含酚废水是一种难以降解的工业废水,严重危害生态环境和人身健康。处理含酚废水的方法,有生物降解法[1,2]、萃取法[3~5]、吸附法[6]和高级氧化法[7]。高级氧化法,是将过氧化物(例如过氧化氢)和过硫酸盐等活化成具有强氧化电势的自由基,将有机污染物矿化成二氧化碳和水。高级氧化法中的湿式催化过氧化氢技术(CWPO),其反应条件温和且成本较低。提高湿式催化氧化性能的关键,在于设计和选择适当的催化剂。传统的金属催化剂,在反应体系中有活性金属离子浸出等问题。碳纳米管(Carbon nanotubes,CNTs)是一种新型的纳米碳材料,具有较大的比表面积、较高的缺陷程度以及丰富的离域π电子,能协同表现出非金属碳基催化剂的优良特性[8],在反应体系中能避免活性金属离子的浸出。杂原子掺杂可调整掺杂表面电荷的自旋分布以改变含碳材料的表面性质和形成均匀的共轭电子网络,使碳纳米管催化剂的催化活性提高。硼元素具有较高的强度、硬度和理想的耐化学性[9]。硼原子半径(0.082 nm)与碳原子半径(0.077 nm)接近。因此,掺杂硼可使碳质材料具有良好的催化性能,打破碳质材料的惰性表面使其催化活性提高[10]。纸状烧结不锈钢微纤载体(Paper-like sintered stainless steel fibers, PSSF)是一种微纤复合材料,负载量大、机械强度高和形状可调,能降低使用中的流动阻力和传质阻力[11]。在纸状烧结不锈钢微纤载体表面构筑碳纳米管膜使其成为微纳复合结构表面,可降低流体传质阻力、减少沟流和返混等不良流体,提高反应接触效率、选择性和稳定性。
1 实验方法
1.1 实验用材料和仪器设备
实验用材料:苯硼酸(97%)、樟脑(96%)、苯酚(≥ 99%)、盐酸(36%~38%质量分数)、碘化钾(分析纯)、甲醇(色谱纯)、去离子水、H2O2和超纯水。
实验用仪器设备:管式炉、配备紫外检测器波长的HPLC色谱仪(Agilent 1100)、场发射扫描电子显微镜(FE-SEM, S-3700N)、场发射透射电子显微镜(TEM, JEM 2100F)、激光拉曼测试仪(Raman, LabRAM Aramis)、综合热分析仪(TG, STA449C)、X射线光电子能谱仪(XPS, Axis Supra+)、傅里叶变换红外光谱仪(FTIR, Nicolet IS50)、Agilent HC-C18 (2)色谱柱、TOC-VCPH总有机碳分析仪、Z-2300型火焰原子吸收分光光度计(AAS)和PH S-25酸度计。
1.2 样品的制备
1.2.1 B-CNTs/PSSF的制备
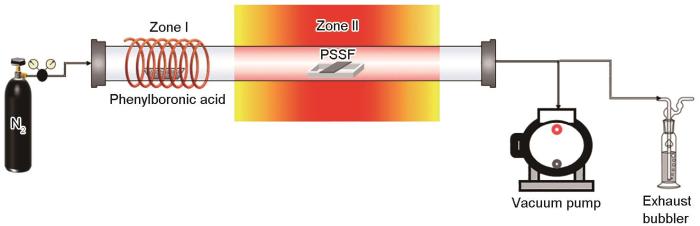
先用湿法造纸工艺和烧结技术制备出PSSF[19,20],然后用化学气相沉积技术合成CNTs/PSSF和B-CNTs/PSSF,使用的装置如图1所示。反应开始前,在氮气(> 99.999%)气氛中进行气体交换以确保管式炉中的惰性环境。将1 g的PSSF放置在反应区的中部,将2 g苯硼酸(97%)放置在辅助加热区。将反应区的温度以5℃·min-1的速率升至750℃并保温10 min,随后打开辅助加热装置使苯硼酸以10℃·min-1的速率升温至130℃使其挥发,生长时间为20 min。反应完成后以5℃·min-1的速率降至室温,得到B-CNTs/PSSF。整个反应在200 sccm的N2气氛中进行。
图1
图1
CVD法制备B-CNTs/PSSF的示意图
Fig.1
Schematic diagram of preparation of B-CNTs/PSSF composite catalysts by CVD method
1.2.2 CNTs/PSSF的制备
以樟脑(96%)为前驱体、以PSSF为载体,用相同的CVD法合成CNTs/PSSF。工艺参数为:用辅助热源将2 g樟脑升温至200℃使其挥发,沉积温度为750℃,沉积时间为20 min。得到的产物用于后续苯酚湿式催化氧化反应,进行对比研究。
1.3 材料的形貌、组成和性能表征
用场发射扫描电子显微镜(FE-SEM, S-3700N)表征材料的微观形貌和结构,操作电压为15 kV,测试前对样品喷金。用场发射透射电子显微镜(TEM, JEM 2100F)观察碳纳米管的形貌和微观结构,测试前将样品在无水乙醇中超声清洗,使碳材料从PSSF载体上脱离。用SEM观察二次煅烧后的PSSF载体、CNTs/PSSF以及B-CNTs/PSSF的微观形貌,用TEM观察B-CNTs/PSSF的管状结构。用FTIR分析CNTs/PSSF和B-CNTs/PSSF的表面结构和官能团。用Raman光谱表征CNTs/PSSF和B-CNTs/PSSF复合材料的缺陷程度及掺杂信息。用TG测试生长在PSSF载体上的CNTs产率和热稳定性。用XPS分析B-CNTs/PSSF表面的元素组成及化学键合形式。用激光拉曼测试仪(Raman, LabRAM Aramis)表征样品表面缺陷和掺杂。用综合热分析仪(TG, STA449C)分析样品的热稳定性和质量损失。用X射线光电子能谱仪(XPS, Axis Supra+)分析样品表面的元素组成、含量和化学键合形式。用傅里叶变换红外光谱仪(FTIR, Nicolet IS50)分析样品表面的官能团。
以苯酚为污染物模拟工业中的含酚废水,在基于B-CNTs/PSSF的结构化固定床中进行苯酚湿式催化氧化实验。实验装置和流程,如图2所示。将B-CNTs/PSSF置于反应器中部充当床层,用玻璃珠填充床层的两端。用去离子水制备体积为1 L的反应液,苯酚和H2O2的浓度分别为1 g/L和30%(质量分数)。将反应液以2 mL·min-1的流速泵入反应器。使用恒温水浴控制反应温度为80℃。每隔1 h取反应液样一次,取样时间为10 min,共取出约20 mL的液体并记录其pH值。
图2
图2
苯酚在结构化固定床反应器上湿式催化氧化实验流程图
Fig.2
Flowchart for phenol catalytic wet peroxide oxidation in structured fixed-bed reactor
用0.22 μm过滤器过滤10 mL反应液,加入少量的MnO2去除过量的H2O2。用配备紫外检测器波长为210 nm的HPLC色谱仪(Agilent 1100)测定苯酚的浓度。使用Agilent HC-C18 (2)色谱柱分离有机物,甲醇和超纯水(VMeOH∶Vwater = 4∶6)为流动相。苯酚转化率为
其中Cphenol(0)为t = 0时刻苯酚的浓度(mg·L-1),Cphenol(t)为t时刻溶液中苯酚的浓度(mg·L-1)。
取15 mL过滤的反应液(废水),用TOC-VCPH总有机碳分析仪测试其中总有机碳(TOC)的含量。TOC转化率为
其中CTOC(0)为t = 0时刻反应液中的TOC总量(mg·L-1);CTOC(0)为t时刻反应液中的TOC总量(mg·L-1)。
用间接碘量法测定H2O2转化率。在5 mL用于过滤的反应液中加入适量的盐酸和碘化钾,将得到的溶液置于黑暗中10 min用Na2S2O3滴定。H2O2的转化率为
其中
用Z-2300型火焰原子吸收分光光度计(AAS)测试反应后稀释10倍的溶液中Fe离子浸出浓度。用PH S-25酸度计测定反应液的pH值。
用(6,6)碳纳米管模拟对H2O2的吸附。在B3LYP/6-31G*水平下进行几何优化,6-311G*基组用于计算单点能。使用Gaussian 16 C.01程序包计算。使用IEFPCM溶剂模型计算,以H2O为溶剂环境。由Multiwfn 3.8 dev计算电子密度差[21],用VMD绘制。吸附能Eads为
式中Eam-sur为吸附结构的总能量(kJ·mol-1),Esur和Eam分别为吸附剂和吸附质的能量(kJ·mol-1)。
2 结果和讨论
2.1 催化剂的表征
2.1.1 CNTs/PSSF和B-CNTs/PSSF的形貌
图3给出了二次煅烧后的PSSF载体、CNTs/PSSF和B-CNTs/PSSF的微观形貌和B-CNTs/PSSF的管状结构。从图3a可见,二次煅烧后的PSSF载体呈现三维网状结构,空隙率较高,表面粗糙度均匀,属于纳米尺度。这些纳米丘壑,可为CNTs的生长提供催化活性位点[22,23]。图3b表明,以樟脑为碳源用CVD法可在PSSF载体上生长出一层均匀致密的膜结构。这些纳米管形成的膜包裹在整个PSSF表面,使PSSF的直径从6 μm增大到十几微米。从图3c、d可观察到,在用苯硼酸为前驱体的CVD过程中在PSSF载体上生长出直径约为50 nm的纳米管膜,完整地包覆在不锈钢微纤表面。值得注意的是,在碳纳米管的空腔结构顶端可观察到痕量铁基催化剂颗粒。这表明,硼掺杂碳纳米管在PSSF表面可能遵循顶端生长机制[24]。Huang等[25]指出,封装管内的金属纳米颗粒可协同强化H2O2的活化效果。同时,碳纳米管的空腔呈现处弯曲和波浪结构,形成类似“肘关节”的微观形貌[26]。有研究表明[27],硼原子在负高斯曲率位点上的稳定性较高,可在碳纳米管上诱导形成“肘关节”。
图3
图3
二次煅烧的PSSF、CNTs/PSSF、B-CNTs/PSSF的SEM图和B-CNTs/PSSF的TEM像
Fig.3
SEM images of bare PSSF, CNTs/PSSF, B-CNTs/PSSF (a~c) and TEM image of B-CNTs/PSSF (d)
2.1.2 CNTs/PSSF和B-CNTs/PSSF的表面结构和官能团
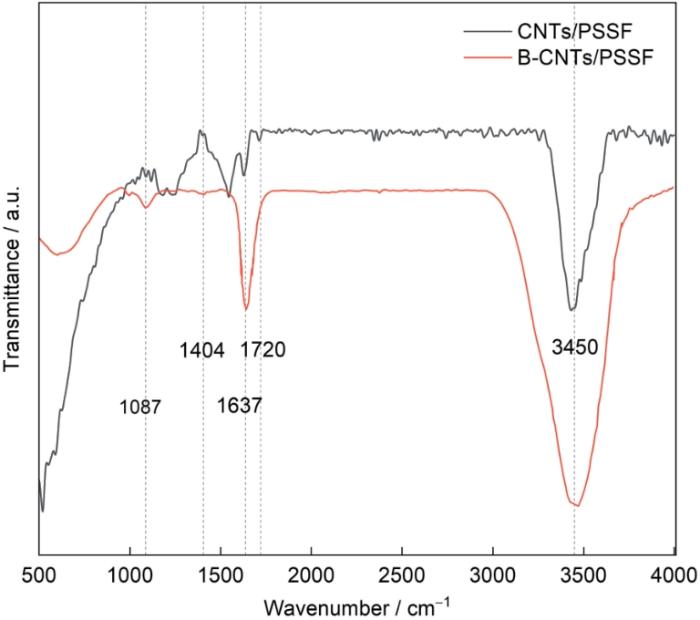
图4给出了CNTs/PSSF和B-CNTs/PSSF的表面结构和官能团。从图4可见,位于3450 cm-1附近的宽肩峰可归因于-OH的拉伸振动[28,29],还可归因于KBr圆盘中的微量水[29]。在2850和2920 cm-1波数处均未发现在石墨平面边缘的-CH2、-CH3基团和非晶态碳的伸缩振动所产生的峰,表明CNTs/PSSF和B-CNTs/PSSF在CVD合成中生成的缺陷较少[30]。特别是,在CNT/PSSF上1720 cm-1处的峰归因于C=O主导的含氧官能团,与其高氧含量对应[31]。这个峰没出现在B-CNTs/PSSF,表明其氧含量较低。出现在1637 cm-1处的峰,通常被归因于水的拉伸振动[32]。位于1404和1087 cm-1处的峰,分别与B-O和C-B伸缩振动有关[33]。
图4
2.1.3 CNTs/PSSF和B-CNTs/PSSF复合材料的缺陷和掺杂信息
图5给出了CNTs/PSSF和B-CNTs/PSSF复合材料的缺陷程度及掺杂信息[34~36]。从图5观察到典型的碳质材料特征谱带:D带(~1347 cm-1)、G带(1576 cm-1)以及2D带(2683 cm-1)。D带与石墨的结构缺陷或无序sp2碳原子有关,G带对应一阶散射E2g模式,与高度有序的石墨有关。而2D带则源于双声子散射,与碳纳米管中石墨层的结晶度有关[10, 37]。可用D带与G带的强度比ID/IG表征碳质材料的无序和缺陷程度。较高的ID/IG值表明,CNTs具有较高的缺陷程度或无序程度。CNTs/PSSF的ID/IG值为0.658,表明合成的CNTs的石墨化水平较高。值得注意的是,在碳纳米管成核过程中氧元素导致氧空位的形成。因此,高氧含量使碳纳米管中的缺陷更多[38]。与CNTs/PSSF相比,B-CNTs/PSSF的含氧量较低,但是其缺陷程度较高。其原因是,掺杂的硼部分取代碳使局部六边形对称性降低,从而产生更多的缺陷[39]。同时,B-CNTs/PSSF的2D峰强度显著降低,可能是杂原子的掺杂破坏了石墨畴的长程有序[40]。
图5
图5
B-CNTs/PSSF和CNTs/PSSF的Raman谱
Fig.5
Raman spectra of CNTs/PSSF catalyst and B-CNTs/PSSF
2.1.4 CNTs/PSSF和B-CNTs/PSSF的碳产率及热稳定性
图6给出了生长在PSSF载体上的CNTs碳产率和热稳定性。可以看出,温度低于200℃时只发生了轻微的质量损失,可能是CNTs表面吸附的水及含氧官能团所致[41],在200~400℃约0.2%的质量损失可归结为无定形碳的氧化[42]。而CNTs发生质量损失的温度约为484℃,表明其热稳定性良好,碳产率约为4.02%。B-CNTs/PSSF在50~300℃的轻微失重可归因于催化剂表面吸附的水和含氧官能团(如-OH和-COOH)的分解[43],其碳产率为3.33%。同时,与CNTs的初始质量损失温度484℃相比,B-CNTs的初始质量损失温度约为388℃,降低了约96℃,其原因可能是硼原子掺杂产生的缺陷使B-CNTs的热稳定性降低。
图6
2.1.5 B-CNTs/PSSF表面的组成和化学键合
B-CNTs/PSSF表面的元素组成及化学键合形式,如图7所示。图7a中的全谱表明,样品表面的元素主要为C、O和B,用XPS定量分析出B-CNTs/PSSF表面的硼原子含量为2.96%。图7b表明,B-CNTs/PSSF的高分辨率C1s谱可分解为5个峰:分别对应283.4 eV处的C-B键、284.8 eV处石墨晶格中的sp2碳、286.2 eV处的羟基(C-OH)、288.0 eV处的羰基(C=O)和290.3 eV处的π-π*电子跃迁[10]。而图7d所示的高分辨O1s XPS谱可反卷积分为三个峰:其结合能位于531.2、532.7和534.4 eV处的峰分别对应羰基(C=O)、羟基(C-OH)和羧基(-COOH)。根据文献[44~46],碳纳米管边缘的羰基是苯酚CWPO的催化活性位点,加强电子转移可促进催化活性。这有利于后续B-CNTs/PSSF在苯酚降解中的应用。进行反卷积拟合高分辨B1s XPS谱,进一步研究了催化剂中硼的种类和含量。如图7c所示,B1s谱可反卷积为四种峰,结合能位于186.7和189.5 eV处的峰分别属于B簇和BC3。结合能位于191.2、192.5 eV附近的峰分别属于BC2O[47~49]、BCO2。这表明,已经将B掺杂进碳纳米管的骨架中。
图7
图7
B-CNTs/PSSF的XPS全谱、C1s、B1s和O1s的高分辨谱
Fig.7
High-resolution XPS spectra of CNTs/PSSF and B-CNTs/PSSF catalysts (a)Full spectra, (b) C 1s, (c) B 1s, (d) O 1s
2.2 B-CNTs/PSSF降解苯酚的性能
在温度为80℃、苯酚流量为2 mL·min-1、床层高度为2 cm的条件下分别考察了H2O2转化率、苯酚转化率、TOC转化率和pH值随反应时间的变化和Fe离子的浸出情况,结果如图8所示。从图8a可以看出,PSSF载体在80℃对H2O2的转化率仅为10%,因为经过二次煅烧氧化处理的PSSF表面生成的Fe2O3氧化膜能在一定程度上催化分解H2O2。而在较高的反应温度(80℃)下,一部分H2O2受热分解为O2[50]。CNTs/PSSF对H2O2的平均转化率仅约为40%,且随着反应的进行出现不断下降的趋势。而B-CNTs/PSSF,在7 h内表现出了对H2O2优异的活化性能。虽然碳基催化剂上的C=O在CWPO过程中发挥了催化活性位点的作用[44~46],但是CNT/PSSF中丰富的C=O显著降低了共轭C=C的含量,从而使边缘碳原子的电子密度降低和π电子传输能力减弱,从而降低了催化活性[40]。图8b表明,以B-CNTs/PSSF为催化剂苯酚的转化率可达100%。值得注意的是,苯酚转化率和H2O2转化率与催化剂之间有显著的相关性。这表明,H2O2 活化分解出的⋅OH对苯酚的湿式催化氧化起决定性作用。图8c表明,B-CNTs/PSSF在7 h内对苯酚的矿化效率优异,TOC转化率稳定在40%以上,最高可达68%。而CNTs/PSSF的矿化能力较差,因为在反应过程仍有一部分苯酚未被降解,且产生了大量的小分子羧酸使剩余的TOC增多[51]。图8d所示的pH值变化,也证实了这一点。从图8e可以看出,在反应7 h内PSSF载体中几乎没有Fe离子浸出。CNTs/PSSF和B-CNTs/PSSF作为催化剂,Fe离子浸出浓度均随时间的推移而降低。Fe离子浸出的原因,是在酸性条件下碳纳米管端口打开,铁基催化剂颗粒直接暴露在液相中[52]。
图8
图8
不同催化剂对苯酚CWPO的催化性能
Fig. 8
Evaluation of catalytic performance of phenol CWPO based on different catalysts (a) H2O2 conversion; (b) phenol conversion; (c) TOC conversion; (d) pH; (e) Fe leaching concentration
2.3 B-CNTs/PSSF降解苯酚的反应机理
根据密度泛函理论研究了湿式催化氧化中碳纳米管与H2O2之前的复杂相互作用,结果如图9所示。B-CNT对H2O2的吸附能为-24.1 kJ·mol-1,表明B-CNT比CNT对H2O2的吸附能力更强,有利于从从溶液中吸附H2O2。在电子密度差图中,绿色为电子密度增加的区域,蓝色为电子密度减少的区域。可以发现,在吸附过程中碳纳米管与H2O2伴随着明显的电子转移现象。B-CNT片段向H2O2片段转移0.048 e,CNT与H2O2间电子转移可以忽略不计(0.007 e)。这意味着,在苯酚降解过程中B-CNTs/PSSF表面有利于H2O2的吸附,并且更多的电子转移有利于打断H2O2的O-O键形成
图9
图9
CNT对H2O2的吸附构型、吸附能和电子密度差图以及B-CNT对H2O2的吸附构型和电子密度差图
Fig.9
Adsorption configuration, adsorption energy and electron density difference of CNT on H2O2 (a), adsorption configuration, adsorption energy and electron density difference of B-CNT on H2O2 (b)
3 结论
(1) 以苯硼酸为前驱体采用化学气相沉积技术可在纸状烧结不锈钢微纤载体表面上生长硼掺杂碳纳米管膜,制备出新型B-CNTs/PSSF复合材料。
(2) B-CNTs/PSSF对苯酚降解具有优异的催化性能,使苯酚转化率达到100%、使TOC转化率达到68%。
(3) BCNT对H2O2的吸附性能比CNT更高,在催化过程中出现明显的电子转移。
参考文献
Efficiency, mechanism, influencing factors, and integrated technology of biodegradation for aromatic compounds by microalgae: a review
[J].
Enhancing biodegradation of toxic industrial wastewaters in a continuous two-phase partitioning bioreactor operated with effluent recycle
[J].
Individually recovery of gallic acid and 3, 5-dimethyl-2, 4-dichlorophenol from industry wastewater by solvent extrac-tion
[D].
溶剂萃取法处理高浓度含酚有机工业废水
[D].
Progress in phenol containing wastewater treatment by extraction
[J].
萃取法处理含酚废水的研究进展
[J].
Study on high efficiency extraction of phenolic-containing wastewater from coal chemical industry
[J].
煤化工含酚废水高效萃取研究
[J].
Research progress on the treatment of industrial phenolic wastewater over advanced adsorbent materials
[J].
先进吸附材料在含酚工业废水中应用的研究进展
[J].
Advanced oxidation processes for the removal of antibiotics from water. An overview
[J].
Surface mechanism of carbon-based materials for catalyzing peroxide degradation of organic pollutants in water
[J].In recent years, compared to metal catalysts, metal-free carbon-based catalysts including traditional carbon materials such as activated carbon (AC), biochar (BC), activated carbon fiber (ACF) and activated carbon cloth (ACC), and new nano-carbon material such as carbon nanotubes (CNT), graphene (GE), ordered mesoporous carbon (OMC), and their surface modified materials are gradually investigated as a new peroxide activator. In the field of water treatment, the above carbon-based materials can be used to catalyze and activate peroxides such as hydrogen peroxide (H<sub>2</sub>O<sub>2</sub>), peroxymonosulfate (HSO<sup>-</sup><sub>5</sub>, PMS) or persulfate (S<sub>2</sub>O<sup>2-</sup><sub>8</sub>, PS) to produce highly active hydroxyl radicals (·OH) or sulfate radicals (SO<sub>4</sub><sup>·-</sup>), which can efficiently degrade organic contaminants through advance oxidation processes (AOPs). What's more, the surface structure of carbon-based materials is rich in functional groups, such as hydroxyl, carboxyl, ketone, pyridine, pyrrole, etc., as well as abundant and varied defect shape, delocalized π electrons, hybrid C orbitals, and so on. They can work together and show the excellent catalytic properties of metal-free carbon-based materials. Therefore, different types of materials and their surface functional groups, surface structure, electron density and other factors play a significant role in the mechanism of carbon-based materials catalyzing peroxides. Accordingly, the progress of this AOP since 2010 and the surface mechanism of the above carbon-based materials in catalyzing peroxide and then degrading organic pollutants in water through the process of adsorption, complexing intermediates and electron transfer are deeply reviewed. Especially, the effects of surface physical and chemical properties on catalyzing mechanisms by the way of oxidation, nitriding, polyatomic in-situ doping and reduction modification are summarized. In addition, the influence mechanism of oxidants on the surface of carbon-based materials is also studied. At the same time, the prospects of the existing problems are pointed out.<br>Contents<br>1 Introduction<br>2 Material differences<br>2.1 Non-nano carbon material<br>2.2 Nano carbon material<br>3 The mechanism of the surface physical and<br>chemical properties<br>3.1 The effect of oxidation<br>3.2 The effect of nitriding<br>3.3 The effect of polyatomic in-situ doping<br>3.4 The effect of reductive treatments<br>4 The influence mechanism of oxidants on the surface of carbon-based materials<br>4.1 Physical properties<br>4.2 Chemical properties<br>5 Conclusion
炭基材料催化过氧化物降解水中有机污染物: 表面作用机制
[J].近几年来,利用传统炭材料(如活性炭(AC)、生物炭(BC)、活性炭纤维(ACF)与活性炭布(ACC))以及纳米碳材料(如碳纳米管(CNT)、石墨烯(GE)、有序介孔碳(OMC)),或其表面改性后的炭基材料,代替金属催化剂,来催化活化过氧化物(包括过氧化氢(H<sub>2</sub>O<sub>2</sub>)、过一硫酸氢盐(HSO<sub>5</sub><sup>-</sup>,PMS)、过二硫酸盐(S<sub>2</sub>O<sub>8</sub><sup>2-</sup>,PS))产生高活性羟基自由基(·OH)或硫酸根自由基(SO<sub>4</sub><sup>·-</sup>),从而氧化去除水中难生物降解有机污染物,成为水处理领域研究热点。在炭表面富含很多起催化作用的官能团,如羟基、羧基、酮基、吡啶、吡咯等,同时具有丰富多样的缺陷形状、离域π电子、杂化C轨道等,能共同协作表现出非金属炭基催化剂的优良特性。因此,不同类型材料及其表面官能团、表面结构、电子密度等因素对炭基材料催化过氧化物的机理发挥显著作用。本文深入分析了上述炭基材料在吸附、络合中间体、电子转移过程中催化过氧化物产生强氧化性的自由基,并高效降解水中有机污染物的作用机理,综述了2010年以来该类高级氧化技术在水处理领域的研究进展,特别是通过总结炭基材料的氧化改性作用、氮化改性作用、多原子原位掺杂作用以及还原改性作用,系统阐述了表面物理化学性质对炭基材料催化过氧化物的表面作用机制的影响,并归纳了氧化剂对炭基材料的表面作用机制,对存在的问题提出了新的研究展望。
Effective defect generation and dual reaction pathways for phenol degradation on boron-doped carbon nanotubes
[J].
Insights into the peroxomonosulfate activation on boron-doped carbon nanotubes: performance and mechanisms
[J].
Adsorption dynamics of phenol in a fixed bed packed with activated carbon and stainless steel fiber-reinforced activated carbon paper
[J].
Boron–carbon nanotubes from the pyrolysis of C2H2-B2H6 mixtures
[J].
The characterization of boron-doped carbon nanotube arrays
[J].
Effect of in-situ boron doping on hydrogen adsorption properties of carbon nanotubes
[J].
CVD growth of single-walled B-doped carbon nanotubes
[J].
Comparison of structural changes in nitrogen and boron-doped multi-walled carbon nanotubes
[J].
Kinetic study of boron doped carbon nanotubes synthesized using chemical vapour deposition
[J].Boron doped carbon nanotubes were synthesized using chemical vapour deposition using acetylene as carbon source, boric acid as boron source and Ferrocene/MgO as catalyst/support. Boric acid was directly used as a solid precursor for doping boron in the CNT lattice. A kinetic model was established by varying the temperature of the reactor, partial pressure of reactants, flow rates and catalyst concentration. The rate controlling steps were studied and the optimal reaction conditions were established so as to synthesize the desired quantity and quality of boron doped carbon nanotubes. Boron doping of 6.31-6.71 at.% was obtained. Two different mechanisms were found to control the rate of reaction at different range of temperatures. The activation energy for the two mechanisms was found to be 18.21 kJ/mol and 6.73 kJ/mol respectively. A mechanism was proposed and validated using the experimental data so as to understand the growth of B-CNTs. The synthesized B-CNTs were purified using concentrated hydrochloric acid and characterized by using SEM, TEM to understand the surface characteristics; FTIR and XPS to detect and quantify the boron present in the sample, Raman spectroscopy and TGA analysis to determine the purity of the product. (C) 2019 Published by Elsevier Ltd.
Permanent boron doped graphene with high homogeneity using phenylboronic acid
[J].
Synthesis of CNTs on stainless steel microfibrous composite by CVD: effect of synthesis condition on carbon nanotube growth and structure
[J].
Degradation of m-cresol over iron loaded carbon nanotube microfibrous composite: kinetic optimization and deactivation study
[J].
Multiwfn: a multifunctional wavefunction analyzer
[J].Multiwfn is a multifunctional program for wavefunction analysis. Its main functions are: (1) Calculating and visualizing real space function, such as electrostatic potential and electron localization function at point, in a line, in a plane or in a spatial scope. (2) Population analysis. (3) Bond order analysis. (4) Orbital composition analysis. (5) Plot density-of-states and spectrum. (6) Topology analysis for electron density. Some other useful utilities involved in quantum chemistry studies are also provided. The built-in graph module enables the results of wavefunction analysis to be plotted directly or exported to high-quality graphic file. The program interface is very user-friendly and suitable for both research and teaching purpose. The code of Multiwfn is substantially optimized and parallelized. Its efficiency is demonstrated to be significantly higher than related programs with the same functions. Five practical examples involving a wide variety of systems and analysis methods are given to illustrate the usefulness of Multiwfn. The program is free of charge and open-source. Its precompiled file and source codes are available from http://multiwfn.codeplex.com.Copyright © 2011 Wiley Periodicals, Inc.
Direct growth of MWCNTs on 316 stainless steel by chemical vapor deposition: effect of surface nano-features on CNT growth and structure
[J].
Carbon nanotube synthesis upon stainless steel meshes
[J].
Preparation of micro-fibrous nitrogen-doped carbon nanotubes coated paper-like sintered stainless steel fibers composite catalyst for catalytic degradation of phenolic wastewater
[D].
微纤复合氮掺杂碳纳米管膜催化剂的制备及其催化降解含酚废水特性研究
[D].
Preparation of novel catalyst-free Fe3C nanocrystals encapsulated NCNT structured catalyst for continuous catalytic wet peroxide oxidation of phenol
[J].
Effect of boron concentration on physicochemical properties of boron-doped carbon nanotubes
[J].
Covalently bonded three-dimensional carbon nanotube solids via boron induced nanojunctions
[J].The establishment of covalent junctions between carbon nanotubes (CNTs) and the modification of their straight tubular morphology are two strategies needed to successfully synthesize nanotube-based three-dimensional (3D) frameworks exhibiting superior material properties. Engineering such 3D structures in scalable synthetic processes still remains a challenge. This work pioneers the bulk synthesis of 3D macroscale nanotube elastic solids directly via a boron-doping strategy during chemical vapour deposition, which influences the formation of atomic-scale "elbow" junctions and nanotube covalent interconnections. Detailed elemental analysis revealed that the "elbow" junctions are preferred sites for excess boron atoms, indicating the role of boron and curvature in the junction formation mechanism, in agreement with our first principle theoretical calculations. Exploiting this material's ultra-light weight, super-hydrophobicity, high porosity, thermal stability, and mechanical flexibility, the strongly oleophilic sponge-like solids are demonstrated as unique reusable sorbent scaffolds able to efficiently remove oil from contaminated seawater even after repeated use.
Evaluation of mild acid oxidation treatments for MWCNT functionalization
[J].
H2SO4/HNO3/HCl—Functionalization and its effect on dispersion of carbon nanotubes in aqueous media
[J].
FTIR studies of nitrogen doped carbon nanotubes
[J].
Effect of chemical oxidation on the structure of single-walled carbon nanotubes
[J].
Nitrogen in graphite and carbon nanotubes: magnetism and mobility
[J].
Evaluation of different oxidizing agents on effective covalent functionalization of multiwalled carbon nanotubes
[J].
Perspectives on Raman spectroscopy of graphene-based systems: from the perfect two-dimensional surface to charcoal
[J].
Raman spectroscopy as a versatile tool for studying the properties of graphene
[J].Raman spectroscopy is an integral part of graphene research. It is used to determine the number and orientation of layers, the quality and types of edge, and the effects of perturbations, such as electric and magnetic fields, strain, doping, disorder and functional groups. This, in turn, provides insight into all sp(2)-bonded carbon allotropes, because graphene is their fundamental building block. Here we review the state of the art, future directions and open questions in Raman spectroscopy of graphene. We describe essential physical processes whose importance has only recently been recognized, such as the various types of resonance at play, and the role of quantum interference. We update all basic concepts and notations, and propose a terminology that is able to describe any result in literature. We finally highlight the potential of Raman spectroscopy for layered materials other than graphene.
Studying disorder in graphite-based systems by Raman spectroscopy
[J].Raman spectroscopy has historically played an important role in the structural characterization of graphitic materials, in particular providing valuable information about defects, stacking of the graphene layers and the finite sizes of the crystallites parallel and perpendicular to the hexagonal axis. Here we review the defect-induced Raman spectra of graphitic materials from both experimental and theoretical standpoints and we present recent Raman results on nanographites and graphenes. The disorder-induced D and D' Raman features, as well as the G'-band (the overtone of the D-band which is always observed in defect-free samples), are discussed in terms of the double-resonance (DR) Raman process, involving phonons within the interior of the 1st Brillouin zone of graphite and defects. In this review, experimental results for the D, D' and G' bands obtained with different laser lines, and in samples with different crystallite sizes and different types of defects are presented and discussed. We also present recent advances that made possible the development of Raman scattering as a tool for very accurate structural analysis of nano-graphite, with the establishment of an empirical formula for the in- and out-of-plane crystalline size and even fancier Raman-based information, such as for the atomic structure at graphite edges, and the identification of single versus multi-graphene layers. Once established, this knowledge provides a powerful machinery to understand newer forms of sp(2) carbon materials, such as the recently developed pitch-based graphitic foams. Results for the calculated Raman intensity of the disorder-induced D-band in graphitic materials as a function of both the excitation laser energy (E(laser)) and the in-plane size (L(a)) of nano-graphites are presented and compared with experimental results. The status of this research area is assessed, and opportunities for future work are identified.
Raman spectra for characterization of defective CVD multi-walled carbon nanotubes
[J].
Effect of pyrrolic-N defects on the capacitance and magnetization of nitrogen-doped multiwalled carbon nanotubes
[J].
Boron-doped carbon nanotubes with uniform boron doping and tunable dopant functionalities as an efficient electrocatalyst for dopamine oxidation reac-tion
[J].
Efficient and continuous removal of phenol by activating PMS via nitrogen doped carbon nanotube membrane in the structured fixed bed
[J].
Different functional groups functionalized hexagonal boron nitride (h-BN) nanoparticles and multi-walled carbon nanotubes (MWCNT) for hydrogen storage
[J].
Study of purification process of single-walled carbon nanotubes by thermoanalytical techni-ques
[J].
Synergistic effects of functional CNTs and h-BN on enhanced thermal conductivity of epoxy/cyanate matrix composites
[J].
Catalytic wet air oxidation of phenol over carbon nanotubes: synergistic effect of carboxyl groups and edge carbons
[J].
Insights into heterogeneous catalysis of persulfate activation on dimensional-structured nanocarbons
[J].
Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: electron transfer mechanism
[J].
Boron doped multi-walled carbon nanotubes as catalysts for oxygen reduction reaction and oxygen evolution reactionin in alkaline media
[J].
Porous boron doped diamonds as metal-free catalysts for the oxygen reduction reaction in alkaline solution
[J].
Boron-doped carbon nanotubes as metal-free electrocatalysts for the oxygen reduction reaction
[J].
Characterization of α-Fe2O3/γ-Al2O3 catalysts for catalytic wet peroxide oxidation of m -cresol
[J].
Graphenes as efficient metal-free fenton catalysts
[J].
Removal of some impurities from carbon nanotubes
[J].