在化学反应中引入催化剂,可将其强化。这表明,使用适当的电催化剂提高硫正极电化学还原和氧化反应速率,从而可提高锂硫电池的性能。Salem等[22]发现,在石墨烯上锚定Pt纳米颗粒,有利于多硫化物的氧化和提高还原反应速率。于是,研究者们在硫正极引入过渡金属的氧化物[22~25]、硫化物[26~28]、磷化物[29~31]、氮化物[32,33]、碳化物[34,35]以及单原子Fe、Co[32,36~38]作为电催化剂,以提高正极反应速率和电池充、放电、循环性能。电催化剂表面的金属或非金属对多硫化锂中硫或锂的化学键吸附,可加速表面反应[39,40]。在上述电催化剂中,过渡金属磷化物优异的电化学性能有利于其能量的储存和转化[41~43]。理论计算结果表明,过渡金属磷化物中的p-带中心更靠近费米能级且与过渡金属原子的d-带中心能级差较小,有利于提高多硫化锂在其表面的氧化还原反应的可逆性[42,44]。DFT计算结果表明,FeP和Ni2P的能级在费米能级附近,具有适中的吸附强度。FeP中Fe原子的d轨道态密度与Pd类似,具有贵金属性质[45]。过渡金属元素掺杂的NiFeP、Ni2Co4P3等磷化物的电导率更高,且通过掺杂调整d-带可进一步提高其催化活性[46~48]。鉴于此,本文将双金属磷化物NiFeP纳米片与Ketjen Black ECP-600JD (KB)超导电炭复合,再与纳米硫材料均匀混合制成S/NiFeP/KB复合材料,将其作为正极活性材料制备锂硫电池正极,研究锂硫电池的性能。
1 实验方法
1.1 制备NiFe-LDH/KB和NiFeP/KB
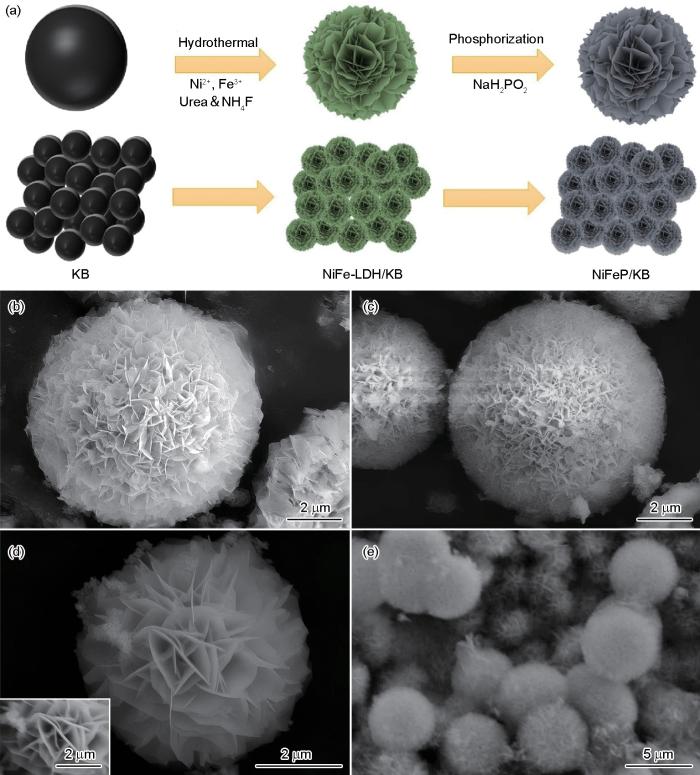
先将0.5 mmol Fe(NO3)3·9H2O、1.5 mmol Ni(NO3)2·6H2O、10 mmol CO(NH2)2和5 mmol NH4F溶解在35 mL去离子水中,然后加入70 mg KB并搅拌均匀,超声分散30 min得到分散均匀的液相混合物。将上述液相混合物转移到聚四氟乙烯内衬水热反应釜中,恒温120℃下反应12 h,冷却后将得到的产物离心分离,用去离子水和乙醇充分洗涤,在60℃干燥12 h得到生长在KB上的镍铁双金属层状氢氧化物NiFe-LDH/KB。按5∶1的质量比将NaH2PO2和制备出的NiFe-LDH/KB顺序放置在管式炉的中段,在惰性气氛中以2℃/min的速率升温到300℃,反应2 h得到NiFeP/KB。合成NiFeP/KB复合材料的示意图,在图1中给出。
图1
图1
NiFeP/KB复合材料合成示意图、NiFe-LDH和NiFeP的SEM照片、NiFe-LDH及NiFeP/KB的高倍SEM照片
Fig.1
Synthesis schematic of NiFeP/K B (a), SEM of NiFe-LDH (b, d), SEM of NiFeP (c) and SEM of NiFeP/KB (e)
1.2 制备纳米S
将200 mL
1.3 制备S/NiFeP/KB和S/KB
将NiFeP/KB和纳米S按质量比3∶7混合后研磨分散,得到S/NiFeP/KB。为了比较,用相同的方法制备S/KB。
1.4 材料性能的表征
用扫描电子显微镜(SEM,VEGA 3 SBH)表征样品的微观形貌结构。用X射线衍射(XRD,D/MAX-2500X)分析样品的物相组成,2θ范围为10°~80°。用X射线光电子能谱仪(XPS,Axis Supra)分析样品表面元素的价态。用TGA实验测量S/NiFeP/KB样品中S的质量分数,测试温度区间为0~400℃,升温速率为10℃/min。用比表面积分析仪(Tristar II Plus)测定样品的比表面积和孔径分布。
1.5 组装电池和测试电化学性能
按8∶1∶1的质量比将S/NiFeP/KB复合材料、聚偏二氟乙烯(PVDF)和KB混合后加入适量的N-甲基吡咯烷酮(NMP)液体,将其剪切分散制成均匀的浆料。用涂膜器将浆料均匀地涂布在铝箔表面,在60℃真空干燥12 h后裁切成直径为12 mm的圆形极片。极片上活性物质硫的负载量为0.8~1.2 mg。以此极片为正极、以锂片为负极,以Celgard 2400聚丙烯膜为隔膜,以双三氟甲基磺酰亚胺锂(LiTFSI,1 mol/L)和二氧戊烷/乙二醇二甲醚(DOL/DME,体积比1∶1)的混合溶液(添加1%LiNO3,质量分数)为电解液,在循环净化手套箱(H2O、O2含量< 1 × 10-7,体积分数)中组装CR2032型号纽扣电池。
使用电池测试系统(LANHE CT2001A、CT30-02A)测试电池的循环充放电和倍率性能。使用电化学工作站(Pine WaveDriver200) 进行循环伏安测试(CV)和电化学阻抗测试(EIS),交流振幅为5 mV,交流阻抗测试频率为10-1~106 Hz。
2 结果和讨论
2.1 NiFeP/KB复合材料催化剂的形貌和结构
图2
图2
纳米硫颗粒的高倍SEM照片和S/NiFeP/KB复合材料的SEM照片
Fig.2
SEM of sulfur nanoparticles (a) and SEM of S/NiFeP/KB (b)
图3给出了样品的XRD谱。可以看出,在NiFe-LDH/KB复合物的谱中2θ为11.41°、22.97°、33.54°、34.43°、38.99°、45.99°、59.94°和61.25°处出现了特征衍射峰,分别对应NiFe-LDH相(Ni0.75Fe0.25(CO3)0.125(OH)2·0.38H2O,PDF卡号:40-0215)的(003)、(006)、(101)、(012)、(015)、(018)、(110)和(113) 晶面,表明已经合成出NiFe-LDH/KB前驱体。在26.60°处出现的特征峰,可归因于KB基底。磷化后,在2θ为28.94°、40.42°、44.60°、47.01°、53.9°和54.07°处出现了明显的特征衍射峰,其中2θ为40.42°、44.60°和47.01°处的峰可归因于Fe2P (PDF卡号:76-0089)和Ni2P (PDF卡号74-1385)的(111)、(201)和(210)晶面。NiFeP/KB的谱中2θ为53.9°和54.07°处的峰归因于Fe2P和Ni2P的(300)、(221)面,在NiFeP/KB的谱中2θ为26°处的宽峰归因于碳载体。XRD谱的结果表明,氢氧化物前驱体已经转化成磷化物。
图3
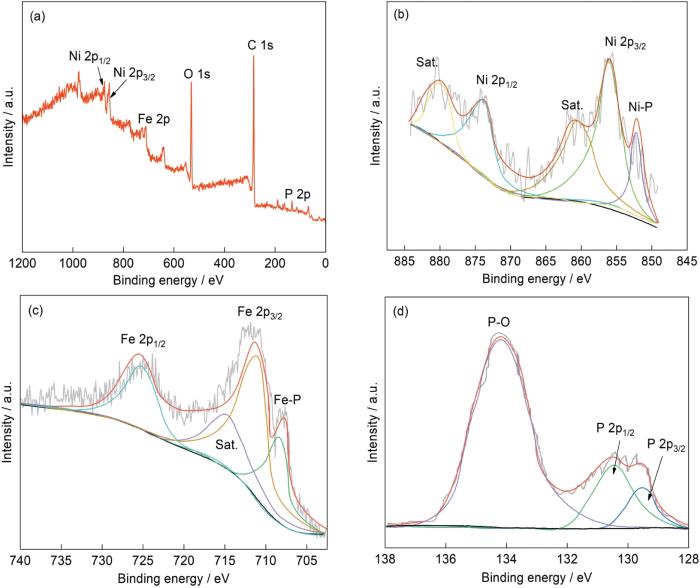
为了分析NiFe-LDH/KB和NiFeP/KB样品的表面组成和价态,进行了XPS表征。从图4a中的全谱可以看出,NiFeP/KBXPS主要由Ni、Fe、O、C和P元素组成。图4b给出了Ni 2p的精细能谱,在856.4、874.1 eV的特征峰分别对应Ni 2p3/2、Ni 2p1/2。出现两个卫星峰的原因,是Ni 2p3/2与Ni 2p1/2的自旋-轨道耦合。与Ni 2p3/2的参考峰(852.7 eV)相比,在峰位为853.3 eV的Ni 2p3/2特征峰发生了正位移,表明样品中含有Ni-P键[49]。图4c给出了Fe 2p的精细能谱,谱中出现了峰位为712.2 eV的Fe 2p3/2特征峰,峰位为725.5 eV的Fe 2p1/2的特征峰和峰位为715.0 eV的卫星峰。与Fe 2p3/2 (707.3 eV)相比,峰位为709.8 eV的Fe 2p3/2的特征峰和峰位为712.2 eV的特征峰也发生了正位移,表明样品中含有Fe-P键。图4d给出了P 2p的轨道谱,P 2p3/2和P 2p1/2的两个峰出现在129.5和130.5 eV处,与元素P 2p3/2的参考峰(130.2 eV)相比发生了负位移,表明有给电子位点和金属磷化物。同时,出现结合能为133.82 eV的峰表明样品中含有P-O键。总之,XPS谱表明,已经合成出NiFeP材料且与KB碳材料结合良好。
图4
图4
NiFeP/KB的XPS全谱、Ni 2p和Fe 2p的精细能谱以及P 2p的轨道谱
Fig.4
XPS pattern of NiFeP/KB (a), fine energy spectrum of Ni 2p (b) and Fe 2p (c) and track spectrum of P 2p (d)
图5
图5
NiFeP/KB和NiFeP/KB/S的氮气吸附-脱附等温曲线和孔径分布图以及 S/NiFeP/KB和S/KB的TGA曲线
Fig.5
N2 adsorption/desorption (a) and pore size distribution curves of NiFeP/KB and S/NiFeP/KB (b) and TGA curves of S/NiFeP/KB and S/KB (c)
进行热重分析 (TGA)测试了正极材料S/NiFeP/KB和S/KB中的硫负载量。如图5c所示,由于硫单质的蒸发,S/NiFeP/KB的TGA曲线在150~400℃发生69.66%的质量损失。这表明,S/NiFeP/KB复合材料的活性硫含量有69.66%,对照组S/KB的活性硫含量为69.58%,与样品制备的比例一致。
2.2 S/NiFeP/KB和S/KB正极电化学性能
S/NiFeP/KB和S/KB两种正极在0.1C下的循环稳定性,如图6a所示。可以看出,S/NiFeP/KB正极的初始放电比容量为1454.5 mAh/g,远高于S/KB (802.0 mAh/g)。S/NiFeP/KB在0.1C下循环200次后仍保持821.1 mAh/g的放电比容量,而S/KB 200次循环后放电比容量只有540 mAh/g。图6b、c给出了不同电流密度下的倍率性能。可以看出,随着电流密度从0.1C提高到2C,S/NiFeP/KB正极的放电比容量分别为1454.5、1342.0、1248.7、1194.1和861 mAh/g,优于S/KB电极。Zhou等[50]制备的多功能磷化铁碳布(FeP/CC)用于锂硫电池,其电流密度为0.1C、0.2C、0.5C、1C、2C时的放电容量分别为1246、1020、830、710、535 mAh/g,低于本文制备的S/NiFeP/KB正极的电流密度。电流密度回到0.1C时仍有1336.9 mAh/g的放电比容量,图中明显的充放电平台证明了其内部氧化还原反应的完整性。0.1C充、放电平台电势差约为0.2 V,也表明以S/NiFeP/KB为正极的锂硫电池具有较高的可逆性和快速的反应动力学。图6d表明,在2C高电流密度下使用S/NiFeP/KB正极的锂硫电池的循环性能优异。在2C电流密度下300次循环后的比容量仍然保持在639.9 mAh/g,容量保持率达到74.7%,平均每次循环衰减率仅为0.08%。
图6
图6
S/NiFeP/KB和S/KB电极在0.1C下的循环性能、S/NiFeP/KB和S/KB电极的倍率性能、S/NiFeP/KB电极的首圈充放电曲线以及S/NiFeP/KB电极在2C下的循环性能
Fig.6
Cycle performances of S/NiFeP/KB and S/KB at 0.1C (a), rate performances of S/NiFeP/KB and S/KB (b), the initial charge/discharge curve of S/NiFeP/KB (c) and cycle performances of S/NiFeP/KB (d)
图7a给出了S/NiFeP/KB和S/KB电极在电压范围为1.7~2.8 V的CV曲线。可以看出,S/NiFeP/KB的两个还原峰位约在2.29和2.01 V,分别代表S8还原为Li2S x (4 ≤ x ≤ 8)和Li2S x (4 ≤ x ≤ 8)还原为不溶物Li2S2和Li2S的电化学过程。同时,S/NiFeP/KB的氧化峰分别位于2.34和2.41 V,表明不溶物Li2S2和Li2S氧化为S8。与S/KB相比,S/NiFeP/KB的氧化还原峰更明显和更尖锐,进一步表明NiFeP保证了氧化还原反应的完整性,并且具有较快的电化学动力学过程。从图中还可见,S/NiFeP/KB的氧化还原峰间的电位差(ΔV1 = 0.05 V)比S/KB的氧化还原峰间的电位差(ΔV2 = 0.15 V)小,也优于Liu等[41]S/Ni/Ni2P@C电极的0.13V氧化还原峰间的电位差,表明S/NiFeP/KB阴极电极的内部氧化还原过程更完整,极化程度更低。同时,如图7b所示,扫速为0.1 mV/s时,随着CV循环圈数的增加正极和负极的峰值电流密度和位置保持稳定,表明S/NiFeP/KB电极的可逆性良好。
图7
图7
S/NiFeP/KB和S/KB电极在0.1 mV/s下的CV曲线、S/NiFeP/KB电极在0.1 mV/s下循环4圈的CV曲线以及S/NiFeP/KB和S/KB电极的电化学阻抗谱
Fig.7
Initial CV curves of S/NiFeP/KB and S/KB at 0.1 mV/s (a), CV curves of S/NiFeP/KB at 0.1 mV/s for four cycles (b), EIS of S/NiFeP/KB and S/KB (c)
电化学阻抗图由半圆和直线组成,其中图像的起始点为超高频区域,其大小代表了电解液电阻的大小,高频区半圆代表接触电阻与电极和电解质之间的电荷转移电阻(Rct),低频区的线性斜率反映Li+的扩散阻抗。图7c给出了S/NiFeP/KB和S/KB电池的阻抗分析。S/NiFeP/KB在高频区的半圆较小,说明由NiFeP电催化剂组成的正极复合材料使电极与电解液的界面电阻降低,具有更优异的电荷转移动力学。
为了研究NiFeP电催化剂对Li+传输速率的影响,测试了实验组和对照组电池扫描速率为0.1~0.5 mV/s时的CV曲线。图8a、b给出了各氧化还原电流峰值对应的I-ν1/2 (I为电流,ν为扫描速率)曲线。可以看出,峰值电流与扫描速率的平方根具有良好的线性关系,表明电化学反应是由Li+扩散控制的。从CV曲线可见,在循环过程中S/NiFeP/KB的电流响应比S/KB更高、峰面积更大。从图8c可见,随着扫速的提高S/KB的还原峰越来越不明显。这表明,NiFeP/KB材料的反应活性与稳定性更高。由此可以推断:NiFeP/KB的引入可提高电池的催化活性和延长使用寿命。还可以看出,S/NiFeP/KB阴极的I-ν1/2斜率比S/KB阴极的大,表明S/NiFeP/KB的Li+转移速率更高。结果表明,引入NiFeP有利于提高锂离子的传输能力和促进电池的反应动力学。同时,随着扫描速率的提高S/NiFeP/KB的电流增大的趋势比S/KB材料的大,与图7a、b给出的结果一致。S/NiFeP/KB在加快多硫化物氧化、还原反应过程的同时,也增大了Li+传质梯度和促进了多硫化物的转化。
图8
图8
S/NiFeP/KB电极和S/KB电极在不同扫速下的CV曲线以及S/NiFeP/KB和S/KB电极峰值电流的线性拟合
Fig.8
CV curves of S/NiFeP/KB (a) and KB/S (b) with different scan rates and linear fits of A, B and C peak currents for S/NiFeP/KB and KB/S (c)
3 结论
(1) 用水热合成NiFe-LDH/KB和低温磷化两步法可制备花球状颗粒和比表面积较高的NiFeP/KB复合材料。NiFeP纳米片颗粒表面的花球状形貌可提供丰富的多硫化锂氧化和还原反应界面。
(2) 与基于S/KB的锂硫电池相比,基于S/NiFeP/KB的锂硫电池具有更优异的首次放电比容量和容量保持率。
(3) NiFeP/KB电催化剂用于锂硫电池可提高多硫化锂的氧化和还原反应速率、改善电极极化和促进电池中锂、硫反应动力学。
参考文献
Bridging the academic and industrial metrics for next-generation practical batteries
[J].Batteries have shaped much of our modern world. This success is the result of intense collaboration between academia and industry over the past several decades, culminating with the advent of and improvements in rechargeable lithium-ion batteries. As applications become more demanding, there is the risk that stunted growth in the performance of commercial batteries will slow the adoption of important technologies such as electric vehicles. Yet the scientific literature includes many reports describing material designs with allegedly superior performance. A considerable gap needs to be filled if we wish these laboratory-based achievements to reach commercialization. In this Perspective, we discuss some of the most relevant testing parameters that are often overlooked in academic literature but are critical for practical applicability outside the laboratory. We explain metrics such as anode energy density, voltage hysteresis, mass of non-active cell components and anode/cathode mass ratio, and we make recommendations for future reporting. We hope that this Perspective, together with other similar guiding principles that have recently started to emerge, will aid the transition from lab-scale research to next-generation practical batteries.
Pathways for practical high-energy long-cycling lithium metal batteries
[J].State-of-the-art lithium (Li)-ion batteries are approaching their specific energy limits yet are challenged by the ever-increasing demand of today's energy storage and power applications, especially for electric vehicles. Li metal is considered an ultimate anode material for future high-energy rechargeable batteries when combined with existing or emerging high-capacity cathode materials. However, much current research focuses on the battery materials level, and there have been very few accounts of cell design principles. Here we discuss crucial conditions needed to achieve a specific energy higher than 350 Wh kg(-1), up to 500 Wh kg(-1), for rechargeable Li metal batteries using high-nickel-content lithium nickel manganese cobalt oxides as cathode materials. We also provide an analysis of key factors such as cathode loading, electrolyte amount and Li foil thickness that impact the cell-level cycle life. Furthermore, we identify several important strategies to reduce electrolyte-Li reaction, protect Li surfaces and stabilize anode architectures for long-cycling high-specific-energy cells.
An exfoliation-evaporation strategy to regulate N coordination number of Co single-atom catalysts for high- performance lithium-sulfur batteries
[J].
Review on high‐loading and high‐energy lithium-sulfur batteries
[J].
Porous carbon framework nested nickel foam as freestanding host for high energy lithium sulfur batteries
[J].
Implanting single Zn atoms coupled with metallic Co nanoparticles into porous carbon nanosheets grafted with carbon nanotubes for high-performance lithium-sulfur batteries
[J].
Liquid electrolyte design for metal‐sulfur batteries: mechanistic understanding and perspective
[J].
Advanced chemical strategies for lithium-sulfur batteries: a review
[J].
Advances in lithium-sulfur batteries based on multifunctional cathodes and electrolytes
[J].
A review of flexible lithium-sulfur and analogous alkali metal-chalcogen rechargeable batteries
[J].
Designing high-energy lithium-sulfur batteries
[J].Due to their high energy density and low material cost, lithium-sulfur batteries represent a promising energy storage system for a multitude of emerging applications, ranging from stationary grid storage to mobile electric vehicles. This review aims to summarize major developments in the field of lithium-sulfur batteries, starting from an overview of their electrochemistry, technical challenges and potential solutions, along with some theoretical calculation results to advance our understanding of the material interactions involved. Next, we examine the most extensively-used design strategy: encapsulation of sulfur cathodes in carbon host materials. Other emerging host materials, such as polymeric and inorganic materials, are discussed as well. This is followed by a survey of novel battery configurations, including the use of lithium sulfide cathodes and lithium polysulfide catholytes, as well as recent burgeoning efforts in the modification of separators and protection of lithium metal anodes. Finally, we conclude with an outlook section to offer some insight on the future directions and prospects of lithium-sulfur batteries.
Progress of the interface design in all-solid-state Li-S batteries
[J].
Advances in lithium-sulfur batteries: from academic research to commercial viability
[J].
Co-Fe mixed metal phosphide nanocubes with highly interconnected-pore architecture as an efficient polysulfide mediator for lithium-sulfur batteries
[J].Lithium-sulfur (Li-S) batteries have been regarded as one of the most promising candidates for next-generation energy storage owing to their high energy density and low cost. However, the practical deployment of Li-S batteries has been largely impeded by the low conductivity of sulfur, the shuttle effect of polysulfides, and the low areal sulfur loading. Herein, we report the synthesis of uniform Co-Fe mixed metal phosphide (Co-Fe-P) nanocubes with highly interconnected-pore architecture to overcome the main bottlenecks of Li-S batteries. With the highly interconnected-pore architecture, inherently metallic conductivity, and polar characteristic, the Co-Fe-P nanocubes not only offer sufficient electrical contact to the insulating sulfur for high sulfur utilization and fast redox reaction kinetics but also provide abundant adsorption sites for trapping and catalyzing the conversion of lithium polysulfides to suppress the shuttle effect, which is verified by both the comprehensive experiments and density functional theory calculations. As a result, the sulfur-loaded Co-Fe-P (S@Co-Fe-P) nanocubes delivered a high discharge capacity of 1243 mAh g at 0.1 C and excellent cycling stability for 500 cycles with an average capacity decay rate of only 0.043% per cycle at 1 C. Furthermore, the S@Co-Fe-P electrode showed a high areal capacity of 4.6 mAh cm with superior stability when the sulfur loading was increased to 5.5 mg cm. More impressively, the prototype soft-package Li-S batteries based on S@Co-Fe-P cathodes also exhibited superior cycling stability with great flexibility, demonstrating their great potential for practical applications.
Electrocatalysis of sulfur and polysulfides in Li-S batteries
[J].
Polypyrrole-enveloped Prussian blue nanocubes with multi-metal synergistic adsorption toward lithium polysulfides: high-performance lithium-sulfur batteries
[J].
Recent advances in chemical adsorption and catalytic conversion materials for Li-S batteries
[J].Owing to their low cost, high energy densities, and superior performance compared with that of Li-ion batteries, Li-S batteries have been recognized as very promising next-generation batteries. However, the commercialization of Li-S batteries has been hindered by the insulation of sulfur, significant volume expansion, shuttling of dissolved lithium polysulfides (LiPSs), and more importantly, sluggish conversion of polysulfide intermediates. To overcome these problems, a state-of-the-art strategy is to use sulfur host materials that feature chemical adsorption and electrocatalytic capabilities for LiPS species. In this review, we comprehensively illustrate the latest progress on the rational design and controllable fabrication of materials with chemical adsorbing and binding capabilities for LiPSs and electrocatalytic activities that allow them to accelerate the conversion of LiPSs for Li-S batteries. Moreover, the current essential challenges encountered when designing these materials are summarized, and possible solutions are proposed. We hope that this review could provide some strategies and theoretical guidance for developing novel chemical anchoring and electrocatalytic materials for high-performance Li-S batteries.
Rational design of two-dimensional nanomaterials for lithium-sulfur batteries
[J].
Exploring and understanding the roles of Li2S n and the strategies to beyond present Li-S batteries
[J].
Catalytic effects in lithium-sulfur batteries: promoted sulfur transformation and reduced shuttle effect
[J].
Recent progress in sulfur cathode for Li-S batteries
[J].
锂硫电池硫正极材料研究进展
[J].
Electrocatalytic polysulfide traps for controlling redox shuttle process of Li-S batteries
[J].Stabilizing the polysulfide shuttle while ensuring high sulfur loading holds the key to realizing high theoretical energy of lithium-sulfur (Li-S) batteries. Herein, we present an electrocatalysis approach to demonstrate preferential adsorption of a soluble polysulfide species, formed during discharge process, toward the catalyst anchored sites of graphene and their efficient transformation to long-chain polysulfides in the subsequent redox process. Uniform dispersion of catalyst nanoparticles on graphene layers has shown a 40% enhancement in the specific capacity over pristine graphene and stability over 100 cycles with a Coulombic efficiency of 99.3% at a current rate of 0.2 C. Interaction between electrocatalyst and polysulfides has been evaluated by conducting X-ray photoelectron spectroscopy and electron microscopy studies at various electrochemical conditions.
Balancing surface adsorption and diffusion of lithium-polysulfides on nonconductive oxides for lithium-sulfur battery design
[J].
Sulphur-TiO2 yolk-shell nanoarchitecture with internal void space for long-cycle lithium-sulphur batteries
[J].
Application of layered Co3O4/C derived from metal-organic framework in lithium-sulfur batteries
[J].
金属有机骨架衍生的层状Co3O4/C在锂硫电池中的应用
[J].
Metallic and polar Co9S8 inlaid carbon hollow nanopolyhedra as efficient polysulfide mediator for lithium-sulfur batteries
[J].
Elastic sandwich‐type rGO-VS2/S composites with high tap density: structural and chemical cooperativity enabling lithium-sulfur batteries with high energy density
[J].
SnS2/ZIF-8 derived two-dimensional porous nitrogen-doped carbon nanosheets for lithium-sulfur batteries
[J].
SnS2/ZIF-8衍生二维多孔氮掺杂碳纳米片复合材料的锂硫电池性能研究
[J].
Three-dimensional P-doped carbon skeleton with built-in Ni2P nanospheres as efficient polysulfides barrier for high-performance lithium-sulfur batteries
[J].
Transition metal phosphides: new generation cathode host/separator modifier for Li-S batter-ies
[J].
Regulating the polysulfide redox conversion by iron phosphide nanocrystals for high-rate and ultrastable lithium-sulfur battery
[J].
Cobalt in nitrogen-doped graphene as single-atom catalyst for high-sulfur content lithium-sulfur batteries
[J].Because of their high theoretical energy density and low cost, lithium-sulfur (Li-S) batteries are promising next-generation energy storage devices. The electrochemical performance of Li-S batteries largely depends on the efficient reversible conversion of Li polysulfides to LiS in discharge and to elemental S during charging. Here, we report on our discovery that monodisperse cobalt atoms embedded in nitrogen-doped graphene (Co-N/G) can trigger the surface-mediated reaction of Li polysulfides. Using a combination of operando X-ray absorption spectroscopy and first-principles calculation, we reveal that the Co-N-C coordination center serves as a bifunctional electrocatalyst to facilitate both the formation and the decomposition of LiS in discharge and charge processes, respectively. The S@Co-N/G composite, with a high S mass ratio of 90 wt %, can deliver a gravimetric capacity of 1210 mAh g, and it exhibits an areal capacity of 5.1 mAh cm with capacity fading rate of 0.029% per cycle over 100 cycles at 0.2 C at S loading of 6.0 mg cm on the electrode disk.
Porous-shell vanadium nitride nanobubbles with ultrahigh areal sulfur loading for high-capacity and long-life lithium-sulfur batteries
[J].Lithium-sulfur (Li-S) batteries hold great promise for the applications of high energy density storage. However, the performances of Li-S batteries are restricted by the low electrical conductivity of sulfur and shuttle effect of intermediate polysulfides. Moreover, the areal loading weights of sulfur in previous studies are usually low (around 1-3 mg cm) and thus cannot fulfill the requirement for practical deployment. Herein, we report that porous-shell vanadium nitride nanobubbles (VN-NBs) can serve as an efficient sulfur host in Li-S batteries, exhibiting remarkable electrochemical performances even with ultrahigh areal sulfur loading weights (5.4-6.8 mg cm). The large inner space of VN-NBs can afford a high sulfur content and accommodate the volume expansion, and the high electrical conductivity of VN-NBs ensures the effective utilization and fast redox kinetics of polysulfides. Moreover, VN-NBs present strong chemical affinity/adsorption with polysulfides and thus can efficiently suppress the shuttle effect via both capillary confinement and chemical binding, and promote the fast conversion of polysulfides. Benefiting from the above merits, the Li-S batteries based on sulfur-filled VN-NBs cathodes with 5.4 mg cm sulfur exhibit impressively high areal/specific capacity (5.81 mAh cm), superior rate capability (632 mAh g at 5.0 C), and long cycling stability.
Cobalt-doped hollow carbon framework as sulfur host for the cathode of lithium sulfur battery
[J].Lithium-sulfur batteries are deemed to be the next generation of cost-effective and high energy density systems for energy storage. However, low conductivity of active materials, shuttle effect and sluggish kinetics of redox reaction lead to serious capacity fading and poor rate performance. Herein, a sodium citrate derived three-dimensional hollow carbon framework embedded with cobalt nanoparticles is designed as the host for sulfur cathode. The introduced cobalt nanoparticles can effectively adsorb the polysulfides, enhance the kinetics of conversion reaction and further improve the cyclic and rate performance. The obtained cathode delivered a high initial discharge capacity of 1280 mAh·g-1 at 0.5C, excellent high-rate performance up to 10C and stable cyclic capacity of 770 mAh·g-1 at 1C for 200 cycles with high Columbic efficiency.
Application of separators modified by carbon nanospheres enriched with α-MoC1- x nanocrystalline in lithium sulfur batteries
[J].
α-MoC1- x 纳米晶富集碳球修饰隔膜对锂硫电池性能的影响
[J].采用自组装及热处理方法合成α-MoC<sub>1-</sub><sub>x</sub>纳米晶富集的纳米碳球(α-MoC<sub>1-</sub><sub>x</sub>/CNS), 并将其涂覆在商用聚丙烯隔膜上, 对隔膜实现了界面修饰。电化学性能显示, 与普通的聚丙烯隔膜相比, 采用修饰的α-MoC<sub>1-</sub><sub>x</sub>/CNS-PP隔膜组装的锂硫电池的循环稳定性和倍率性能均得到明显提升, 在0.5C条件下, 电池首周放电比容量提升至1129.7 mAh/g, 经过100周充放电循环后, 电池仍具有855.5 mAh/g的放电比容量, 且在此循环过程中, 库伦效率始终大于98%。在自放电测试中, 电池经过48 h静置后的容量损失率仅为7.7%。结合α-MoC<sub>1-</sub><sub>x</sub>/CNS的微观形貌及XPS分析可知, 在锂硫电池充放电过程中, α-MoC<sub>1-</sub><sub>x</sub>/CNS修饰层有效地阻挡了多硫化锂向负极侧的扩散迁移, 且当α-MoC<sub>1-</sub><sub>x</sub>与多硫离子接触时能产生Mo-S键、硫代和连多硫酸根产物, 进一步巩固了活性物质被约束的程度, 从而使电池性能得到提升。
Strengthened d-p orbital hybridization through asymmetric coordination engineering of single-atom catalysts for durable lithium-sulfur batteries
[J].
Recent progress of single-atom catalytic materials for lithium-sulfur batteries
[J].
单原子催化剂在锂硫电池中的研究进展
[J].
Application of metal compounds in cathode materials and interlayers for lithium-sulfur batteries
[J].
金属化合物在锂硫电池正极材料及夹层中的应用
[J].
Tuning the architecture of hierarchical porous CoNiO2 nanosheet for enhanced performance of Li-S batteries
[J].
Regulating Li+ migration and Li2S deposition by metal-organic framework-derived Co4S3-embedded carbon nanoarrays for durable lithium-sulfur batteries
[J].
Novel Ni/Ni2P@C hollow heterostructure microsphere as efficient sulfur hosts for high-performance lithium-sulfur batteries
[J].
Mechanistic understanding of metal phosphide host for sulfur cathode in high-energy-density lithium-sulfur batteries
[J].For solving the drawbacks of low conductivity and the shuttle effect in a sulfur cathode, various nonpolar carbon and polar metal compounds with strong chemical absorption ability are applied as sulfur host materials for lithium-sulfur (Li-S) batteries. Nevertheless, previous research simply attributed the performance improvement of sulfur cathodes to the chemical adsorption ability of polar metal compounds toward lithium polysulfides (LPS), while a deep understanding of the enhanced electrochemical performance in these various sulfur hosts, especially at the molecular levels, is still unclear. Herein, for a mechanistic understanding of superior metal phosphide host in Li-S battery chemistry, an integrated phosphide-based host of CF/FeP@C (carbon cloth with grown FeP@C nanotube arrays) is chosen as the model, and this binder-free cathode can exclude interference from the binder and conductive additives. With a systematic electrochemical investigation of the loading sulfur in such oxide- and phosphide-based hosts (CF/FeO@C and CF/FeP@C), it is found that CF/FeP@C@S shows much superior Li-S performances. The greatly enhanced performance of CF/FeP@C@S suggests that FeP can well suppress the shuttle effect of LPS and accelerate their transformation during the charge-discharge process. The first-principles calculations reveal the performance variations of FeO and FeP in Li-S batteries mainly because the shifts of the p band of the FeP could accelerate the interfacial electronics transfer dynamics by increasing the electronic concentration in the Fermi level of adsorbed LiS. The current work sheds light on the promising design of superior Li-S batteries from both theoretical and experimental aspects.
Surface chemistry in cobalt phosphide-stabilized lithium-sulfur batteries
[J].Chemistry at the cathode/electrolyte interface plays an important role for lithium-sulfur batteries in which stable cycling of the sulfur cathode requires confinement of the lithium polysulfide intermediates and their fast electrochemical conversion on the electrode surface. While many materials have been found to be effective for confining polysulfides, the underlying chemical interactions remain poorly understood. We report a new and general lithium polysulfide-binding mechanism enabled by surface oxidation layers of transition-metal phosphide and chalcogenide materials. We for the first time find that CoP nanoparticles strongly adsorb polysulfides because their natural oxidation (forming Co-O-P-like species) activates the surface Co sites for binding polysulfides via strong Co-S bonding. With a surface oxidation layer capable of confining polysulfides and an inner core suitable for conducting electrons, the CoP nanoparticles are thus a desirable candidate for stabilizing and improving the performance of sulfur cathodes in lithium-sulfur batteries. We demonstrate that sulfur electrodes that hold a high mass loading of 7 mg cm and a high areal capacity of 5.6 mAh cm can be stably cycled for 200 cycles. We further reveal that this new surface oxidation-induced polysulfide-binding scheme applies to a series of transition-metal phosphide and chalcogenide materials and can explain their stabilizing effects for lithium-sulfur batteries.
Deciphering the modulation essence of p bands in Co-based compounds on Li-S chemistry
[J].
Unraveling the structure and composition sensitivity of transition metal phosphide toward catalytic performance of C2H2 semi-hydrogenation
[J].
Efficient Ni2Co4P3 nanowires catalysts enhance ultrahigh‐loading lithium-sulfur conversion in a microreactor‐like battery
[J].
NiFeP anchored on rGO as a multifunctional interlayer to promote the redox kinetics for Li-S batteries via regulating d-bands of Ni-based phosphides
[J].
Unveiling the synergistic catalysis essence of trimetallic Fe-Co-Ni phosphides for lithium-sulfur chemistry
[J].
Ni/Fe ratio dependence of catalytic activity in monodisperse ternary nickel iron phosphide for efficient water oxidation
[J].
Efficient catalytic conversion of polysulfides in multifunctional FeP/Carbon cloth interlayer for high capacity and stability of lithium-sulfur batteries
[J].
多功能磷化铁碳布(FeP/CC)中间层高效催化多硫化物实现锂硫电池的高容量与高稳定性
[J].锂硫电池高的比能量密度(2600 Wh•kg<sup>−1</sup>), 使其成为一种很有前景的储能系统. 然而, 在氧化还原反应中, 中间产物多硫化物(LiPSs)的穿梭效应, 以及缓慢的电化学反应动力学导致阴极和阳极的严重降解, 使容量迅速衰减. 在此, 制备了一种多功能磷化铁碳布(FeP/CC)中间层, 为锂硫电池提供了更多的活性位点, 不仅可以物理捕获多硫化物(LiPSs)以抑制穿梭效应, 确保稳定的循环, 而且对LiPSs具有催化能力, 有助于提高电化学反应动力学. 带有FeP/CC中间层的锂硫电池可实现1329 mAh•g<sup>−1</sup>的首圈放电容量, 在100圈循环后, 仍然能保持在1100 mAh•g<sup>−1</sup>的可逆容量, 并且FeP/CC表现出优异的对LiPS吸附与催化转化能力. 这种多功能FeP/CC中间层为高稳定性以及高容量的锂硫电池提供了一种可行的思路.