纤维素是地球上储量最丰富的可再生聚合物资源,是植物体光合作用的主要多糖产物,其碳含量占植物界的50%以上[4]。用纤维素制备纤维素基吸附材料,已广泛应用于水污染治理。但是,这种吸附材料有吸附能力差和功能单一等不足。因此,通过对纤维素进行物理化学改性,掺入其他物质或在其羟基位置上引入活性官能团,从而制备出吸附性能较优的纤维素基吸附材料[5,6]。单宁是一种含有酚类结构的天然酚醛聚合物,具有特异性金属络合能力,能与二价和三价阳离子生成稳定的配合物;同时,单宁与阴离子吸附物之间也可能发生螯合。许多氧阴离子在水溶液中能与多元醇反应,生成具有环结构的阴离子络合物。单宁的邻酚羟基易与金属发生螯合作用,利用该特性可在材料中有效引入金属元素以实现材料的金属负载改性[7,8]。研究表明,将金属作为电子缺失官能团引入到吸附剂表面,通过络合或结构配体等形式的作用力可增强吸附剂与污染物间的亲和力,提高吸附性能[9,10]。Luo等[11]制备的单宁薄膜覆盖的磁性纳米粒子(Fe3O4@TA-Fe3+),在pH =6.0的水溶液中对Pb2+和Hg2+吸附量分别达到1115和279.4 mg/g。廖卉[12]以胶原纤维固化杨梅单宁为载体制备的铁镍双金属负载H-NZFNP,在pH = 5.0时对U(VI)的去除率高于98%。
本文以纤维素粉为原料,用碱/尿素水体系将其溶解,再以环氧氯丙烷为交联剂将杨梅单宁引入到纤维素骨架上制备纤维素固化杨梅单宁复合气凝胶(CBT)。进一步以九水合硝酸铁作为铁源,创新性地利用铁离子与单宁酚羟基间的络合作用实现水体中铁离子在材料上的富集和均匀分散,再通过原位还原反应实现铁离子在纤维素基底上的固定,制得铁负载纤维素/单宁复合吸附剂(Fe-CBT)。以典型抗生素FQs为目标污染物研究吸附剂的吸附性能并探究其中的吸附机理。
1 实验方法
1.1 实验用试剂和仪器设备
实验用试剂:纤维素粉(CP),尿素(Urea),环氧氯丙烷(ECH),氢氧化钠(NaOH),九水合硝酸铁(Fe(NO)3·9H2O),硼氢化钠(NaBH4),杨梅单宁(BT)以及去离子水。
实验用仪器:傅里叶变换红外光谱仪(AVAT-AR 360);钨灯丝扫描电子显微镜(Quanta250);自动氮吸附仪(3H-2000PS1);紫外-可见分光光度计(UV-1780);X射线光电子能谱仪(Kα);热重分析仪(STA449F3);X射线粉末衍射仪(PW-183);Zeta电位与纳米粒度仪(NanoPlus3)。
1.2 铁负载纤维素/杨梅单宁复合吸附剂的制备
(1) 纤维素溶液的制备:按照m(NaOH)∶m(Urea)∶m(H2O)的质量比为7∶12∶81的比例在塑料烧杯中配制纤维素溶解体系,在其中加入纤维素粉继续搅拌至分散均匀后密封,置于超低温冰箱中冷冻过夜,然后在室温下机械搅拌融化使其成为均一透明状溶液。
(2) 纤维素/单宁复合材料(CBT)的制备:在250 mL三口烧瓶中倒入50 g溶解好的纤维素溶液和2 g杨梅单宁(BT)粉末,充分搅拌均匀后加入10 g环氧氯丙烷并剧烈搅拌10 min。将三口烧瓶转移至温度为60℃的烘箱中静置反应6 h。反应结束后用超纯水浸泡以洗去残余反应物使洗涤液呈中性,冷冻干燥后得到纤维素/杨梅单宁复合气凝胶(CBT)。
(3) 铁负载纤维素/单宁复合吸附剂(Fe-CBT)的制备:将0.6 g的 CBT浸泡在100 mg·L-1的九水合硝酸铁Fe3+溶液中,放入恒温摇床中震荡6 h至吸附饱和。在N2氛围中缓慢滴加100 mL的硼氢化钠溶液(n(NaBH4)∶n(Fe3+) = 0.1∶1.00),浸泡30 min进行原位还原。将产物用无水乙醇和超纯水反复浸泡清洗后冷冻干燥,制得铁负载纤维素单宁复合吸附剂Fe-CBT。
1.3 吸附实验
分别配制1000 mg·L-1的氟喹诺酮类(FQs)抗生素诺氟沙星(NOR)、盐酸洛美沙星(LOM)和盐酸左氧氟沙星(LVX)贮备液,并将其稀释到所需要的浓度。
将25 mL的贮备液转移至100 mL锥形瓶中,加入10 mg Fe-CBT样品并密封后置于恒温振荡器中以150 r/min的转速进行吸附反应。吸附前后分别用5 mL的注射器吸取适量上清液,稀释后用0.45 μm的滤头过滤,用紫外-可见分光光度计分别测量溶液中NOR、LOM和LVX的质量浓度,根据公式
分别计算NOR、LOM和LVX的吸附量和去除率。其中qe为平衡吸附容量(mg·g-1);C0为吸附体系的污染物初始质量浓度(mg·L-1);Ce为吸附平衡时污染物的质量浓度(mg·L-1);V为溶液体积(L);m为吸附剂投加质量(g);w为去除率(%)。
1.4 材料的表征
用Quanta250型扫描电镜-X射线能谱仪(SEM-EDS)表征铁固定前后CBT和Fe-CBT的表面形态。用PW-183型X射线粉末衍射仪(XRD)和AVAT-AR 360型红外光谱仪测试CP、CBT和Fe-CBT的XRD谱和红外光谱。用Kα型X-射线光电子能谱仪(XPS)分析表面元素组成和价态;用3H-2000PS1型自动氮吸附仪(BET)测试两种气凝胶的N2吸附脱附,以定量表征CBT、Fe-CBT的比表面积并说明其孔隙结构。用STA449F3型热重分析仪测试材料的TG、DTG曲线。
2 结果与讨论
2.1 理化性能
2.1.1 CBT和Fe-CBT的形貌和EDS分析
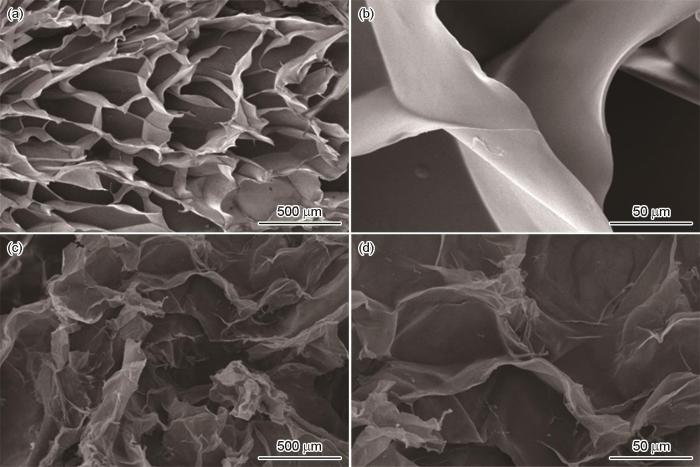
图1给出了CBT和Fe-CBT的SEM照片。可以看出,CBT呈现出三维多孔交连的网状结构。交联反应引起纤维素链的聚集并在溶剂中再生,这种化学自组装生成的孔壁为材料的网络提供了支撑结构。而在原位负载铁离子改性后,材料中的孔洞发生了一定程度的坍塌,孔径略有增大,孔壁表面变得粗糙,但是多孔结构仍然存在,表面褶皱增多,有利于增大吸附剂与吸附质间的接触面积和吸附反应的进行。
图1
图1
CBT和Fe-CBT的扫描电镜照片
Fig.1
SEM images of CBT and Fe-CBT (a, b) CBT; (c, d) Fe-CBT
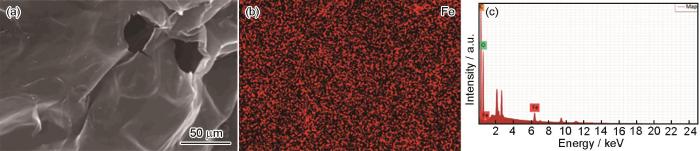
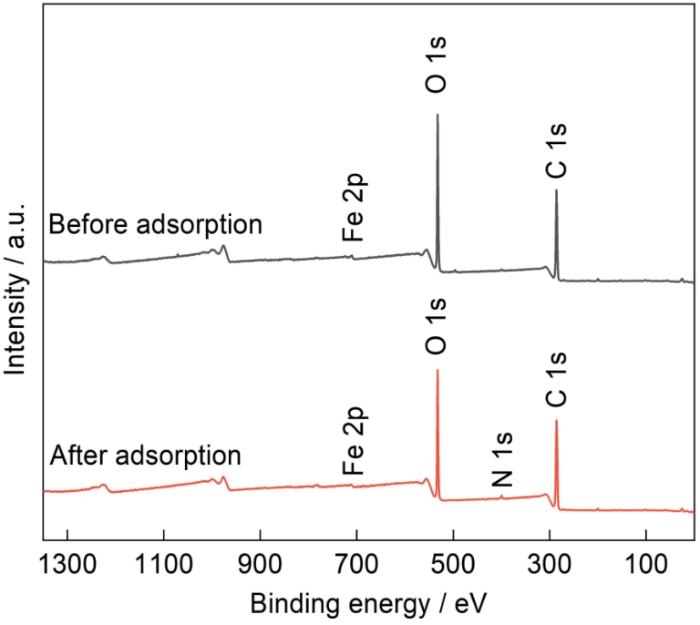
图2给出了Fe-CBT的扫描电镜照片、EDS元素分布分析及EDS图谱,由此可得Fe-CBT上C、O和Fe三种元素的占比分别为50.22%、47.72%和2.07%,证实在CBT表面成功地引入了金属元素Fe。
图2
图2
Fe-CBT的扫描电镜照片、EDS元素分布分析及EDS图谱
Fig.2
SEM images of Fe-CBT (a), EDS analytical results of element distribution of Fe-CBT (b) and EDS patterns of Fe-CBT (c)
2.1.2 CP、CBT和Fe-CBT的结构
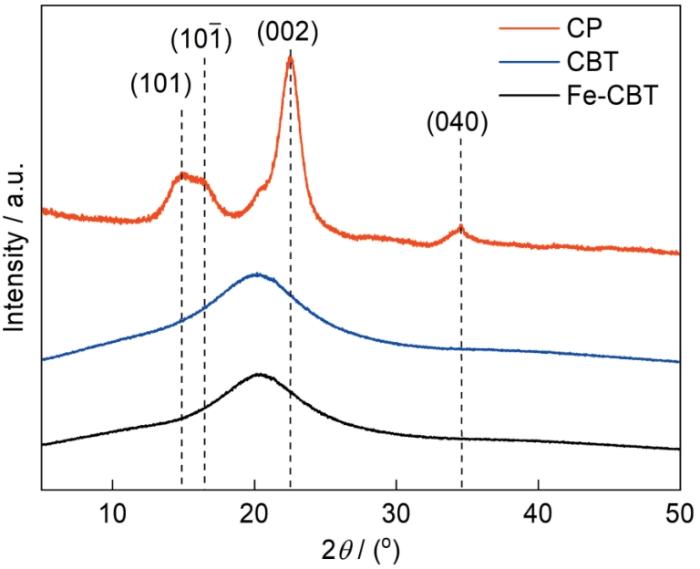
图3给出了材料的x射线衍射谱。可以看出,原料纤维素粉末CP呈现相对有序的结构,在谱中2θ值分别为14.9°、16.4°、22.5°和34.5°处出现了对应于天然纤维素I型结构中的典型晶面(101)、(10
图3
2.1.3 红外(FT-IR)光谱分析
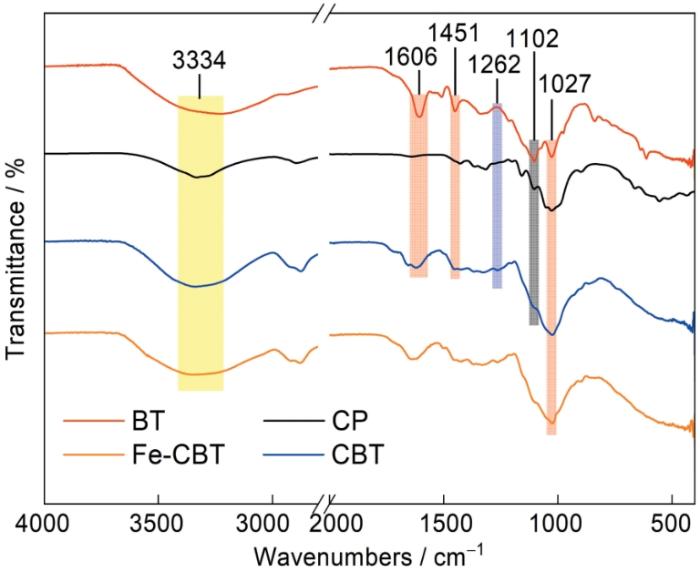
图4给出了CP、BT、CBT和Fe-CBT的红外光谱。可以看出,在CP和BT谱中的3334 cm-1附近都出现了宽羟基带,在改性后的CBT的谱中该处的峰比CP的强度提高且变宽,可归因于材料中引入了单宁。BT的红外光谱中1606和1451 cm-1处出现的吸附峰分别对应芳环中C=C基团的对称和反对称伸缩振动[19]。这些峰在CBT的谱中中仍然存在,但是峰的位置轻微偏移。1027 cm-1附近的峰归属于材料中C-O的伸缩振动。而在CP谱中1102 cm-1处出现的吸收峰,对应纤维素上的伯醇羟基-OH的伸缩振动。在CBT的谱中该吸收峰的强度明显降低,在1262 cm-1处出现一个对应芳醚的=C-O-C的反对称拉伸吸收峰。这些结果都表明,ECH作为交联剂通过与单宁中的酚羟基和纤维素中的醇羟基进行醚化反应实现了单宁在纤维素纤维上的固化[20,21]。与CBT相比,在Fe-CBT谱中的3334 cm-1附近的宽羟基带变宽强度减弱,在1027 cm-1附近的峰强度降低且发生了轻微蓝移。这些变化可归因于CBT中的羟基与铁离子之间发生了络合反应[22,23],而且络合反应在铁离子负载中起了重要的作用。
图4
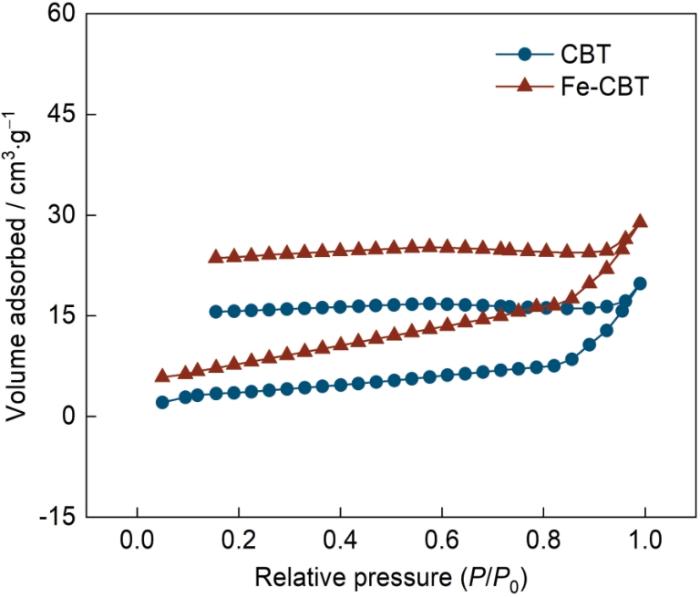
2.1.4 CBT、Fe-CBT的比表面积和孔隙结构
图5
图5
CBT和Fe-CBT的N2吸附-脱附等温曲线
Fig.5
N2 adsorption-desorption isotherms of CBT and Fe-CBT
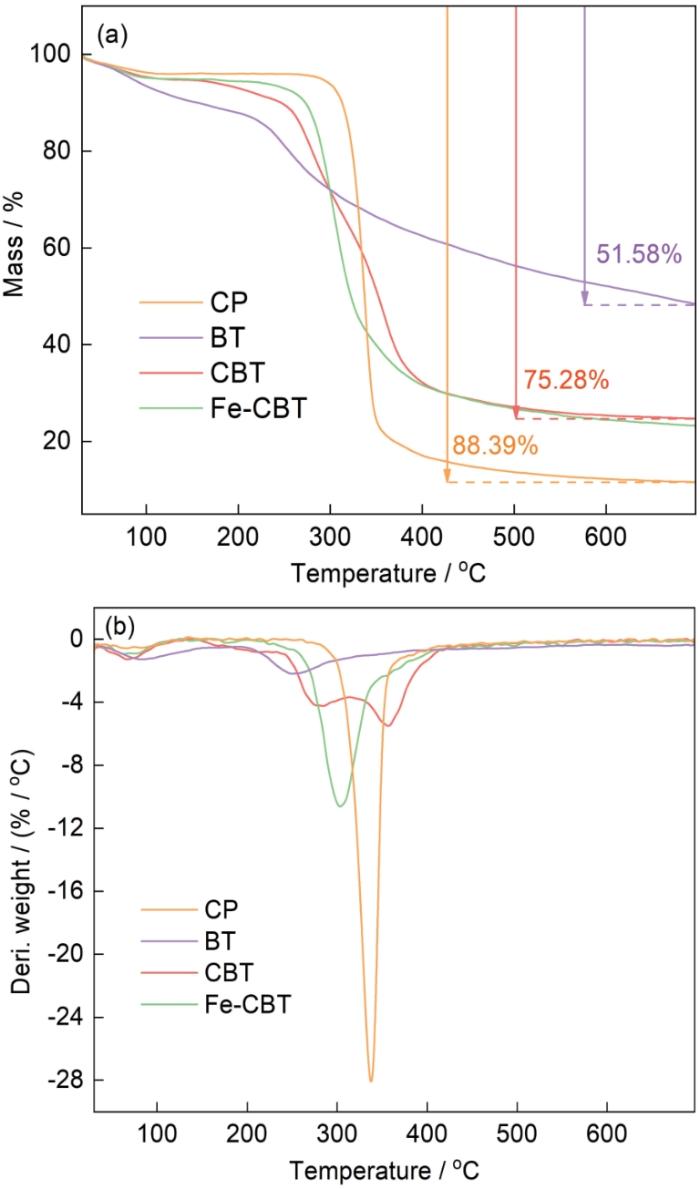
2.1.5 CP、BT、CBT和Fe-CBT的TG和DTG分析
材料的TG和DTG曲线如图6所示。从图6a的TG曲线可以看出,随着温度的升高原始CP出现了三个阶段的质量损失。在室温至100℃阶段,CP发生了轻微质量损失,可归因于样品表面的结晶水和物理吸附的自由水的蒸发[24,25]。虽然样品经过了冷冻干燥,但是因氢键相互作用难以完全去除所有的水分子。在260~400℃阶段CP出现了较大的质量损失,因为纤维素的氢键和结晶结构被破坏,热解为D-吡喃葡萄糖和自由基[26]。在第三阶段质量损失减慢,对应纤维素的缓慢炭化、生成碳和灰分。BT的热解质量损失在大约200℃开始,质量损失率缓慢持续下降,与纤维素单宁粉末的高残留量相比,是多酚支化大分子的顽固特性升高的结果。与纤维素相比,这种热降解模式突出了单宁更复杂的化学结构。从图6b中的DTG曲线可以看出,复合材料CBT的热解曲线不是单一组分曲线的简单重叠。在纤维素溶解再生进行均相改性的过程中,其氢键结构受到破坏,单宁分子诱导了样品的早期降解而使CBT的热分解温度降低[16]。但是,CBT仍遵循纤维素的热解模式,表明纤维素仍然是CBT的主要成分[14]。根据TG结果,计算出CBT复合材料的BT负载率为35.62%(质量分数)。负载金属离子后的Fe-CBT其第二阶段起始温度低于纯CBT,表明铁负载后的吸附剂其热稳定性纯CBT的低。
图6
图6
CP,BT,CBT和Fe-CBT的热重曲线
Fig.6
Thermogravimetric curves of CP, BT, CBT and Fe-CBT (a) TG curves; (b) DTG curves
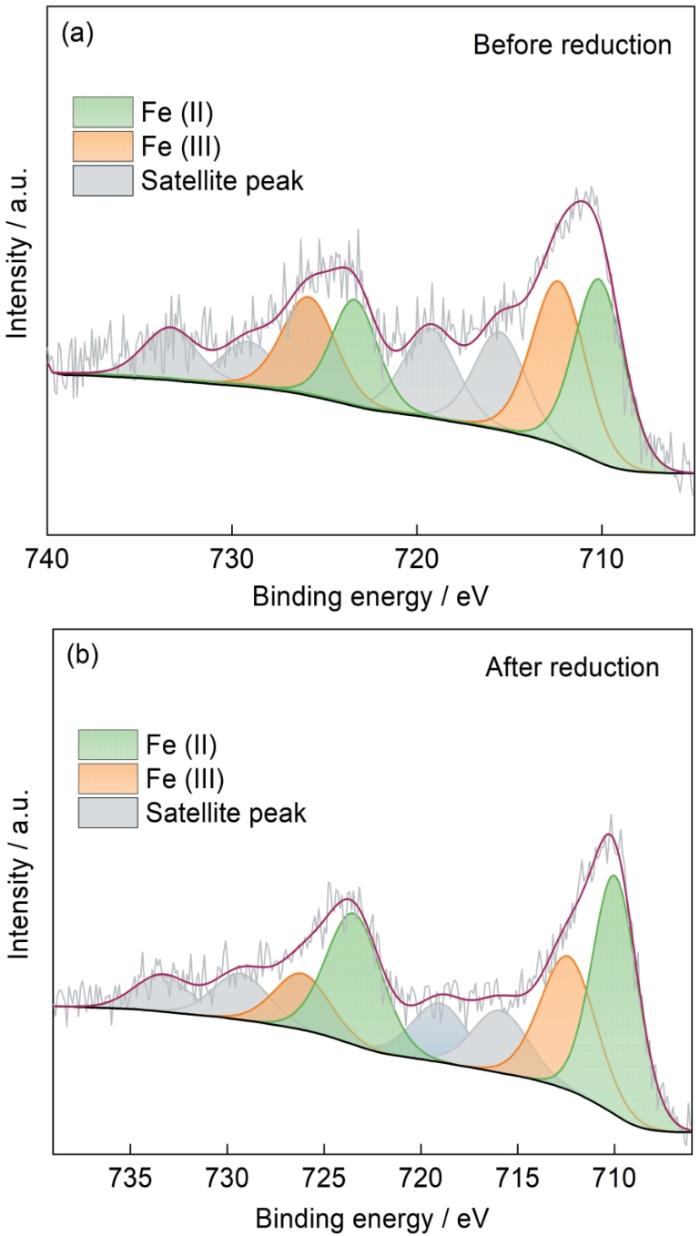
2.1.6 XPS光谱分析
图7
图7
硼氢化钠还原前后吸附剂的XPS高分辨谱
Fig.7
XPS high resolution scan of adsorbents before and after sodium borohydride reduction (a) Fe-CBT before reduction and (b) after reduction of Fe2p
2.2 Fe-CBT对氟喹诺酮类抗生素的吸附
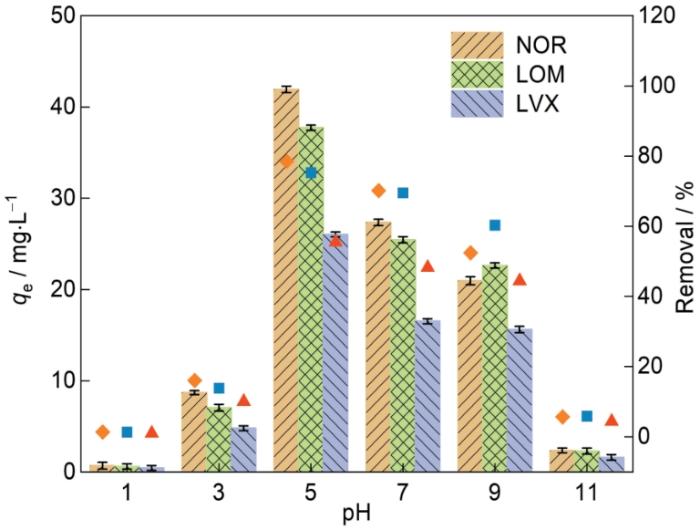
2.2.1 溶液初始pH值对吸附性能的影响
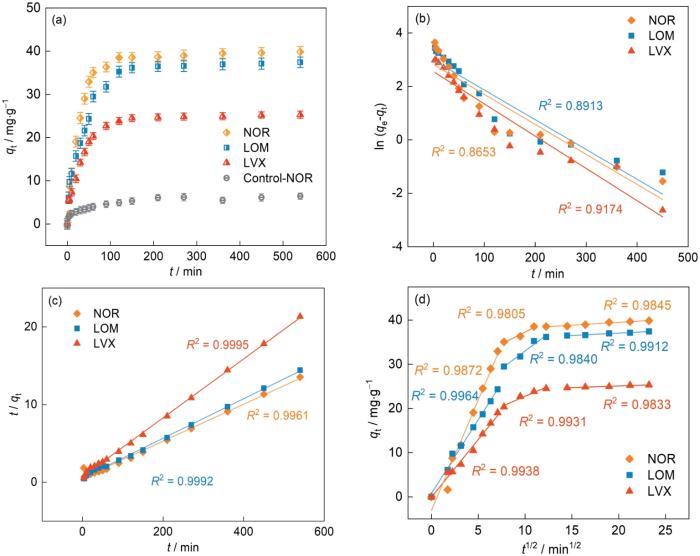
溶液的pH值影响吸附剂和抗生素分子之间的相互作用。图8给出了在溶液初始pH值不同的条件下Fe-CBT对FQs的吸附性能。在pH值为1.0~11.0的范围内FQs在Fe-CBT上的吸附对pH值表现出明显的依赖性。在pH值为1.0~3.0的极酸条件下,Fe-CBT对FQs的去除效果较差;随着pH值由3.0增加到5.0,吸附效果显著提高;当pH值为5.0~9.0时Fe-CBT对FQs的吸附能力缓慢下降,pH值为11.0时吸附能力显着降低。因此,在后续FQs吸附实验中取pH = 5.0。
图8
图8
初始溶液的pH值对NOR、LOM和LVX在Fe-CBT上的吸附性能的影响
Fig.8
Effect of solution pH on the adsorption performance of NOR, LOM and LVX on Fe-CBT
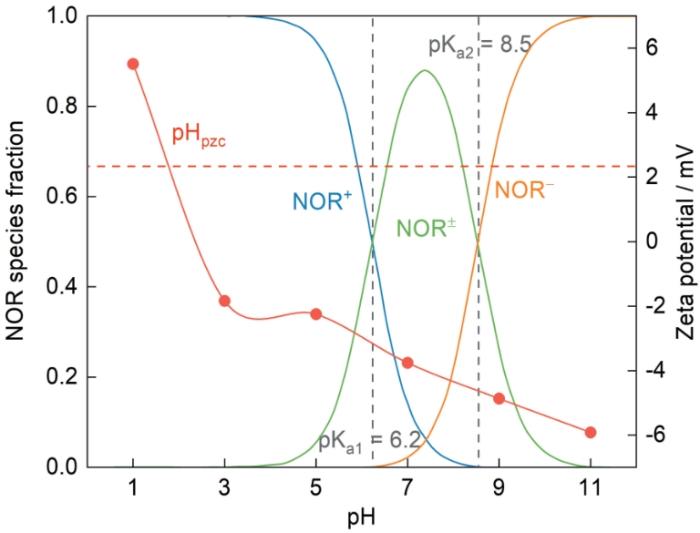
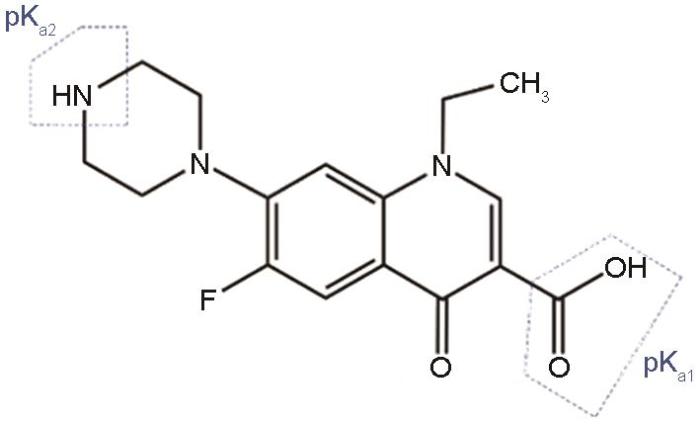
为了进一步研究pH值对Fe-CBT结构形态的影响及其与FQs间的相互作用机制,对Fe-CBT进行了Zeta电位分析。图9给出了pH值为1.0~11.0 NOR分子存在形态的变化和Fe-CBT的Zeta电位数据。FQs有两个pKa值,分别对应其分子结构中的哌嗪环上羧基和氨基。这表明,溶液中FQs的存在形式对pH值有明显的依赖。以NOR为例,NOR的分子结构如图10所示[29,30]。当溶液pH值小于pKa1 (pH = 6.2)时诺氟沙星主要以NOR+状态存在,当pH值介于pKa1和pKa2 (6.2 < pH < 8.5)之间时诺氟沙星主要以两性NOR±形式存在,当pH值大于pKa2 (pH = 8.5)时诺氟沙星分子的主要存在形式是NOR-。另一方面,吸附剂Fe-CBT的表面电荷随着溶液pH值的变化而变化。Fe-CBT的等电点pHpzc ≈ 1.8,当溶液的pH值> 1.8时Fe-CBT表面带正电;而当溶液的pH值< 1.8时,Fe-CBT表面带负电。因此,溶液的pH值影响吸附剂和FQs分子之间的相互作用。
图9
图9
在不同pH值条件下NOR的分子形态和Fe-CBT的Zeta电位
Fig.9
Molecular formula of NOR and zeta potentials of Fe-CBT
图10
溶液的pH值为1.0时Fe-CBT表面携带正电荷,NOR+与吸附剂之间存在静电斥力,对吸附剂与污染物之间接触的抑制使吸附量降低。随着pH值的增大吸附表面逐渐去质子化携带负电荷,与溶液中的NOR+通过静电引力相结合,吸附剂对污染物的吸附能力逐渐增大,pH值提高到5.0时吸附量达到最大值。上述实验结果表明,在pH值为5.0的条件下吸附剂对三种FQs的吸附能力大小的排序为:NOR >LOM > LVX。Zhang等[31]的结果表明,三者pKa1的排序为NOR > LOM > LVX,即在pH值为5.0的条件下NOR比LOM和LVX更多以阳离子形式存在,比去质子化携带负电荷的Fe-CBT间的静电引力更大,因此其吸附量较高。在6.0 < pH < 8.0范围内NOR主要以分子态存在,静电引力比在酸性条件下减弱。在接近中性条件下NOR±的羧基电离为COO-,更容易与金属物种生成表面络合物,因此其吸附性能很高。随着pH值超过pKa2增加到9.0,溶液中的污染物主要以NOR-形式存在,吸附剂表面严重去质子化,表面携带负电荷而使吸附剂与吸附质间存在明显的静电斥力,显著干扰污染物和吸附剂的结合。在此条件下吸附剂对污染物的去除效果虽然降低但仍有一定的效果,表明静电引力不是唯一的作用力。随着pH值的进一步增大吸附剂表面聚集大量负电荷,与同样带负电的NOR-产生强大的静电斥力,过多的OH-也与NOR-形成竞争,使NOR的吸附能力骤降。这些结果表明,在极酸和极碱条件下静电作用干扰其他吸附作用力,从而显著干扰吸附性能。而在偏中性范围内,NOR在Fe-CBT上的吸附是静电相互作用和表面络合的共同作用的共同结果。
2.2.2 吸附等温线
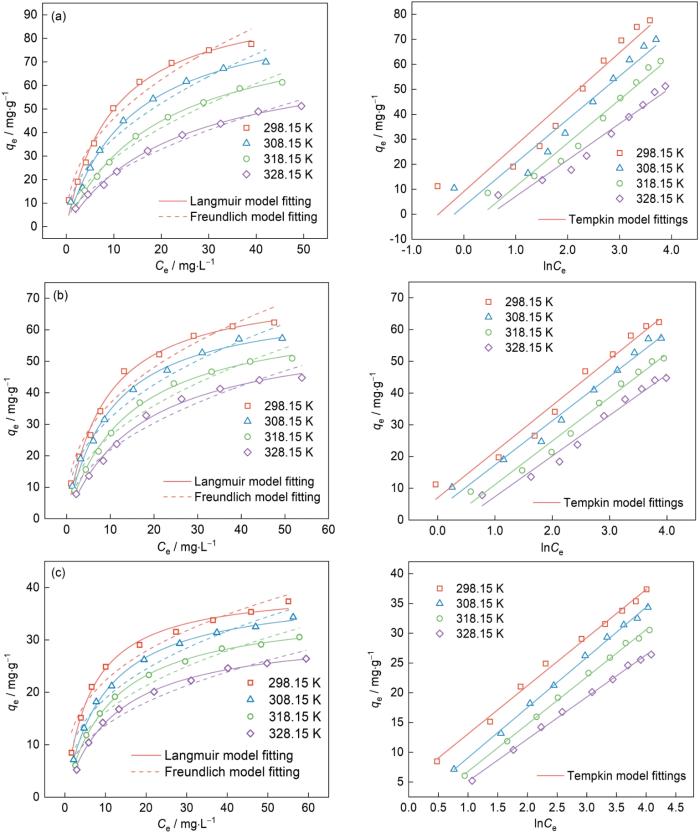
图11
图11
Fe-CBT在不同温度下对FQs的吸附等温模型拟合
Fig.11
Adsorption isotherm model fittings of FQs by Fe-CBT at different temperatures (a) NOR, (b) LOM, (c) LVX
表1 在不同温度下Fe-CBT对NOR、LOM和LVX的等温吸附模型拟合参数
Table 1
| Pollutant | T / K | Langmuir | Freundlich | Tempkin | |||||
|---|---|---|---|---|---|---|---|---|---|
| qm / mg·g-1 | KL | R2 | n | Kf | R2 | R2 | |||
| NOR | 298.15 | 99.07 | 0.1014 | 0.9896 | 2.26 | 16.60 | 0.9643 | 0.9636 | |
| 308.15 | 93.71 | 0.0745 | 0.9927 | 2.07 | 12.30 | 0.9683 | 0.9653 | ||
| 318.15 | 88.36 | 0.0517 | 0.9977 | 1.87 | 8.45 | 0.9815 | 0.9807 | ||
| 328.15 | 75.67 | 0.0431 | 0.9959 | 1.83 | 6.38 | 0.9877 | 0.9791 | ||
| LOM | 298.15 | 74.17 | 0.1179 | 0.9911 | 2.57 | 15.02 | 0.9636 | 0.9870 | |
| 308.15 | 69.36 | 0.0995 | 0.9917 | 2.44 | 12.48 | 0.9781 | 0.9917 | ||
| 318.15 | 65.95 | 0.0719 | 0.9960 | 2.22 | 9.32 | 0.9670 | 0.9901 | ||
| 328.15 | 60.70 | 0.0588 | 0.9929 | 2.10 | 7.31 | 0.9565 | 0.9881 | ||
| LVX | 298.15 | 40.14 | 0.1574 | 0.9943 | 3.09 | 10.58 | 0.9506 | 0.9974 | |
| 308.15 | 39.49 | 0.1043 | 0.9986 | 2.67 | 7.96 | 0.9572 | 0.9990 | ||
| 318.15 | 36.20 | 0.0891 | 0.9983 | 2.55 | 6.56 | 0.9522 | 0.9990 | ||
| 328.15 | 31.83 | 0.0810 | 0.9964 | 2.49 | 5.43 | 0.9508 | 0.9988 | ||
可以看出,温度的升高不利于吸附剂FQs吸附的进行,随着温度的升高Fe-CBT对NOR、LOM和LVX的平衡吸附量都呈现降低的趋势。以NOR为例,比较不同等温吸附模型拟合结果。Langmuir等温吸附模型(R2 = 0.9896、0.9927、0.9977和0.9959)比Freundlich等温吸附模型(R2 = 0.9643、0.9683、0.9815和0.9877)能更准确地描述其在Fe-CBT上的吸附过程。基于Langmuir等温吸附模型的假设,吸附剂Fe-CBT表面各部分的吸附性能相同,吸附的NOR、LOM和LVX主要为单分子层。另外,Freundlich经验常数n在0.1~1的参考范围内,表明Fe-CBT对FQs的吸附效果良好[32]。用Tempkin模型拟合得到的相关系数(R2 = 0.9636、0.9653、0.9807、0.9791)也比较高,表明吸附过程符合Tempkin吸附等温线模型,也表明吸附质和吸附剂之间存在强烈的分子间作用。从根据表1中的Langmuir模型拟合计算结果可以推算出,Fe-CBT在298 K对NOR、LOM和LVX的最大去除能力分别达到99.07、74.17和40.14 mg/g,高于文献报道的性能(表2)。与NOR相比,LOM和LVX的分子尺寸较大,可能阻碍多个分子在表面活性位点上的吸附,从而降低表面覆盖率,因此Fe-CBT对NOR的吸附效果更好。
表2 与其它吸附剂对FQs类抗生素吸附能力的对比
Table 2
2.2.3 吸附热力学
表3 Fe-CBT对NOR、LOM和LVX的吸附热力学参数
Table 3
| Pollutant | T/K | Result | ΔG / kJ·mol-1 | ΔS / J·(mol·K)-1 | ΔH / kJ·mol-1 |
|---|---|---|---|---|---|
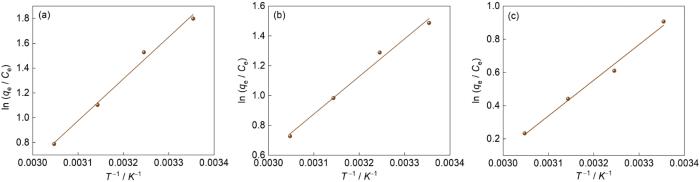
| NOR | 298.15 | y = 3374x - 9.483 R2 = 0.9954 | -4.549 | -78.82 | -28.05 |
| 308.15 | -3.761 | ||||
| 318.15 | -2.973 | ||||
| 328.15 | -2.185 | ||||
| LOM | 298.15 | y = 2520x - 6.936 R2 = 0.9951 | -3.749 | -57.70 | -20.95 |
| 308.15 | -3.172 | ||||
| 318.15 | -2.595 | ||||
| 328.15 | -2.018 | ||||
| LVX | 298.15 | y = 2148x - 6.319 R2 = 0.9953 | -2.190 | -52.54 | -17.86 |
| 308.15 | -1.664 | ||||
| 318.15 | -1.139 | ||||
| 328.15 | -0.614 |
图12
图12
Van't Hoff热力学方程拟合结果
Fig.12
Fitting results of Van't Hoff equation (a) NOR; (b) LOM; (c) LVX
2.2.4 吸附动力学
图13
图13
Fe-CBT对NOR、LOM和LVX的吸附动力学
Fig.13
Adsorption kinetics results of NOR, LOM and LVX on Fe-CBT (a) adsorption kinetics data; (b) pseudo-first-order kinetic linear fittings; (c) pseudo-second-order kinetic linear fittings; (d) intra-particle diffusion model fittings
结合表4中的拟合参数可知,准二级动力学模型的拟合结果(R2 = 0.9961、0.9992和0.9995)明显优于准一级动力学模(R2 = 0.8653、0.9174和0.8913)。准二级动力学拟合得到的计算值qe更与实验结果接近,表明准二级动力学模型更适用于描述该吸附过程。这也表明,在Fe-CBT对FQs的吸附反应中化学吸附是主要的控速因素。由颗粒内扩散模型拟合结果可见,三种FQs的qt和t1/2之间包括三个不通过原点的线性关系,说明吸附过程涉及多个阶段[39]。第一阶段,是污染物从溶液向吸附剂富集的外部扩散环节,FQs分子通过Fe-CBT表面的液膜到达吸附剂的外表面;第二阶段是内部扩散阶段,抗生素进一步向吸附剂的孔洞结构内扩散;第三个阶段是吸附反应阶段,抗生素进一步固定在吸附剂表面和内部孔壁,拟合直线趋于平坦,表明吸附过程达到了平衡[40]。
表4 Fe-CBT对NOR、LOM和LVX的吸附动力学参数
Table 4
| Pollutant | Pseudo-first-order kinetic model | Pseudo-second-order kinetic model | ||||
|---|---|---|---|---|---|---|
| K1 | qe | R2 | K1 | qe | R2 | |
| NOR | 1.123 × 10-2 | 16.87 | 0.8653 | 1.023 × 10-3 | 42.11 | 0.9961 |
| LOM | 1.114 × 10-2 | 19.74 | 0.8913 | 1.132 × 10-3 | 39.28 | 0.9992 |
| LVX | 1.209 × 10-2 | 12.90 | 0.9174 | 1.970 × 10-3 | 26.39 | 0.9995 |
2.2.5 无机阳离子对吸附性能的影响
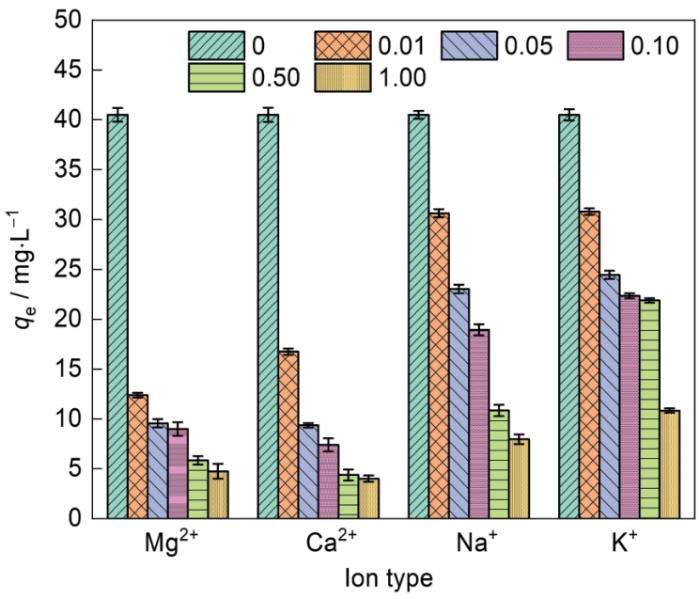
阳离子(如Mg2+、Ca2+、Na+和K+)干扰吸附剂对污染物吸附。调节四种阳离子的浓度分别为0、0.01、0.05、0.10、0.50和1.00 mol/L,研究阳离子的存在对Fe-CBT的NOR吸附效果的影响,结果如图14所示。可以看出,无机阳离子明显的抑制对NOR的吸附。随着水溶液中Mg2+、Ca2+、Na+和K+的浓度从0提高到1 mol/L,NOR在Fe-CBT上的吸附量分别减少了88.28%、90.09%、80.34%和73.26%。其主要原因是,共存的阳离子Mg2+、Ca2+、Na+和K+与FQs分子的阳离子电荷竞争吸附剂表面带负电荷的活性吸附位点,使FQs的吸附性能降低,证实了FQs与Fe-CBT之间存在静电相互作用[41]。同时,引入的金属阳离子部分取代Fe-CBT表面的质子,削弱了Fe-CBT与NOR之间氢键的作用[42]。另一方面,Mg2+和Ca2+比Na+和K+对NOR吸附的抑制更明显,在较低浓度就产生了较强的干扰。其原因不仅是二者带有较高的正电荷,还因为Mg2+、Ca2+与NOR间存在络合作用,易与NOR分子上的羰基的氧原子和羧基的两个氧原子结合形成稳定的六元环,抑制了NOR与吸附剂的接触[42]。上述结果表明,溶液中的共存阳离子抑制NOR在Fe-CBT上的吸附,也进一步证明吸附过程中污染物分子与吸附剂之间存在氢键作用和静电相互作用。
图14
图14
无机阳离子对Fe-CBT的NOR吸附性能的影响
Fig.14
Effect of inorganic cation on the adsorption of NOR by Fe-CBT
2.2.6 吸附剂的循环使用性能
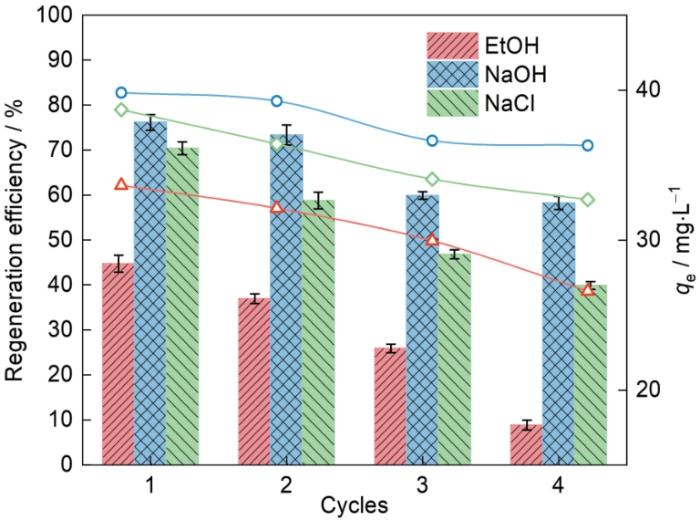
用95%的EtOH、0.1 mol/L的NaOH溶液和0.1 mol/L的NaCl溶液对吸附后的Fe-CBT进行洗脱,结果如图15所示。可以看出,用三种洗脱溶液进行洗脱再生,Fe-CBT都表现出可观的可重复使用性能。对NOR的四次再生效率分别为62.15%、57.05%、49.81%和38.63%;82.76%、82.01%、72.19%和72.11%;73.52%、71.37%、63.52%和62.25%。与NaOH和NaCl溶液相比,EtOH的洗脱效果较差。这表明,在吸附过程中与Fe-CBT与NOR之间的静电作用和氢键及π-π堆积相互作用相比,疏水作用的效果很小。经过4次再生后NaOH洗脱的Fe-CBT保持了高于初始吸附量70%的吸附能力,表明其具有一定的可重复使用性和可再生性。
图15
图15
Fe-CBT对NOR吸附的循环再生性能
Fig.15
Cyclic regeneration results of NOR adsorption by Fe-CBT
2.3 吸附机理
图16
图16
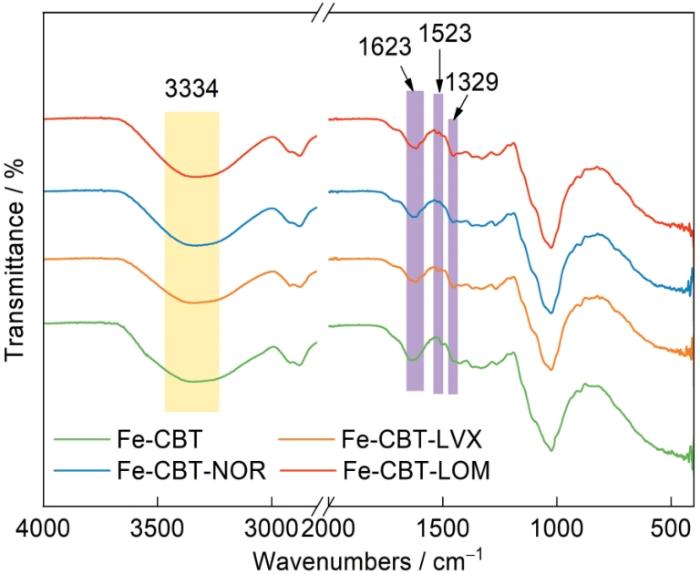
Fe-CBT吸附NOR、LOM和LVX前后的FT-IR谱
Fig.16
FTIR spectra of Fe-CBT before and after NOR, LOM, and LVX adsorption
图17
图17
Fe-CBT吸附NOR前后的XPS谱
Fig.17
XPS spectra of Fe-CBT before and after NOR adsorption
图18
图18
Fe-CBT吸附TCs前后的XPS高分辨谱
Fig.18
High-resolution XPS spectra of Fe-CBT. C1s of (a) before and (b) after adsorption; O1s of (c) before and (d) after adsorption; Fe2p (e) before and (f) after adsorption
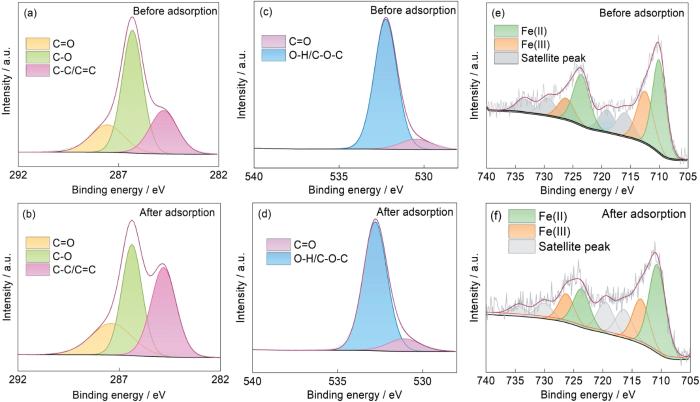
吸附前Fe-CBT的C1s的高分辨谱可分解成三个不同的组分峰,结合能分别为284.75、286.31和287.57 eV,对应C-C/C=C、C-O和C=O。其中吸附后的C-C/C=C和C=O占比分别从26.09%和19.57%提高到38.67%和22.27%,可归因于NOR分子引入在Fe-CBT上;而C-O官能团的含量从54.35%下降到39.06%,推测是C-O参与氢键的形成所致;另一方面,吸附后三个组分峰的结合能都发生了不同程度的变化,可推测是Fe-CBT与NOR之间电子云的移动引起的。O1s可分解成530.47和532.25 eV两处的单独成分峰,分别对应C=O和O-H/C-O-C的结合能。吸附NOR后对应位置的结合能分别增大了0.63和0.54 eV,推测是NOR分子中的氨基、羧基和氟基等基团与吸附剂上的羟基强π-π相互作用和形成氢键所致 [48]。Fe2p的峰出现在705~740 eV,其中709.74 eV处的峰属于Fe2+而在712.39 eV的峰对应Fe3+ [27,28]。NOR吸附后两处的结合能分别增大到710.63和713.28 eV,表明Fe-CBT表面的铁离子可能与NOR之间存在表面络合作用,产生的电子转移使电子云的密度提高从而产生结合能位移。
根据上述实验结果和讨论,Fe-CBT对FQs的吸附机制涉及氢键作用、π-π相互作用、表面络合和静电引力,其中的静电引力可能是造成三者吸附性能不同的主要原因。
3 结论
(1) 吸附剂Fe-CBT对FQs的吸附符合Langmuir等温吸附模型,吸附剂表面各部分的吸附性能相同,主要为单分子层吸附,吸附质和吸附剂之间有强烈的分子间作用。Fe-CBT在298 K下对NOR、LOM和LVX的最大理论吸附量可达到99.07、74.17和40.14 mg/g。在实验温度下三种FQs在吸附过程是自发的放热反应,温度的降低更有利于吸附实验的进行。吸附过程中化学吸附是主要的限速步骤。
(2) 初始溶液pH值的改变引起的Fe-CBT和FQs间静电相互作用变化,显著影响Fe-CBT的吸附性能;无机阳离子通过竞争吸附位点和与污染物间的络合干扰对NOR的去除;洗脱剂氢氧化钠和氯化钠溶液对Fe-CBT的洗脱效果很好,表现出良好的再生性能。
(3) Fe-CBT与NOR分子间的静电引力、表面络合、氢键和π-π堆积的协同作用,是从水溶液中去除NOR的机制。
参考文献
Topoisomerase inhibitors: fluoroquinolone mechanisms of action and resistance
[J].
Treatment technologies to mitigate the harmful effects of recalcitrant fluoroquinolone antibiotics on the environment and human health
[J].
Cellulose: fascinating biopolymer and sustainable raw material
[J].
Cellulose nanofibers-based green nanocomposites for water environmental sustainability: A review
[J].
Cellulose-based nanomaterials for water and wastewater treatments: A review
[J].
Tannic acid-Fe coordination derived Fe/N-doped carbon hybrids for catalytic oxidation processes
[J].
Nature-inspired chemistry toward hierarchical superhydrophobic, antibacterial and biocompatible nanofibrous membranes for effective UV-shielding, self-cleaning and oil-water separation
[J].
Chemically modified sawdust as renewable adsorbent for arsenic removal from water
[J].
Sulfonated graphene nanosheets as a superb adsorbent for various environmental pollutants in water
[J].
Tannic acid modified Fe3O4 core-shell nanoparticles for adsorption of Pb2+ and Hg2+
[J].
Biomass-supported monodisperse n-Fe0@M0 preparation and basic research on application of uranium adsorption and reduction
[D].
生物质负载型单分散性n-Fe0@M0制备及其铀吸附还原应用基础研究
[D].
Classical theory and electron-scale view of exceptional Cd(II) adsorption onto mesoporous cellulose biochar via experimental analysis coupled with DFT calculations
[J].
Nanocellulose-tannin films: From trees to sustainable active packaging
[J].
Study on the preparation and adsorption performance of polyethyleneimine modified cellulose adsorbent
[D].
聚乙烯亚胺改性纤维素重金属吸附剂的制备及其吸附性能研究
[D].
Preparation of cellulose adsorbent materials and study on their adsorption performance towards hexavalent chromium and p-Arsanilic acid
[D].
纤维素吸附材料的制备及其对六价铬和对氨基苯胂酸吸附性能研究
[D].
Selective catalytic hydrogenation and depolymerization of lignin with Ni/MgO
[D].
Ni/MgO催化木质素选择性加氢解聚
[D].
Simultaneous abatement of NO and N2O with CH4 over modified Al2O3 supported Pt, Pd, Rh
[J].
Adsorption of Cu(II), Pb(II) and Cr(VI) from aqueous solutions using black wattle tannin-immobilized nanocellulose
[J].
Development of tannin-immobilized cellulose fiber extracted from coconut husk and the application as a biosorbent to remove heavy metal ions
[J].
Research on preparation and adsorption property of tannin-immobilized cellulose resin
[D].
纤维素固化单宁树脂的制备及吸附性能研究
[D].
Removing Pb(II) ions from aqueous solution by a promising absorbent of tannin-immobilized cellulose microspheres
[J].Tannin/cellulose microspheres (T/C) were successfully prepared via a facile homogeneous reaction in a water/oil (W/O) emulsion for removing Pb(II) ions from aqueous solution. The structure of the microspheres was characterized by scanning electron microscopy (SEM), Fourier-transform infrared (FTIR) spectroscopy, and a zeta potential test. The effects of pH, adsorbent dosage, contact time, and temperature on adsorption ability were investigated. The results showed that T/C microspheres could combine Pb(II)ions via electrostatic attractions and physical adsorption. Adsorption kinetics could be better described by the pseudo-second-order kinetic model. The adsorption behaviors were in agreement with the Langmuir adsorption isotherm model with a fitting correlation coefficient of 0.9992. The maximum adsorption capacity was 23.75 mg/g from the Langmuir isotherm evaluation at 308K with an initial pH of 5. The results suggested that tannin/cellulose microspheres could be a low-cost and effective adsorbent for removing Pb(II) ions from aqueous solution.
Studies on synthesis, characterization, and metal adsorption of mimosa and valonia tannin resins
[J].
Durable antibacterial finishing on kapok fibrous nonwovens with Polydopamine/PEI assisted immobilization of silver
[J].
A new cotton functionalized with iron(III) trimer-like metal framework as an effective strategy for the adsorption of triarylmethane dye: An insight into the dye adsorption processes
[J].
Flexible polypyrrole/copper sulfide/bacterial cellulose nanofibrous composite membranes as supercapacitor electrodes
[J].
Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials
[J].
Soft nano-wrapping on graphene oxide by using metal-organic network films composed of tannic acid and Fe ions
[J].Graphene oxide (GO) nanosheets were easily covered with uniform metal-organic network films composed of tannic acid and Fe ions. The surface morphology of the wrapped GO sheets was elucidated using atomic force, scanning electron, and transmission electron microscopy measurements. The GO sheets covered with the TA-Fe films on a substrate were reduced chemically without the collapse of the wrapped nanostructure of the TA-Fe/GO sheets. The modified GO sheets covered with TA-Fe were highly stable in water and easy to handle, which made it possible for placing on a microelectrode array for conductivity measurements.
Sorption of the antimicrobial ciprofloxacin to aluminum and iron hydrous oxides
[J].
Sorption of veterinary pharmaceuticals in soils: A review
[J].
Adsorption and oxidation of fluoroquinolone antibacterial agents and structurally related amines with goethite
[J].Seven members (ciprofloxacin, enrofloxacin, norfloxacin, ofloxacin, lomefloxacin, pipemidic acid, and flumequine) of the popular fluoroquinolone antibacterial agents (FQs) were found to adsorb strongly to goethite with 50-76% of the added FQ adsorbed under the experimental conditions. The adsorption isotherms fitted well to the Langmuir model. Adsorption was accompanied by slow oxidation of the FQs (except for flumequine) by goethite yielding a range of hydroxylated and dealkylated products. The oxidation kinetics showed different stages in reaction rate, mostly likely caused by accumulation of Fe(II) species on the oxide surface that slowed the reaction. Structurally related amines 1-phenylpiperazine, N-phenylmorpholine, aniline, and N,N-dimethylaniline were found to be oxidized by goethite without significant adsorption. The results strongly indicate that the carboxylic group of FQs is critical for adsorption while the piperazine ring is susceptible to oxidation. A radical mechanism is proposed for the oxidation of FQs by goethite which involves formation of a surface complex between the FQ and surface-bound Fe(III) through adsorption, and initial oxidation at the piperazinyl N1 atom to form radical intermediates that ultimately lead to the final products. This study indicates that Fe oxides in aquatic sediments may well play an important role in the natural attenuation of fluoroquinolone antibacterial agents.
Carbon-based adsorbents for fluoroquinolone removal from water and wastewater: A critical review
[J].
Adsorption of levofloxacin onto mechanochemistry treated zeolite: Modeling and site energy distribution analysis
[J].
Sorption characteristics of different forms of lomefloxacin on kaolinite
[J].We carried out batch experiments to investigate the sorption characteristics of different forms of lomefloxacin (LOM) on kaolinite. Sorption kinetics were pseudo-second-order, and all equilibrium data could be well described by the Langmuir equation. As solution pH increased, the amount of LOM sorption increased first and then decreased. Maximum sorption was achieved at pH values between pKa1 and pKa2 of LOM. The sorption capacity of different forms of LOM on kaolinite ranked as LOM±>LOM+>LOM-. The ionic strength of LOM solution and inorganic cations had little effect on LOM+ sorption, but both factors significantly inhibited LOM± sorption. The sorption capacity of LOM± decreased with increasing ionic strength, and the inhibition potency of different inorganic cations was in the order of Mg2+>Ca2+>K+>Na+. The sorption mechanisms were mainly inner-sphere complexation and cation exchange for LOM+ or cation exchange, hydrogen bonding and electrostatic attraction for LOM± on kaolinite. LOM- sorption on kaolinite is very small due to strong repulsive electrostatic effect; the small sorption may resulted from outer-sphere surface complexation.
不同形态洛美沙星在高岭土上的吸附特性
[J].本研究采用批实验方法探究了不同形态洛美沙星(LOM)在高岭土上的吸附特性。LOM吸附动力学结果符合准二级反应动力学方程,吸附等温数据可用Langmuir方程很好地拟合。随着溶液pH值增大,洛美沙星吸附量先增大后减小,且pH值在洛美沙星pKa1与pKa2间吸附量达到最大。不同形态LOM在高岭土上的吸附量排序为LOM±>LOM+>LOM-。溶液离子强度和无机阳离子种类对LOM+在高岭土上的吸附影响十分微弱,但均明显抑制了LOM±的吸附,且离子强度越大,抑制作用越明显。不同无机阳离子抑制程度排序为Mg2+>Ca2+>K+>Na+。LOM+在高岭土上的吸附机理主要是内层络合和阳离子交换;LOM±在高岭土上的吸附机理主要是阳离子交换、氢键作用和静电引力作用;LOM-与高岭土表面存在较大静电斥力,导致吸附量很小,可能是外层表面络合引起少量的吸附。
Coating magnetic biochar with humic acid for high efficient removal of fluoroquinolone antibiotics in water
[J].
Rapid nitrogen-rich modification of Calotropis gigantea fiber for highly efficient removal of fluoroquinolone antibiotics
[J].
Exploration of functional MOFs for efficient removal of fluoroquinolone antibiotics from water
[J].
Adsorption of quinolone, tetracycline, and penicillin antibiotics from aqueous solution using activated carbons: Review
[J].
Removal of Pb, Cd, Cu and Ni from aqueous solution using nano scale zero valent iron particles
[J].
Manganese dioxide/iron oxide/acid oxidized multi-walled carbon nanotube magnetic nanocomposite for enhanced hexavalent chromium removal
[J].
Optimization adsorption of norfloxacin onto polydopamine microspheres from aqueous solution: Kinetic, equilibrium and adsorption mechanism studies
[J].
Adsorption characteristics and mechanism of norfloxacin in water by γ-Fe2O3@BC
[J].
Green synthesis of stable Fe, Cu oxide nanocomposites from loquat leaf extracts for removal of Norfloxacin and Ciprofloxacin
[J].Mass production of nanomaterials to remove pollutants from water still faces many challenges, mainly due to the complexity of the synthesis methods involved and the use of dangerous reagents. The green method of preparation of nanomaterials from plants can effectively solve these problems. Fe,Cu oxide nanocomposites (Fe-Cu-NCs) were synthesized by a green and single-step method using loquat leaf extracts, and were used as an adsorbent for removal of Norfloxacin (NOR) and Ciprofloxacin (CIP) from aqueous solution. The synthesized adsorbent showed excellent adsorption properties for NOR and CIP. The experimental equilibrium data fitted the Redlich-Peterson and Koble-Corrigan models well and the maximum adsorption capacities of Fe-Cu-NCs calculated by the Langmuir model for NOR and CIP were 1.182 mmol/g and 1.103 mmol/g, respectively, at 293 K. Additionally, the morphologies and properties of Fe-Cu-NCs were characterized by transmission electron microscopy (TEM), scanning electron microscopy X-ray energy-dispersive spectroscopy (SEM-EDS), X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR) analysis and the adsorption mechanism of NOR and CIP onto Fe-Cu-NCs was discussed. Thermodynamic parameters revealed that the adsorption process was spontaneous and endothermic. This study indicated that Fe-Cu-NCs are a potential adsorbent and provide a simple and convenient strategy for the purification of antibiotics-laden wastewater.
Iron/zinc and phosphoric acid modified sludge biochar as an efficient adsorbent for fluoroquinolones antibiotics removal
[J].
Difunctional covalent organic framework hybrid material for synergistic adsorption and selective removal of fluoroquinolone antibiotics
[J].
Terbium-based coordination polymer nanoparticles for detection of ciprofloxacin in tablets and biological fluids
[J].
Utilizing low-cost natural waste for the removal of pharmaceuticals from water: Mechanisms, isotherms and kinetics at low concentrations
[J].