金属在工业领域发挥着重大的作用,然而金属腐蚀的问题十分普遍。腐蚀会严重降低金属的性能,导致金属使用寿命缩短并造成严重的安全隐患和经济损失,因此金属的腐蚀防护非常重要[1]。腐蚀主要是由水分、氧气、电解质等环境因素引起的,一般最先在金属表面发生。因此,在金属表面进行涂层防护,阻隔腐蚀介质进入,是目前广泛采用的腐蚀控制方法[2]。水性环氧树脂涂料是常见的环保涂料,具有优异的耐腐蚀性、耐化学性和较强的机械性能,已广泛应用于金属的保护[3~5]。但是水性环氧涂层残留的亲水基团在固化后会形成极性通道,导致涂层内水渗透加快,引发腐蚀,从而降低耐腐蚀性能[6]。在水性环氧树脂涂层中添加填料可以有效提高其耐蚀性能。石墨烯因其独特的二维纳米结构和优越的物理化学性质可以作为防腐材料,而且可以以薄膜、层状结构或复合材料的形式独立地或协同地作为防止金属腐蚀的屏障[7~10],将其作为填料加入涂层中可以显著提升防腐蚀性能。
在石墨烯结构中引入杂原子是提高石墨烯复合涂层防腐性能的有效方法,因为杂原子容易在金属表面自发吸附,使金属表面形成抑制膜。对石墨烯进行磷掺杂后制备的涂层比石墨烯涂层具有更强的粘附性能,可以进一步提高涂层的耐腐蚀性[11]。Wang等[12]以富含磷酸基团的植酸(PA)为磷源对石墨烯进行掺杂,将其作为填料加入环氧树脂中显著提升了防腐蚀性能。这是因为磷酸基团可以螯合金属离子,生成稳定的配合物沉积在金属表面[13]。在石墨烯结构中引入氮原子也可以提高涂层的耐蚀性能。氮原子中存在的孤电子对可以作为亲核中心,与金属的d轨道形成配位短键,从而提高涂层的防腐性能[14]。Ren等[15]采用CVD法在铜表面制备氮掺杂石墨烯薄膜,通过电化学实验证明了由于N原子的掺入,薄膜/Cu界面上的电化学反应被抑制,腐蚀区域收缩,提高了涂层的耐蚀性能。
对石墨烯进行氮、磷共掺杂可以结合两者的优点,充分发挥氮磷的协同作用。Ma等[16]通过热解法制备了氮磷共掺杂石墨烯气凝胶(NPGA)。由于氮和磷共掺杂的协同效应使NPGA对氧化还原反应产生较高的电催化活性,使其在催化剂领域具有极大的应用价值。目前氮磷共掺杂石墨烯(NPGO)应用在防腐蚀领域还鲜见报道。
本文以氧化石墨烯(GO)为原料,植酸(PA)为磷源,氨水(NH3·H2O)为氮源,采用低温溶液法制备NPGO。再以水性环氧树脂(EP)为成膜物制备复合防腐蚀涂层,研究了复合涂层的耐蚀性以及NPGO添加量对防腐蚀性能的影响。期望能够充分发挥氮磷共掺杂的协同作用,进一步提高石墨烯/水性环氧复合涂层的防腐蚀性能。
1 实验方法
1.1 氧化石墨烯(GO)的制备
氧化石墨烯(GO)的制备参照文献[17]。
1.2 氮磷石墨烯(NPGO)的制备
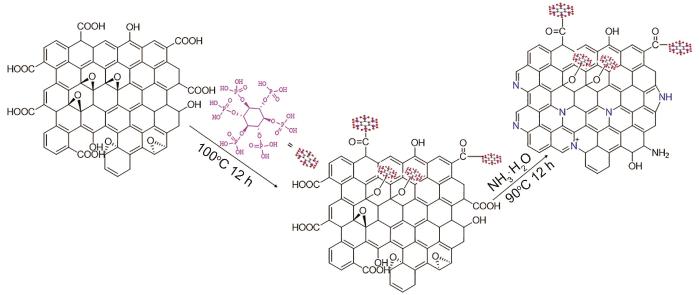
称取0.1 g GO,加入35 g去离子水,超声分散至均匀,加入0.5 g植酸(PA),再次超声分散至均匀,100℃下回流加热12 h,得含磷石墨烯(PGO)分散液。在上述PGO分散液中加入10 mL NH3·H2O,90℃回流加热12 h,然后进行真空抽滤,去离子水多次洗涤直至中性。过滤产物在40℃下干燥8 h得到氮磷石墨烯(NPGO),NPGO的合成路线如图1所示。为了对比,按照上述相同工艺,在GO分散液中只加入PA不加NH3·H2O,经抽滤、洗涤、干燥制备PGO;另外,在GO分散液中不加入PA只加NH3·H2O,经抽滤、洗涤、干燥制备含氮石墨烯(NGO)。
图1
1.3 NPGO/EP复合涂层的制备
称取一定量NPGO粉末进行充分研磨,再以水性环氧树脂乳液(EP)为成膜物,加入EP用量的16%(质量分数)的水性固化剂和EP用量的14% (质量分数)的去离子水,充分搅拌后制备不同含量的氮磷石墨烯/环氧树脂乳液(NPGO/EP)复合涂层材料。Q235钢电极或钢片表面经过打磨抛光处理,并用乙醇、丙酮多次擦拭。采用滚涂法将NPGO/EP复合涂层材料涂于钢表面,放入40℃烘箱中进行固化。经过多次滚涂控制涂层厚度在300 ±10 μm,随后继续在40℃烘箱中固化48 h。按照上述方法分别制备纯EP涂层和GO/EP、PGO/EP、NGO/EP复合涂层。不同复合涂层石墨烯用量(干膜质量分数)见表1。
表1 不同复合涂层石墨烯用量
Table 1
| GO | PGO | NGO | NPGO | NPGO | NPGO | NPGO | |
|---|---|---|---|---|---|---|---|
| Amount / g | 0.03 | 0.03 | 0.03 | 0.01 | 0.02 | 0.03 | 0.04 |
| Content / % | 1.5 | 1.5 | 1.5 | 0.5 | 1.0 | 1.5 | 2.0 |
1.4 NPGO的表征
采用美国Perkin Eimer公司Spectrum one型红外光谱仪分析样品结构,扫描范围为500~4000 cm-1。采用美国Thermo公司ESCALAB250Xi型X-射线光电子能谱仪分析样品结构,采用Al Kα线为X射线光源,功率为300 W。使用德国BRUKER-AXS公司的D8型X射线衍射仪进行测样,扫描速度5 (°)/min,扫描角度5°~60°。采用日本日立S-4300场发射扫描电子显微镜观察样品表面微观形貌,将样品进行喷金处理,电压为20 kV。采用日本日立H-7650透射电子显微镜观察微观形貌,将样品制备为分散液,电压为200 kV。
1.5 NPGO/EP复合涂层性能测试
通过接触角和吸水率测试复合涂层的疏水性能。采用静滴法在JY-82型接触角测定仪上测定涂层表面对水的接触角,水滴体积约5 μL,测试涂层不同位置5次取平均值。将涂覆涂层的不锈钢片置于去离子水中浸泡72 h (室温),按下式计算吸水率(%):
其中,X是吸水率,m0和m1分别是涂层吸水前、后的质量。
采用美国Gamry公司INTERFACE1000型电化学工作站测试涂层的电化学阻抗谱,选用三电极系统:以Ag/AgCl电极作为参比电极,Pt柱电极作为辅助电极,涂覆涂层的Q235钢电极作为工作电极(电极裸露面积为1 cm2),以3.5% (质量分数)的NaCl水溶液为腐蚀介质,电化学阻抗谱测试在开路电位下进行,频率范围为10-2~105 Hz,交流幅值为10 mV。
2 结果与讨论
2.1 NPGO的结构与形貌
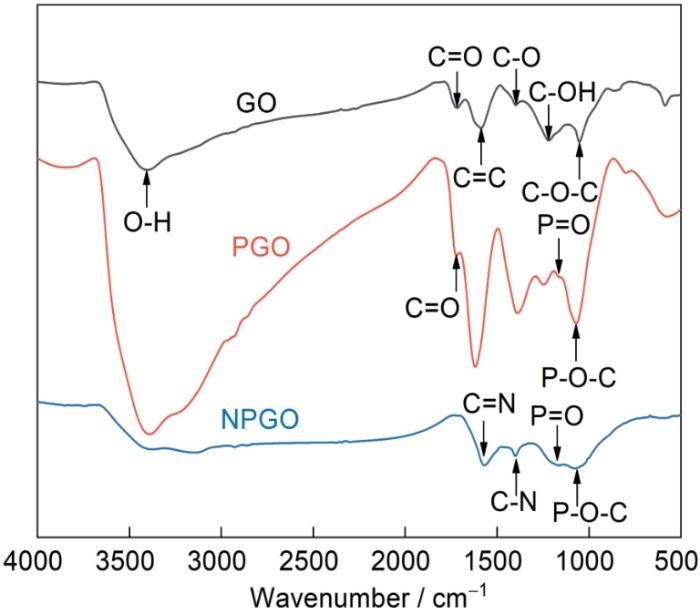
图2分别为GO、PGO和NPGO的红外光谱图。如图所示,GO的FTIR光谱在3410 cm-1处的宽峰为O-H的伸缩振动吸收峰,1718 cm-1处存在C=O的伸缩振动吸收峰,1583 cm-1处存在C=C的伸缩振动吸收峰,1385、1218、1071 cm-1处分别对应于C-O、C-OH和C-O-C的伸缩振动。这些峰的存在证明了GO的成功制备[20]。PGO的FTIR光谱在1161、1057 cm-1处的吸收峰分别归属于P=O和P-O-C的伸缩振动,证明了含磷基团成功引入GO[21]。与GO相比,NPGO在1563、1390、1166、1058 cm-1出现了新的特征峰,分别对应C=N、C-N[22,23]、P=O、P-O-C键[24]。这些特征峰的出现说明氮和磷被引入到GO中,成功地制备了NPGO。
图2
图3为NPGO的XPS谱图。为了确定在GO上添加P源和N源后NPGO的元素组成以及掺杂类型,对样品进行了XPS分析。图3a为NPGO的XPS全谱图,在NPGO的全谱中可以观察到C 1s、O 1s、P 2p、N 1s四个特征峰。为了研究成键情况,对C、P、N元素进行了分峰处理。如图3b,C 1s能谱在284.8,285.3,286.5,288.5和289.5 eV五个吸收峰分别为C-C[25],C-N[26],C-O-P,C=O和O-C=O[27]的吸收峰。图3c显示了P 2p能谱在约134 eV被分为两个峰,分别是132.8 eV处的P=O和134.5 eV处的P-O[28]。P-O键的存在说明磷原子被成功的掺杂进入了石墨烯,有两种存在结构和形式。如图3d所示,N 1s可以被分为不同类型的N键构型相关的四个峰,在398.9、400.0、401.3和403.5 eV处的吸收峰分别对应吡啶氮(Pyridinic N)、吡咯氮(Pyrrolic N)、石墨氮(Graphilic N)和氧化氮(Oxidized N)[29~31]。结合NPGO的全谱分析以及P 2p和N 1s的分峰结果,说明N和P成功地引入到GO中。
图3
图4
图5
图6
2.2 NPGO/EP复合涂层的疏水性
图7
图7
NPGO含量对复合涂层接触角和吸水率的影响
Fig.7
Variations of the contact angle and water absorption of the composite coating with the content of NPGO
2.3 涂层的附着力
图8为不同填料及NPGO不同添加量的复合涂层的附着力数据,涂层附着力越高表明涂层与金属的结合力越高,涂层对金属的保护效果越好。从图8a中可以看出,纯EP涂层的附着力为12.3 MPa,加入石墨烯后,由于石墨烯可以填补涂层与金属界面间的孔隙,使系列复合涂层的附着力均有提高。其中,相比GO/EP复合涂层,NGO/EP和PGO/EP复合涂层的附着力又有提高,表明N掺杂或P掺杂对复合涂层的附着力提升均有促进作用。这是因为NGO中的氮原子可以作为亲核中心,与金属的d轨道形成配位短键;PGO表面的磷酸基团可以与金属离子螯合生成配合物,从而使涂层与金属间的结合力增强。对于NPGO/EP复合涂层,由于充分发挥了N、P共掺杂的协同作用,使NPGO/EP复合涂层的附着力达到最大值。另外,NPGO在EP中的添加量对复合涂层的附着力有一定的影响。从图8b中可以看出,当NPGO添加量为1.5% (干膜质量分数)时,涂层的附着力达到了最大值为20.6 MPa,这是因为适量的NPGO在涂层中能够分散良好,充分发挥了N、P共掺杂的作用,填补涂层与金属界面间的孔隙,极大的提高了涂层的附着力。NPGO添加量较少时,作用发挥不理想;继续增加NPGO的量,又会降低NPGO在涂层中分散的均匀性,导致涂层的附着力降低。
图8
图8
不同填料对涂层附着力的影响
Fig.8
Effects of various fillers on the adhesion strength of the composite coating
2.4 涂层的耐蚀性能
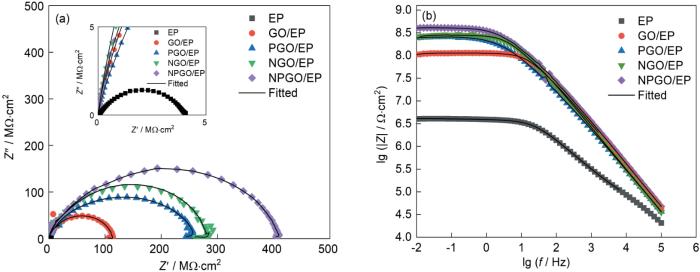
图9为EP涂层、GO/EP、NGO/EP、PGO/EP以及NPGO/EP复合涂层在3.5% (质量分数) NaCl溶液中浸泡24 h之后测得的电化学阻抗谱图(EIS),包括Nyquist和Bode图。Nyquist图中的容抗弧的大小可以反应涂层的耐腐蚀性能,容抗弧的半径越大,涂层的防腐蚀效果越好。如图9所示,与EP涂层相比,添加了系列GO的复合涂层的容抗弧半径均增大,其中NGO/EP复合涂层和PGO/EP复合涂层较GO/EP复合涂层的半径大,而NPGO/EP复合涂层的容抗弧半径最大,说明GO起到了屏蔽腐蚀粒子的作用,且由于N、P的共同作用使NPGO/EP复合涂层的阻抗最大,防腐蚀性能最佳。同时,Bode图中低频率区的阻抗模值(|Z| f = 0.01 Hz)也表示了涂层的阻抗大小。在0.01 Hz频率下,EP、GO/EP、NGO/EP、PGO/EP以及NPGO/EP复合涂层的|Z|依次增大,其中,NPGO/EP复合涂层的|Z|为最大,达到了4.92 × 108 Ω·cm2,说明NPGO/EP复合涂层的防腐蚀性能最好。
图9
图9
EP涂层和GO/EP、NGO/EP、PGO/EP、NPGO/EP复合涂层在3.5% (质量分数) NaCl溶液中浸泡24 h后的Nyquist和Bode图
Fig.9
(a) Nyquist and (b) Bode plots of EP, GO/EP, NGO/EP, PGO/EP, NPGO/EP coatings after immersion in 3.5%NaCl solution for 24 h
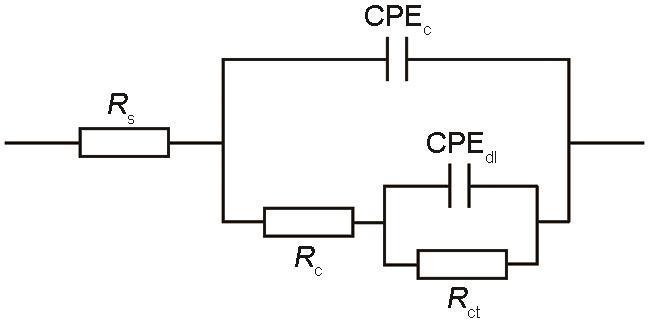
为了进一步分析EIS结果,采用Zview软件对EIS数据进行拟合,得到的电化学数据如表2所示,等效电路如图10所示。其中Rc和Rct分别代表涂层电阻和电荷转移电阻,CPEc和CPEdl为电容器的恒相元件,Rs为电解质的电阻[33]。涂层的Rc反应了涂层的致密性,Rc越大涂层的致密性就越高,表示涂层的防腐性能越好。涂层的Rct反应了腐蚀反应的发生趋势,Rct值越高,表示钢表面的腐蚀反应就更难发生。由拟合数据可以看出,相比纯EP涂层,GO/EP复合涂层的Rc和Rct值均明显增大,说明GO的添加对EP涂层的防腐蚀性能有很好的提升作用。相比GO/EP复合涂层,NGO/EP和PGO/EP复合涂层的Rc和Rct值又有所增大,表明N或P的引入进一步提高了复合涂层的防腐性能。当在EP中添加N、P共掺杂的NPGO时,由于结合了N的配位作用和P的螯合作用,使NPGO/EP复合涂层的Rc和Rct均高于NGO/EP和PGO/EP复合涂层,证明NPGO/EP复合涂层的防腐蚀性能最好。
表2 EIS拟合数据
Table 2
| Sample | Rs / Ω·cm2 | Rc / Ω·cm2 | CPEc | Rct / Ω·cm2 | CPEdl | ||
|---|---|---|---|---|---|---|---|
| Y0 / Ω·cm-2·s n | nc | Y0 / Ω·cm-2·s n | nc | ||||
| EP | 35673 | 1.32 × 106 | 8.12 × 10-9 | 1.442 | 3.72 × 106 | 4.25 × 10-9 | 0.7936 |
| GO/EP | 28254 | 1.03 × 108 | 1.35 × 10-10 | 0.9358 | 1.04 × 107 | 6.13 × 10-10 | 0.8393 |
| NGO/EP | 32261 | 2.61 × 108 | 4.02 × 10-10 | 0.7754 | 3.97 × 107 | 2.66 × 10-11 | 1.986 |
| PGO/EP | 26477 | 2.52 × 108 | 2.65 × 10-10 | 0.9364 | 2.78 × 107 | 1.93 × 10-10 | 0.9238 |
| NPGO/EP | 32870 | 3.98 × 108 | 2.39 × 10-10 | 0.8145 | 4.41 × 107 | 2.22 × 10-10 | 1.071 |
图10
图10
电化学阻抗数据拟合等效电路
Fig.10
Equivalent circuit model for fitted EIS of various coatings
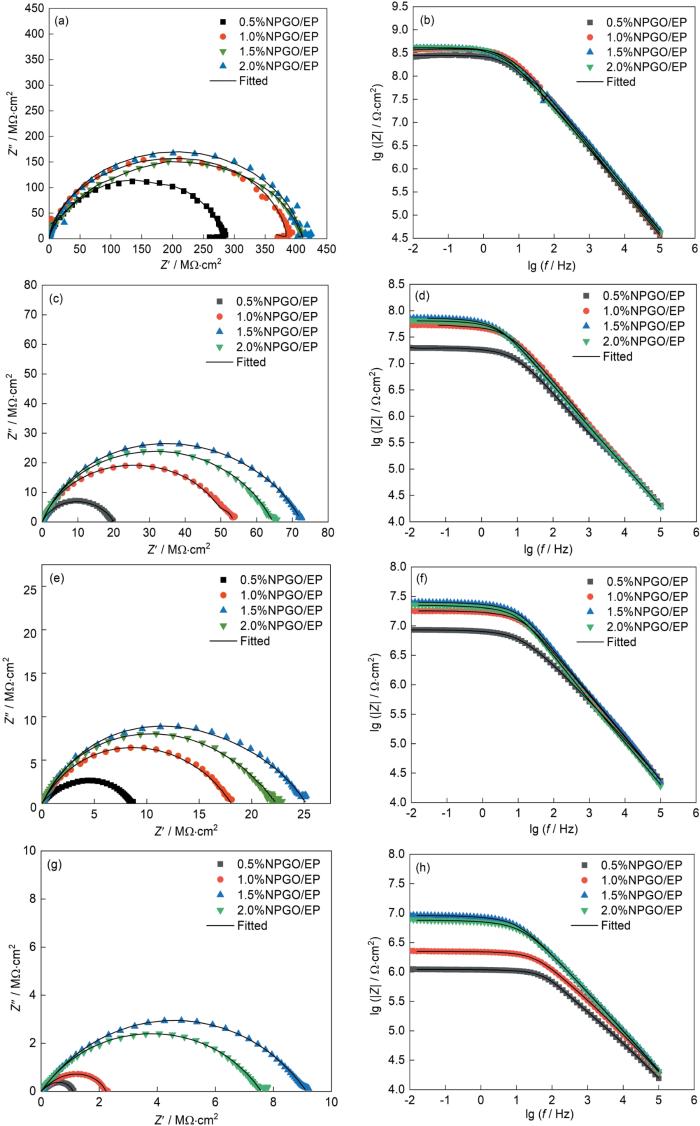
图11
图11
不同含量的NPGO/EP复合涂层在3.5%NaCl溶液中浸泡不同时间的Nyquist和Bode图
Fig.11
(a, c, e, g) Nyquist and (b, d, f, h) Bode plots of NPGO/EP composite coatings with different contents of NPGO after immersion in 3.5%NaCl solution for (a, b) 24 h, (c, d) 240 h, (e, f) 480 h, (g, h) 720 h
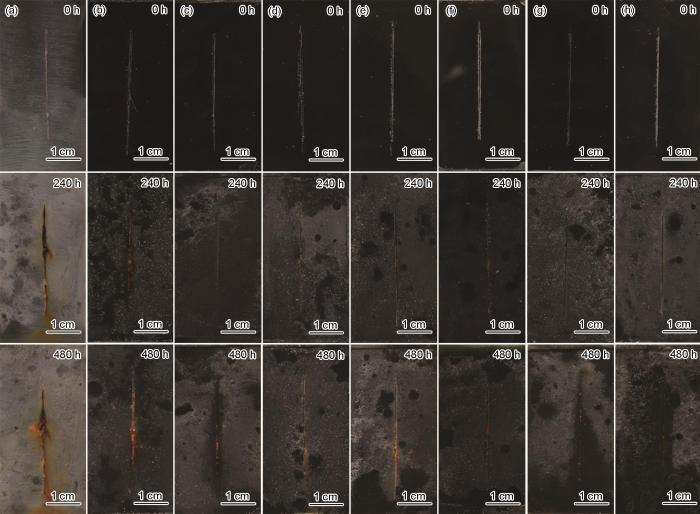
图12是EP、GO/EP、PGO/EP、PGO/EP以及不同含量的NPGO/EP涂覆在Q235钢表面后在盐雾试验0、240和480 h时的照片。从图12可以看出,随着浸泡时间的延长,EP涂层的腐蚀变得严重,划痕处有大量的锈痕,并且向里扩散。而添加GO、PGO、NGO和NPGO的复合涂层在划痕处的腐蚀产物明显减少,腐蚀扩散也逐渐减少。其中,当NPGO含量达到1.5%时,NPGO/EP复合涂层在划痕处的金属表面的腐蚀产物最少,涂层的防腐性能最佳。这是由于,适量的填料能够均匀分散在涂层中,填补了EP内部的空隙,一方面使得腐蚀介质(Na+、Cl-、O2和H2O等)难以渗透到金属的表面对金属腐蚀;另一方面,划痕处的腐蚀也不易扩散。但当NPGO含量过多时会引起团聚现象,导致涂层防腐性能下降。
图12
图12
不同涂层经不同时间的盐雾实验后的宏观形貌照片
Fig.12
Macro-photos of (a) EP, (b) GO/ EP, (c) NGO/EP, (d) PGO/EP, (e) 0.5%NPGO/EP, (f) 1.0%NPGO/EP, (g) 1.5%NPGO/EP and (h) 2.0%NPGO/EP coatings after salt spray test for different time
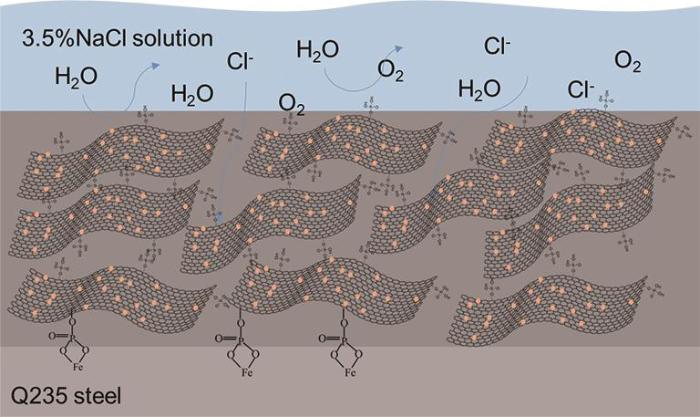
2.5 NPGO/EP复合涂层的防腐蚀机理
图13为NPGO/EP复合涂层的防腐蚀机理示意图。结合防腐蚀测试结果进行分析,复合涂层具有较好防腐蚀性能主要是因为以下几点:首先,石墨烯固有的不渗透性产生了屏障效应,同时石墨烯片层结构能够延长腐蚀介质在涂层中的渗透路径[34];适量的NPGO分散在环氧树脂中能够发挥物理屏蔽作用,有效的弥补环氧树脂涂层中的空隙和缺陷,使复合涂层的防腐蚀性有效提升。其次NPGO中的磷酸基团可以与金属离子螯合生成配合物,在金属表面沉积,起到防腐蚀的作用;NPGO中的氮原子易与金属生成配位短键,同时氮原子的存在有助于复合涂层在钢表面的快速吸附。第三,NPGO能够充分发挥氮磷的协同作用,进一步提升复合涂层的防腐蚀性能。综上,NPGO/EP涂层的防腐蚀效果明显优于EP、GO/EP、PGO/EP、NGO/EP涂层,是NPGO片层的阻隔作用、NPGO中磷酸基团对金属的螯合作用以及氮原子对金属的配位作用协同发挥的结果。
图13
图13
NPGO/EP复合涂层防腐蚀机理示意图
Fig.13
Schematic diagram of corrosion resistant mechanism of NPGO/EP composite coating
3 结论
(1) 以氧化石墨烯(GO)为原料,植酸(PA)为磷源,氨水(NH3·H2O)为氮源成功制备了氮磷共掺杂石墨烯(NPGO)。XPS、SEM和TEM等表征表面N和P成功地引入到GO结构中,且NPGO具备褶皱的片层结构。
(2) 电化学测试等结果表明,在纯环氧树脂(EP)中添加GO、PGO、NGO和NPGO均能使复合涂层的阻抗增大,有效地提高复合涂层的防腐蚀性能。
(3) 由于N和P的协同作用,使NPGO/EP复合涂层具有最佳的附着力、疏水性和防腐蚀性能。当NPGO的添加量为1.5%时,附着力为20.6 MPa,比EP涂层提高67.5%;接触角为77.26°,比EP涂层提高39.4%;吸水率5.4%,比EP涂层降低了48.1%。NPGO/EP复合涂层的低频阻抗值为4.85 × 108 Ω·cm2,比EP涂层提高了2个数量级;盐雾实验480 h后,只有轻微腐蚀,可以达到对金属长期防腐的效果。
参考文献
Enhanced adhesion and corrosion resistance of reduced graphene oxide coated-steel with iron oxide nanoparticles
[J].
Research progress of bionic surface/coating in metal corrosion protection
[J].
仿生表面/涂层在金属腐蚀防护中的研究进展
[J].
A novel hydroxyl epoxy phosphate monomer enhancing the anticorrosive performance of waterborne graphene/epoxy coatings
[J].
Thermoresponsive polyaniline nanoparticles: preparation, characterization, and their potential application in waterborne anticorrosion coatings
[J].
Corrosion resistance of a self-curing waterborne epoxy resin coating
[J].Waterborne coatings have gained more and more attention as a method to protect the environment. However, the application of waterborne coatings to metals, especially for sufficient long-term protection in harshly corrosive environments, is still limited. Here, we report a self-curing waterborne coating with good corrosion resistance. First, a self-curing waterborne epoxy resin (SWEP) was synthesized by a chemical reaction between the epoxy resin and titanium diisopropoxide bisacetylacetonate in water. The structural characterizations via Fourier transform infrared spectroscopy and C-13 nuclear magnetic resonance spectrsocopy confirmed the successful preparation of the SWEP resin. Differential scanning calorimetry and thermogravimetric analysis showed that the SWEP resin could be cured by heating without the use of any curing agents, and the cured coating exhibited good thermal resistance. The mechanical properties of the cured coatings were discussed in the context of the corresponding standards. The results confirmed that the physical properties of the SWEP coating were as good as those of a traditional two-component waterborne epoxy resin (DCWEP) coating. The chemical resistance analysis revealed that the water, acid and alkaline resistances of the SWEP coating all have significant improvements compared to those of the DCWEP coating. The electrochemical impedance spectroscopy and potentiodynamic polarization test implied that the SWEP coating provided better corrosion protection for metal substances than the DCWEP coating.
Anti-corrosion and wear-resistant coating of waterborne epoxy resin by concrete-like three-dimensional functionalized framework fillers
[J].
Corrosion resistance of graphene-reinforced waterborne epoxy coatings
[J].Graphene (G) was dispersed uniformly in water and used as an inhibitor in waterborne epoxy coatings. The effect of dispersed G on anticorrosion performance of epoxy coatings was evaluated. The composite coatings displayed outstanding barrier properties against H2O molecule compared to the neat epoxy coating. Open circuit potential (OCP), Tafel and electrochemical impedance spectroscopy (EIS) analysis confirmed that the corrosion rate exhibited by composite coatings with 0.5 wt% G was an order of magnitude lower than that of neat epoxy coating. Salt spray test results revealed superior corrosion resistance offered by the composite coating.
Graphene nanocoating provides superb long-lasting corrosion protection to titanium alloy
[J].The presence of metallic species around failed implants raises concerns about the stability of titanium alloy (Ti-6Al-4V). Graphene nanocoating on titanium alloy (GN) has promising anti-corrosion properties, but its long-term protective potential and structural stability remains unknown. The objective was to determine GN's anti-corrosion potential and stability over time.GN and uncoated titanium alloy (Control) were challenged with a highly acidic fluorinated corrosive medium (pH 2.0) for up to 240 days. The samples were periodically tested using potentiodynamic polarization curves, electrochemical impedance spectroscopy and inductively coupled plasma-atomic emission spectroscopy (elemental release). The integrity of samples was determined using Raman spectroscopy, X-ray photoelectron spectroscopy, atomic force microscopy and scanning electron microscopy. Statistical analyses were performed with one-sample t-test, paired t-test and one-way ANOVA with Tukey post-hoc test with a pre-set significance level of 5%.There was negligible corrosion and elemental loss on GN. After 240 days of corrosion challenge, the corrosion rate and roughness increased by two and twelve times for the Control whereas remained unchanged for GN. The nanocoating presented remarkably high structural integrity and coverage area (>98%) at all time points tested.Graphene nanocoating protects titanium alloy from corrosion and dissolution over a long period while maintaining high structural integrity. This coating has promising potential for persistent protection of titanium and potentially other metallic alloys against corrosion.Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Corrosion-resistant SiO2-graphene oxide/epoxy coating reinforced by effective electron beam curing
[J].
Preparation of amino-functionalized graphene oxide/sulfonated polyaniline and its application in waterborne epoxy anticorrosive coatings
[J].
氨基化氧化石墨烯/磺化聚苯胺的制备及在水性环氧防腐涂料的应用
[J].
Functionalization of graphene oxide coatings with phosphorus atoms and their corrosion resistance in sodium chloride environment
[J].
Phytic acid intercalated graphene oxide for anticorrosive reinforcement of waterborne epoxy resin coating
[J].
Preparation of phosphorus-containing graphene and corrosion resistance of composite coating
[J].The phosphorus-containing graphene (PhA-G) was prepared by pyrolysis method with phytic acid (PhA) as raw material, and next the PhA-G/SiR composite anticorrosion coating was prepared with silicone resin (SiR) as film forming material. The structure and morphology of phosphorus-containing graphene was characterized by means of Raman spectroscope, X-ray photoelectron spectroscope (XPS), scanning electron microscope (SEM), transmission electron microscope (TEM) and atomic force microscope (AFM). The prepared coatings with the different additive amount of PhA-G (1%~4% in mass fraction), were comparatively examined by means of measurements of contact angle, water absorption, potentiodynamic polarization curves, electrochemical impedance spectroscopy (EIS), as well as salt spray test. The results show that the protectiveness of PhA-G/SiR composite coatings for metal substrate is greater than that of the plain SiR coating, and graphene oxide/silicone resin (GO/SiR) coating. The PhA-G/SiR composite coating exhibits good hydrophobicity and excellent corrosion resistance when the mass fraction of PhA-G is 3%. Correspondingly, which exhibits hydrophobic contact angle of 103.5° and water absorption rate of 3.72%, while corrosion current density of 3.53×10-10 A/cm2 and electrochemical impedance as high as 3.82×107 Ω·cm2 in 3.5%(mass fraction) NaCl solution. Furthermore, the PhA-G/SiR composite coating presents excellent resistance to salt spray testing, up to above 960 h.
含磷石墨烯的制备及复合涂层的耐蚀性能
[J].以植酸(PhA)为原料,采用热解法制备含磷石墨烯(PhA-G),并以硅树脂(SiR)为成膜物制备含磷石墨烯/硅树脂(PhA-G/SiR)复合防腐蚀涂层。通过拉曼光谱和XPS分析含磷石墨烯的结构,通过SEM、TEM和AFM观察含磷石墨烯的形貌,通过接触角、吸水率、电化学阻抗谱、极化曲线和盐雾实验等研究复合涂层的耐蚀性能。结果表明:相比于纯SiR涂层和氧化石墨烯/硅树脂(GO/SiR)复合涂层,PhA-G/SiR复合涂层对金属的保护作用更好;当含磷石墨烯添加量为3%(质量分数)时,PhA-G/SiR复合涂层表现出较好的疏水性和优异的防腐蚀性能,其接触角为103.5°,吸水率为3.72%;腐蚀电流密度为3.53×10<sup>-10</sup> A/cm<sup>2</sup>,电化学阻抗值达到3.82×10<sup>7</sup> Ω·cm<sup>2</sup>,耐盐雾达到960 h。
Effect of Fe3O4-decorated N-doped reduced graphene oxide nanohybrid on the anticorrosion performance of epoxy composite coating
[J].The present study explores the synergistic effect of Fe3O4 and heteroatom doped reduced graphene oxide (rGO) nanohybrid on anti-corrosion performance of epoxy composite coating. In this connection, Fe3O4 nanoparticles were decorated over nitrogen doped rGO (Fe3O4-NRG) and characterized by Fourier transform infrared spectroscopy (FT-IR), Raman spectroscopy, X-ray diffraction (XRD), X-ray Photoelectron spectroscopy (XPS), and Thermogravimetric analysis (TGA). This approach to modulating the surface properties of graphene and hence the interaction with polymer matrix were quite distinct from traditional surface functionlization or decoration in a sense that the doped nitrogen atom facilitate the growth of Fe3O4 particles and at the same time augmented the interaction of rGO with polymer matrix. The prepared nanohybrid was dispersed in epoxy matrix through mechanical mixing and coated over mild steel surface via spin coating technique. The potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) study revealed superior anti-corrosion performance of Fe3O4-NRG/epoxy coating in 3.5 wt% NaCl solution. The corrosion inhibition was found to improve by similar to 98.5% with 0.5 wt% loading of Fe3O4-NRG. We believe that this simple solvent free approach to prepare composite coating can be exploit for practical application and thus deserves special attention..
N-doping of graphene: toward long-term corrosion protection of Cu
[J].
Three-dimensional nitrogen and phosphorous co-doped graphene aerogel electrocatalysts for efficient oxygen reduction reaction
[J].
Preparation of graphitic oxide
[J].
Improvement and enhancement of natural gas hydrate formation process by Hummers' graphene
[J].
Highly compressible three-dimensional graphene hydrogel for foldable all-solid-state supercapacitor
[J].
Preparation of N-doped graphene by hydrothermal method and interpretation of N-doped mechanism
[J].
Fabrication of anti-corrosion nitrogen doped graphene oxide coatings by electrophoretic deposition
[J].
Amphiphilic PA-induced three-dimensional graphene macrostructure with enhanced removal of heavy metal ions
[J].
Fluorometric detection of pH and quercetin based on nitrogen and phosphorus co-doped highly luminescent graphene-analogous flakes
[J].
Ultrahigh rate capability supercapacitors based on tremella-like nitrogen and phosphorus co-doped graphene
[J].
Fabrication of N, P-codoped reduced graphene oxide and its application for organic dye removal
[J].
Preparation and structure study of phosphorus-doped porous graphdiyne and its efficient lithium storage application
[J].
p-type doping of graphene with cationic nitrogen
[J].Tailoring electrical properties of graphene by nitrogen doping is currently of great significance in a broad area of advanced applications. Bonding configuration of nitrogen atoms in graphene plays a vital role in controlling its electrical, chemical, and optical properties. Here, we report for the first time a simple bottom-up synthesis of a novel cationic nitrogen doped graphene (CNG) by a solution plasma (SP). A mixture of ionic liquid and organic solvent was used as starting precursor. CNG exhibited an orthorhombic structure possibly due to the presence cationic nitrogen in hexagonal graphene lattice. Nitrogen doping content was found to be as high as 13.4 atom %. Electrical characterization demonstrated that the CNG exhibited a p-type semiconducting behavior with superior electrical conductivity and carrier concentration. Such unique electrical characteristics of CNG are mainly attributed to the presence of cationic nitrogen with preserved planar structure.
Chloride corrosion resistant nitrogen doped reduced graphene oxide/platinum electrocatalyst for hydrogen evolution reaction in an acidic medium
[J].
Production of P, N co-doped graphene-based materials by a solution process and their electrocatalytic performance for oxygen reduction reaction
[J].
Three-dimensional phosphorus-doped graphene as an efficient metal-free electrocatalyst for electrochemical sensing
[J].
Synthesis of Ti3C2 MXene@PANI composites for excellent anticorrosion performance of waterborne epoxy coating
[J].
The role of graphene in anti-corrosion coatings: a review
[J].