistance equivalent number,PREN)为PREN = %Cr + 3.3Mo + 13%~30%N-0.25Ni[5]。这表明,少量的N就能使不锈钢的耐点蚀性显著提高。N提高抗点蚀性能的机制包括:(1) 质子消耗机制。钝化膜局部破裂形成亚稳态蚀坑,N溶解形成铵根离子。这一过程消耗了蚀坑中的质子,缓冲蚀坑内的局部pH值,使蚀坑内局部化学环境的侵蚀性降低,从而抑制了亚稳态蚀坑通过自催化过程的进一步扩展,在蚀坑生长为稳定点蚀坑前使其发生再钝化[6~8]。也有学者提出,在含有次氯酸等高价氯的溶液中铵和铵根离子与N反应生成氯胺,抑制高价氯化物引起的腐蚀[9]。(2) 界面富集机制。在含N不锈钢中,N的含量通常为0.1%~0.45%。但是阳极极化后N在厚度约为3 nm的金属基体一侧富集,其最高含量可达基体含量的7倍[10]。N在界面处偏聚,是金属原子选择性溶解所致,这可能是其抗点蚀性能提高的原因[6]。N在表面的偏聚可能生成FeN而抑制Fe的溶解并填充蚀坑,不会促进点蚀自催化发展所需的局部化学环境[11]。Marcus等发现,N不但在金属表面富集,而且还进入钝化膜中[12]。在金属与钝化膜的界面处生成CrN,稳定界面活性和阻碍金属离子透过钝化膜的溶解过程,从而使腐蚀受到抑制。还有一些学者提出膜内形成氮化物稳定钝化膜结构的机制,以解释不锈钢耐蚀性的提高[13~15]。(3) 合金元素N与Mo间的协同作用机制。Truman等[16]发现,Cr含量高于20%、Mo含量高于1.5%以及N含量高于0.15%时,N对点蚀萌生的抗性非常显著,N含量的微弱提高就能使抗点蚀性能显著提高。Olsson[17]发现,在铁素体相N含量很低的2205双相不锈钢中,N的单独作用不足以提高钝化膜的稳定性和耐蚀性,但是N-Mo的协同作用可使其表面结构稳定性提高。
目前合金氮化的主要方法有:(1) 冶炼时维持较高的N分压(Smelting nitriding)。即在高温冶炼时通入含氮气体或加入氮化物,例如通入NH3制备含量高达3%的高氮奥氏体不锈钢[18]。(2) 等离子氮化(Plasma nitriding)。这种不锈钢表面氮化技术需要在至少300~500℃的温度下保持5 h,能大幅度提高不锈钢的耐蚀性[19~23]。(3) 电化学渗氮(Electrochemical nitriding)。这种方法可在不锈钢表面引入N,最早由Willenbruch等在盐酸-硝酸钠溶液中进行,使不锈钢的耐蚀性显著提高[24]。用硝酸替代盐酸,更有利于电化学渗氮。Wang[14,25]等报道,在0.1 mol/L HNO3 + 0.5 mol/L KNO3溶液中进行电化学渗氮可使不锈钢的耐蚀性显著提高,更适应质子交换膜燃料电池双极板所处的酸性、有电流通过的三相复杂环境。许多研究者报道了电化学渗氮对不锈钢耐蚀性的影响,用XPS和电化学测试等方法证实电化学渗氮在不锈钢表面引入N和提高不锈钢耐蚀性[13,25~30]。
N影响不锈钢腐蚀性能作用机制的假说表明,N的作用主要是在不锈钢中的分布特征、存在形式以及腐蚀过程中发生的反应改变了局部腐蚀环境。电化学渗氮过程涉及电化学反应,而发生电极反应的不锈钢其表面结构在电化学渗氮过程中不可避免地发生变化。研究电化学渗氮过程中表面结构特征的演变,有助于理解不锈钢表面的电极过程和优化电化学渗氮工艺。本文对304L不锈钢进行电化学渗氮,使用原子力显微镜、透射电子显微镜、测试电化学极化曲线和电化学阻抗谱,研究电化学渗氮对材料的表面性质和腐蚀性能的影响。
1 实验方法
实验用304L型不锈钢的成分为:18.29%Cr、8.10%Ni、0.44%Si、1.30%Mn、0.006%S、0.078%P,其余为Fe。将尺寸为2 mm × 2 mm × 1.8 mm的不锈钢试样用水磨砂纸逐级打磨至1500#,然后在-20℃的10%高氯酸酒精溶液中进行电化学抛光,然后封装成电极。
使用AUTOLAB PGSTAT302N型电化学工作站进行电化学渗氮及电化学测试,传统的三电极体系包括不锈钢工作电极,Pt片辅助电极和饱和甘汞电极(SCE)参比电极。文中的电极电位都是相对于SCE参比电极的电位。
在常温常压下进行电化学渗氮。将试样在0.1 mol/L HNO3 + 0.5 mol/L KNO3溶液中极化4 h,恒电位为-0.7 V。电化学渗氮结束后,用去离子水冲洗试样的表面并吹干水分。
在3.5%NaCl(质量分数)溶液中进行开路电位(OCP)的监测和极化曲线的测试;在0.5 mol/L H2SO4溶液中测试电化学阻抗谱(EIS),测试前将样品在溶液中稳定300 s。在开路电位下进行EIS测试,加载正弦波作为扰动信号,频率范围为0.1~105 Hz,振幅为10 mV。用Cypher ES型原子力显微镜(AFM)探测电化学渗氮后304L不锈钢的表面结构,探测位置包括渗氮样品表面的边缘和中部。Cypher ES型AFM具有自动马达控制悬臂激光对准系统、Koher照明系统和3.1 M像素CMOS相机,用于快速准确地扫描试样的表面结构。用Spectra 300和Talos F200X G2型透射电子显微镜表征电化学渗氮前后不锈钢样品的截面。
2 实验结果和讨论
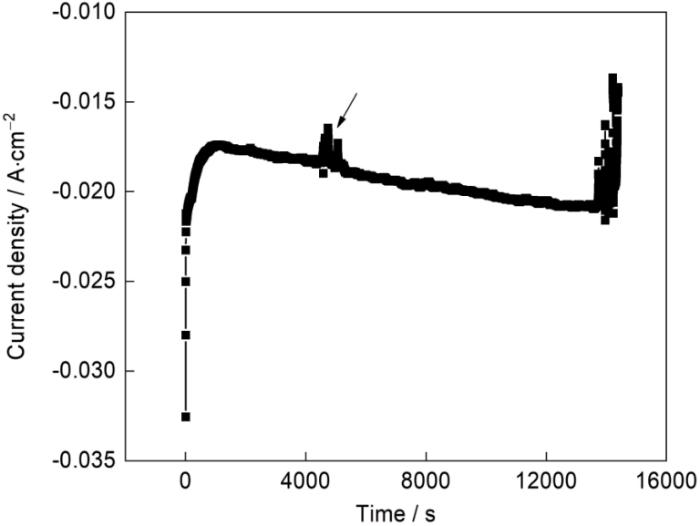
2.1 电化学渗氮的电流密度
图1
图1
电化学渗氮电流密度随渗氮时间的变化
Fig.1
Anodic current density vs. time curve in electrochemical nitriding
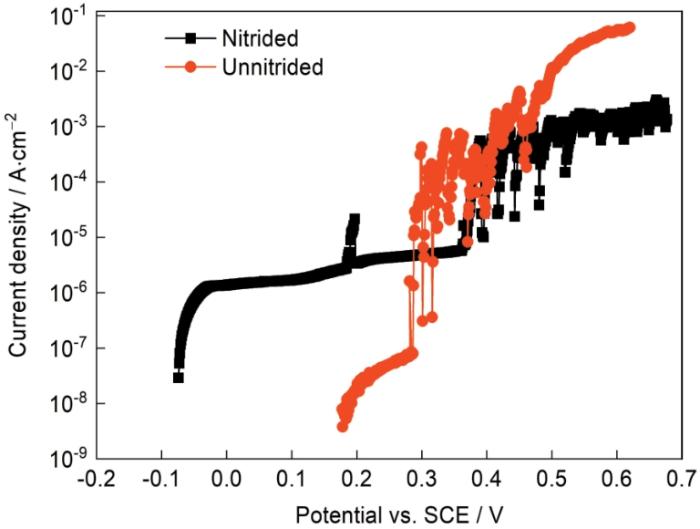
2.2 阳极极化曲线
图2
图2
304L不锈钢电化学渗氮前后在3.5%NaCl溶液中的阳极极化曲线
Fig.2
Anodic polarization curves of 304L stainless steel with as well as free of electrochemical nitriding in 3.5% (mass fraction) NaCl solution
2.3 表面结构
2.3.1 AFM的结构表征
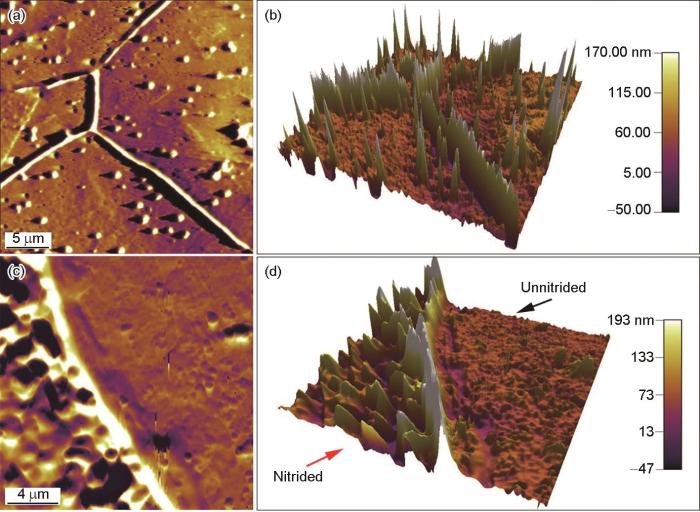
渗氮后304L不锈钢的AFM表面形貌像如图3a、b所示。可以看出,在渗氮后的304L表面,尤其是晶界处,出现了明显的起伏。样品渗氮区域和封装区域的AFM成像如图 3c、d所示,右侧是封装电极时用松香石蜡覆盖的区域,渗氮时没有接触电解质溶液,因此电化学抛光后保持平整,在AFM形貌图中最大起伏不超过30 nm;而渗氮区域,其表面最大起伏超过100 nm。电化学渗氮后表面的明显起伏,表明渗氮过程中不锈钢表面发生了局部溶解或反应产物局部沉积。在-0.7 V的阴极渗氮电位下,不锈钢表面状态不均一,并没有完全处于阴极保护状态,部分表面的平衡电位较低,在-0.7 V电位极化下可能发生阳极溶解,而相对平衡电位更高的位置则作为还原反应的场所发生了硝酸根离子的还原,钝化膜也可能还原溶解而暴露出膜下的基体,从而转变为可发生溶解的微阳极区。也就是在渗氮过程中电极表面并没有都发生阴极反应,其表面存在微阴极和微阳极区,且随着反应的进行处于动态变化中;同时,溶解的金属离子也可能再沉积而形成具有微观起伏特征的表面。电化学渗氮造成的表面微观起伏使不锈钢的比表面积增大,新暴露的表面增加了发生渗氮电极反应的位置,从而使渗氮电流随时间逐渐增大(图1)。评价渗氮对抗点蚀性能的影响时(图2)发现,虽然渗氮后点蚀电位显著提高,但是钝化电流密度却增大了近一个数量级。AFM表征结果表明,渗氮后比表面积显著增加,但是计算电流密度时使用的面积仍然是样品的远低于实际表面积的宏观表面积,,其结果是图2中的钝化电流密度偏大。
图3
图3
电化学渗氮对304L不锈钢表面结构的影响
Fig.3
AFM characterization illustrating the effect of electrochemical nitriding on the surface morphology of 304L stainless steel (a) AFM image showing the morphology of the nitrided surface which include grain boundaries, (b) three-dimensional (3D) morphology corresponding to the zone shown in Fig.3a (c) AFM image showing the morphology of the site of nitride zone adjacent to the unaffected zone and (d) 3D morphology corresponding to the zone shown in Fig.3c
2.3.2 TEM的结构表征
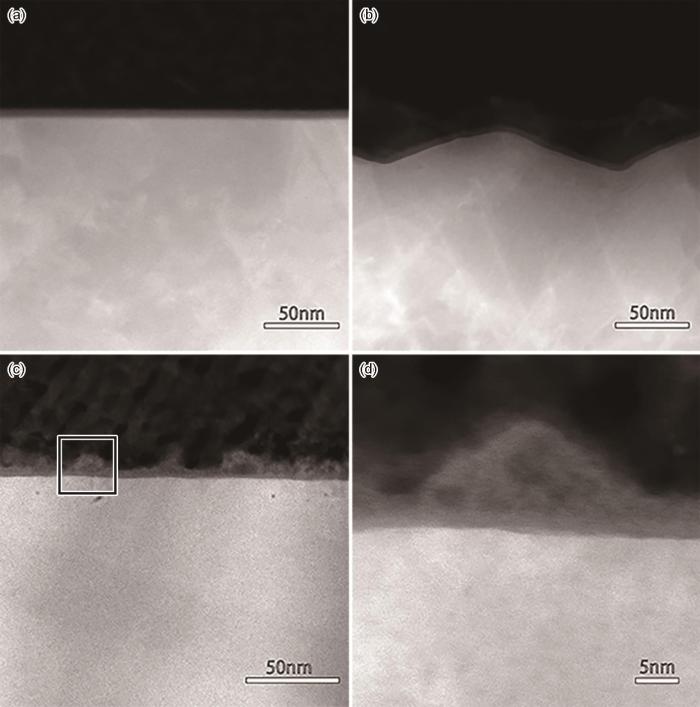
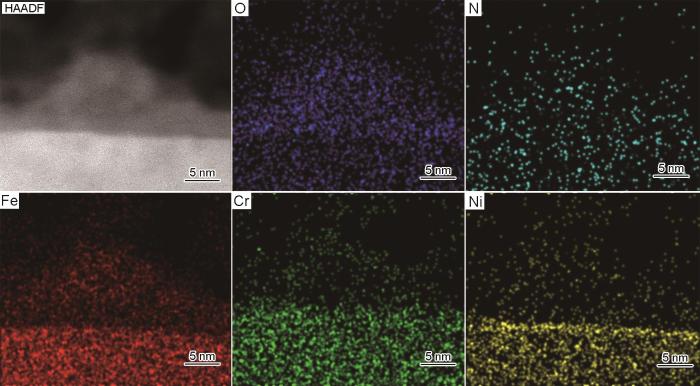
用AFM技术探测到电化学渗氮产生了表面的微观起伏。用扫描透射(STEM)成像技术并结合超级能谱(Super-EDS)分析,从垂直于渗氮表面的纵深方向探测渗氮造成的表面结构特征的变化。高角环形暗场模式下的扫描透射电镜(HAADF-STEM)模式是通过高角环形探测器接收高角非弹性散射电子进行成像,其图像衬度与原子序数的平方近似成正比,原子序数越大衬度越亮。将经过电化学渗氮的不锈钢样品进行对粘,预磨薄化后继续离子减薄制成TEM截面样品。图4给出了渗氮前后样品的HAADF-STEM图像。可以看出,电化学抛光处理的样品表面平滑、钝化膜与基体界面清晰且平直(图4a),而经过电化学渗氮的样品表面状态不均一,一些局部位置原来的平滑表面变为起伏表面,出现几十纳米的起伏(图4b)。同时,在如图4c所示的局部位置,疏松的产物沉积在原来的平直表面上;图4d给出了对应图4c框选区域的放大图像,可见沉积物的衬度比基体更暗且不均一,表现出疏松的结构特征。对沉积物的能谱分析结果,如图5所示。图5表明,沉积物的组成主要是Fe的氧化物,紧邻氧化膜下的金属基体的富Ni特征也清晰可见。表面起伏和沉积物的出现,进一步佐证了在“电化学渗氮过程中伴随微阳极位置的溶解和在微阴极位置产物沉积”。图4b所示的起伏位置对应微阳极区。即使是微阳极区,其阳极溶解速率仍然存在差异,从而形成了微观起伏的表面特征;而图4c所示的产物沉积位置对应微阴极区,与之相邻的微阳极区溶解形成的金属阳离子传输至此发生沉积。沉积产物下的基体,仍然保持着未溶解的平直表面特征。能谱分析结果没有给出明显的渗氮层,其原因可能是N含量较低,原子序数小且分布不均匀,而EDS 面扫描分析的区域又很小,导致在能谱面扫描结果中表现出噪音信号。但是,TEM和EDS分析清晰地给出了电化学渗氮所导致的表面结构特征的变化。
图4
图4
304L不锈钢电化学渗氮前后的剖面显微图像
Fig.4
HAADF-STEM images from the cross-sectional direction showing the structural evolution induced by the electrochemical nitriding (a) HAADF-STEM image of 304L stainless steel free of nitriding showing a straight and sharp interface,(b) HAADF-STEM image showing the undulating surface induced by the electrochemical nitriding, (c) HAADF-STEM image showing some loose deposits with darker contrast cover the original smooth surface, (d) the zoom-in image corresponding to the zone labeled with the red rectangle in panel Fig.4c
图5
图5
电化学渗氮后沉积在不锈钢表面的沉积物能谱
Fig.5
Element EDS mapping analysis showing that the products deposited on the surface after electrochemical nitriding is mainly comprised of iron oxide
2.4 电化学阻抗谱
电化学阻抗谱,是通过施加周期性扰动信号采集系统输出的响应信号,根据输入输出信号的变化分析电极材料在特定电化学系统中的表面特征。电化学极化曲线和显微结构表征表明,电化学渗氮影响不锈钢表面的形貌和耐蚀性。用酸性溶液模拟不锈钢渗氮前后在酸性环境,用EIS测试表面性质的变化,以深入了解电化学渗氮影响不锈钢腐蚀性能的机制。
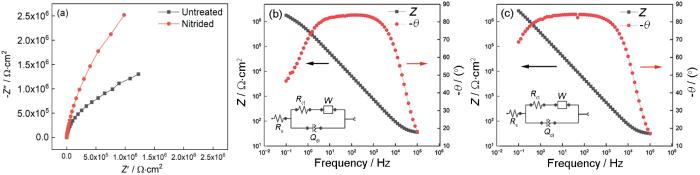
图6a给出了渗氮前后的304L不锈钢的阻抗谱Nyquist图,可见渗氮显著增大了容抗弧的直径。这表明,渗氮处理表面产生了更大的电荷转移电阻,表明其具有更高的耐蚀性,与极化曲线测试结果一致。Bode图中高频段相位与表面缺陷和溶液电阻相关,中频段的相位角度与表面氧化膜的内在性质相关,低频段的相位与氧化膜和金属界面相关[31]。对比图6b、c,可见渗氮前后的中高频段相位角度类似,均表现出电容特性;渗氮后低频段相位角度更明显地接近90°,如表1所示,模拟电路的电荷转移电阻(Rct)由渗氮前1.33 MΩ·cm2提高到渗氮后7.53 MΩ·cm2,表明渗氮后在金属基体与氧化膜界面处发生电极反应的电荷转移更困难。这个结果,与有些学者提出的N富集表面[32~34]使界面稳定和提高耐蚀性的机制一致。
图6
图6
304L不锈钢渗氮前后的电化学阻抗谱
Fig.6
Electrochemical impedance spectra of 304L stainless steel before and after nitriding (a) Nyquist diagram, (b) electrochemical pre-nitriding Bode diagram, (c) Bode diagram after electrochemical nitriding
表1 电化学渗氮前后EIS曲线拟合的参数值
Table 1
| Materials | Rs / Ω·cm2 | Rct / MΩ·cm2 | Qdl / nΩ-1·s N | N | W / nΩ-1·s0.5 |
|---|---|---|---|---|---|
| Unnitrided | 33.2 | 1.33 | 377 | 0.935 | 879 |
| Nitrided | 29.8 | 7.53 | 505 | 0.934 | 464 |
3 结论
(1) 在电化学渗氮过程中304L不锈钢的表面变得粗糙,表面起伏幅度约为100 nm。
(2) 在电化学渗氮电位下电极表面伴随着微阳极溶解和金属离子在微阴极的再沉积,从而产生了微观起伏的粗糙表面。
(3) 电化学渗氮提高了304L不锈钢的抗点蚀性能,提高了金属/氧化膜的界面性能,支持了N使不锈钢耐蚀性提高的界面富集机制。
参考文献
Medium- to long-term nickel price forecasting using LSTM and GRU networks
[J].
High-nitrogen steel
[J].
Overview: high-nitrogen alloying of stainless ste-els
[J].
The influence of nitrogen and grain size on yield strength in type AISI 316L austenitic stainless steel
[J].
Pitting and crevice corrosion of superaustenitic stainless steels
[J].
Properties of nitrogen-containing stainless alloy designed for high resistance to pitting
[J].This paper describes the following features of a recently developed high nitrogen-bearing, highly corrosion resistant stainless steel: tensile properties, stress corrosion characteristics in boiling magnesium chloride solution, effect of sensitizing heat treatment on the nature and extent of grain boundary attack, and electrochemical polarization behavior in sulfuric acid and low pH chloride solutions.
Passivity and its breakdown on iron and iron-base alloys
[R].
Role of nitrogen on the corrosion behavior of austenitic stainless steels
[J].
Cathodic reactions involved in metallic corrosion in chlorinated saline environments
[J].
Surface enrichment of nitrogen during passivation of a highly resistant stainless steel
[J].
Synergism of alloying elements and pitting corrosion resistance of stainless steels
[J].
The role of nitrogen in the passivity of austenitic stainless steels
[J].
Formation of a protective nitride layer by electrochemical nitridation on 316L SS bipolar plates for a proton exchange membrane fuel cell (PEMFC)
[J].
Modifying a stainless steel via electrochemical nitridation
[J].
Characterization and corrosion behavior of high-nitrogen HP-13Cr stainless steel in CO2 and H2S environment
[J].
Note on the influence of nitrogen content on the resistance to pitting corrosion of stainless steels
[J].
The influence of nitrogen and molybdenum on passive films formed on the austenoferritic stainless steel 2205 studied by AES and XPS
[J].
Fabrication of ultra high nitrogen austenitic stainless steel by NH3 solution nitriding
[J].
On the effects of H2 and Ar on dual layer formed by plasma nitrocarburizing on austenitic stainless steels
[J].
Wear and corrosion studies of duplex surface-treated AISI-304 steel by a combination of cathodic cage plasma nitriding and PVD-TiN coating
[J].
Plasma nitrided type 349 stainless steel for polymer electrolyte membrane fuel cell bipolar plate-part I: nitrided in nitrogen plasma
[J].
Active screen plasma nitriding-an overview
[J].Extensive researches carried out over the past few years have shown that the novel active screen plasma nitriding (ASPN) technique can be used to treat low alloy steels, stainless steels, tool steels and other steels to achieve identical nitriding effects as the conventional DC plasma nitriding technology. Importantly, the ASPN technique provides the possibilities of treating non-electrical conducting materials such as steel with an oxidised surface and polymeric materials which are unattainable with a conventional DC plasma system. Experimental results presented in this overview further demonstrate that sputtering and deposition play important roles in nitrogen mass transfer in ASPN. In order to achieve a desirable metallurgical response, materials for the active screen and the amount of bias applied to the component have to be considered in applications of active screen plasma processing. The distance between the screen and the component surface also needs to be considered if the components to be treated are placed in a floating potential.
Structure and corrosion resistance of plasma nitrided stainless steel
[J].AISI316 stainless steel has been plasma nitrided at 570°C over a range of processing conditions, and the resultant corrosion properties have been investigated by a potentiodynamic polarization technique. X-ray diffraction and transmission electron microscopy studies have been used to map the process parameters under which a duplex surface compound layer of γ’ phase and austenite is formed. This surface compound layer has better corrosion resistance than a plasma nitrided stainless steel surface, where the normal hardened layer consists of austenite’ and chromium nitride precipitates. It has been found that the improvement in corrosion resistance is related to the presence of the γ’ nitride. Furthermore; low temperature plasma nitriding at 400° C produces a nitrided layer which has a corrosion resistance equivalent to that of the original material.
An XPS and electrochemical study of the influence of molybdenum and nitrogen on the passivity of austenitic stainless steel
[J].
Electrochemical nitridation of a stainless steel for PEMFC bipolar plates
[J].
On the influence of cold working and electrochemical nitridation on the corrosion behaviour of 316L austenitic stainless steel in acidic environment
[J].
The effect of electrochemical nitridation on the corrosion resistance of the passive films formed on the 2205 duplex stainless steel
[J].
Corrosion and interfacial contact resistance behavior of electrochemically nitrided 316L SS bipolar plates for proton exchange membrane fuel cells
[J].
Effect of grain refinement and electrochemical nitridation on corrosion resistance of the 316L stainless steel for bipolar plates in PEMFCs environment
[J].
Modifying a stainless steel for PEMFC bipolar plates via electrochemical nitridation
[J].
Corrosion protection of CrN/TiN multi-coating for bipolar plate of polymer electrolyte membrane fuel cell
[J].
High temperature solution-nitriding and low-temperature nitriding of AISI 316: effect on pitting potential and crevice corrosion performance
[J].
The evaluation of the repassivation tendency of Cr-Mn and Cr-Ni steels using scratch technique
[J].