价廉的AlOOH比表面积大,有利于去除污染物[10,11]。Zhou等一步合成了羧基修饰的镍掺杂AlOOH纳米花,吸附多种过渡金属离子后可用作析氧反应的电催化剂[11]。Tao等用草酸改性Ce-AlOOH去除废水中的氟,吸附容量可达90 mg·g-1。草酸改性Ce-AlOOH对氟的吸附符合Freundlich模型和拟二阶模型[12]。Ma等合成了可回收废水中磷的AlOOH嵌粒壳聚糖,吸附容量达到45.82 mg·g-1[13]。用无模板法和模板辅助法均可合成不同形貌的AlOOH。无模板法需添加柠檬酸盐和有机溶剂等Al3+配合剂,用模板辅助法可制备形貌可控、孔径可调的材料,但是需用溶剂萃取或煅烧去除模板。制备AlOOH常用的原料有Al(NO3)9·9H2O[11,12],较少使用Al2(SO4)3。
镍湿法冶金中的铝渣是有价的二次资源,合理利用可提高资源利用效率和避免环境污染。本文用铝渣制取Al2(SO4)3溶液,用简易的水热法制备空心球形AlOOH,阐明空心球形AlOOH的形成过程和对刚果红的吸附性能,研究Al3+/尿素摩尔比、水热温度、水热时间及Al3+浓度对AlOOH形貌和结构的影响。
1 实验方法
1.1 空心球形AlOOH的制备
将干燥后的Al(OH)3用稀硫酸溶解,配置成浓度为0.05~0.40 mol·L-1的溶液。
将铝渣碱浸、一次碳分净化和二次碳分,制备成纯度较高的Al(OH)3,酸溶后得到Al2(SO4)3溶液。按照Al3+/尿素(AR)摩尔比为1∶1.5~3.5的配比称取尿素,及其溶解于60 mL的蒸馏水中。将60 mL的Al2(SO4)3溶液置于烧杯中,在搅拌条件下将尿素溶液加入其中得到混合溶液。再将混合溶液移至200 mL的聚四氟乙烯内衬反应釜中。然后将反应釜置于已升温至120~220℃的烘箱中,反应一段时间后取出使其降温。将得到的物料过滤分离,用蒸馏水将产物充分洗涤后再用无水乙醇充分洗涤,然后将其在100℃干燥8 h,即得到空心球形AlOOH。
1.2 性能表征
吸附性能的测试:在磁力搅拌下将50 mg空心球形AlOOH加入到0~50℃的50 mL刚果红溶液中,刚果红的浓度为100~400 mg·L-1。按照设定的时间间隔取样,根据上清液浓度的测定值计算吸附容量。
用日本理学Ultima IV型X-射线衍射仪表征样品的结构:Cu靶辐射、加速电压40 kV、扫描速率6°·min-1。用日本日立8010型扫描电子显微镜观察样品的形貌。用TU-1900型紫外分光光度计测定上清液的吸光度。
2 结果和讨论
2.1 Al3+/尿素摩尔比对空心球形AlOOH结构和形貌的影响
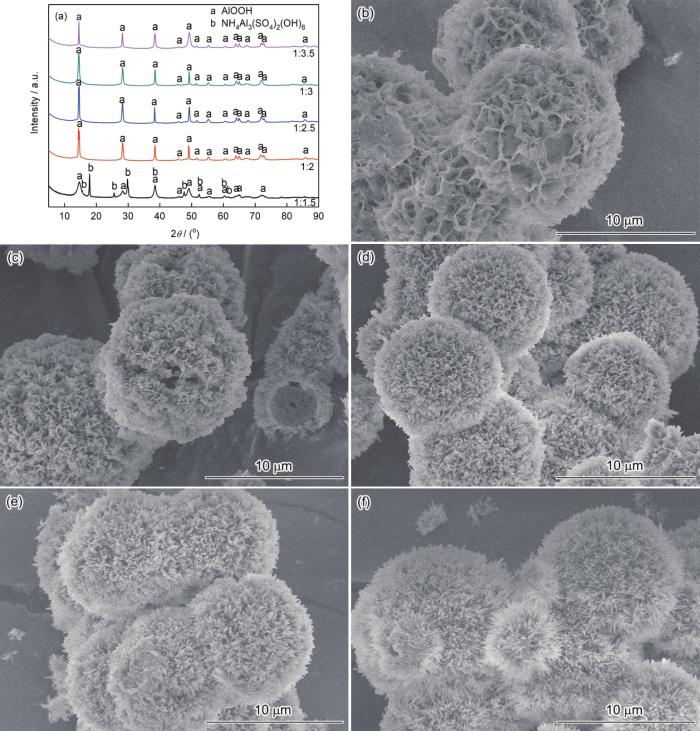
图1给出了Al3+/尿素摩尔比对样品结构和形貌的影响,Al3+的浓度为0.2 mol·L-1、反应温度为180℃、反应时间为12 h。可以看出,Al3+/尿素摩尔比1∶1.5的样品,是AlOOH和NH4Al3(SO4)2(OH)6的混合物。Al3+/尿素摩尔比为1∶2~1∶3.5的样品,其衍射数据与JCPDS No.21-1307 (a=0.3700 nm, b=1.2227 nm, c=0.2868 nm)一致,为勃姆石AlOOH。摩尔比为1∶2.5的样品是直径约为9 μm的规则球形颗粒,形貌的变化明显。其原因是,尿素量不足使晶核过少,有利于长成大颗粒。随着尿素量的增加在初始阶段即生成大量的晶核,有利于AlOOH晶体规则生长。但是过量的尿素使体系的碱度提高,导致AlOOH晶体过度生长。摩尔比为1∶3.5时AlOOH纳米片已长成针状。选择Al3+/尿素摩尔比为1∶2.5。
图1
图1
Al3+/尿素摩尔比不同的AlOOH的XRD谱和SEM形貌
Fig.1
XRD patterns and SEM images of AlOOH obtained in conditions of Al3+/urea molar ratios (a) XRD patterns, (b) 1∶1.5, (c) 1∶2, (d) 1∶2.5, (e) 1∶3 and (f) 1∶3.5
2.2 水热温度的影响
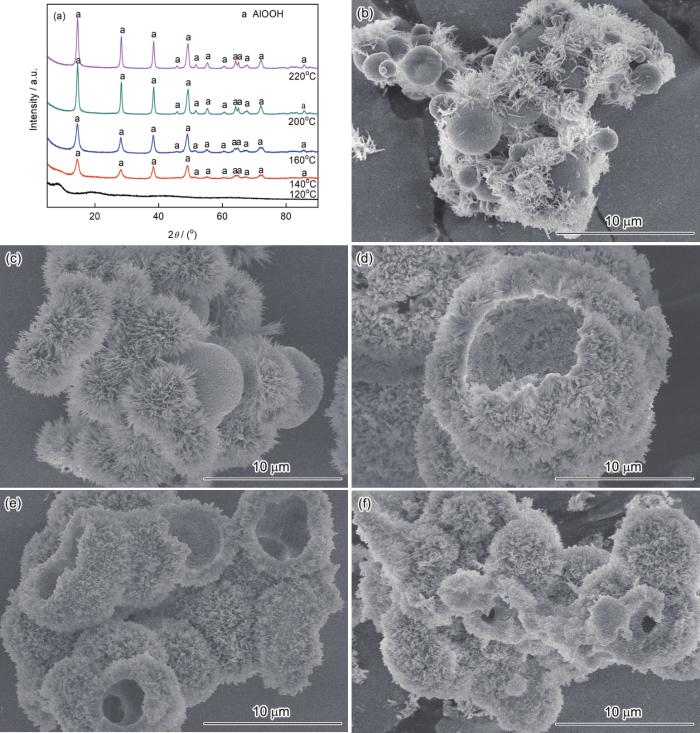
图2给出了水热温度对样品结构和形貌的影响,Al3+浓度为0.2 mol·L-1、Al3+/尿素摩尔比为1∶2.5、反应时间为12 h。可以看出,水热温度为120℃的样品是无定形的,水热温度高于140℃时的样品为勃姆石AlOOH。随着水热温度的升高,样品的形貌发生显著的变化。无定形Al(OH)3为球形,粒径为1~6 μm,表面包覆着花状纤维。水热温度为140℃是得到球形与花状颗粒共存的样品,花状颗粒由针状纳米颗粒组成。水热温度为160℃和180℃时得到的样品形貌类似,为空心球形结构,内表面光滑。水热温度升高到200℃,空心球形AlOOH颗粒受到破坏。水热温度进一步升高到220℃,则空心球形结构崩塌,是Ostwald熟化作用所致。可以看出,水热温度是影响晶体生长速率的主要因素。较低的水热温度使反应速度低,颗粒仍处于生长阶段(图2b)。水热温度升高使空心球形结构生长,但是水热温度过高则使空心结构过度生长而崩塌。因此,选择水热温度为180℃。
图2
图2
反应温度不同的样品的XRD谱和SEM形貌
Fig.2
XRD patterns and SEM images of specimens obtained at different reaction tempeerature (a) XRD patterns, (b) 120℃, (c) 140℃, (d) 160℃, (e) 200℃ and (f) 220℃
2.3 水热反应时间的影响
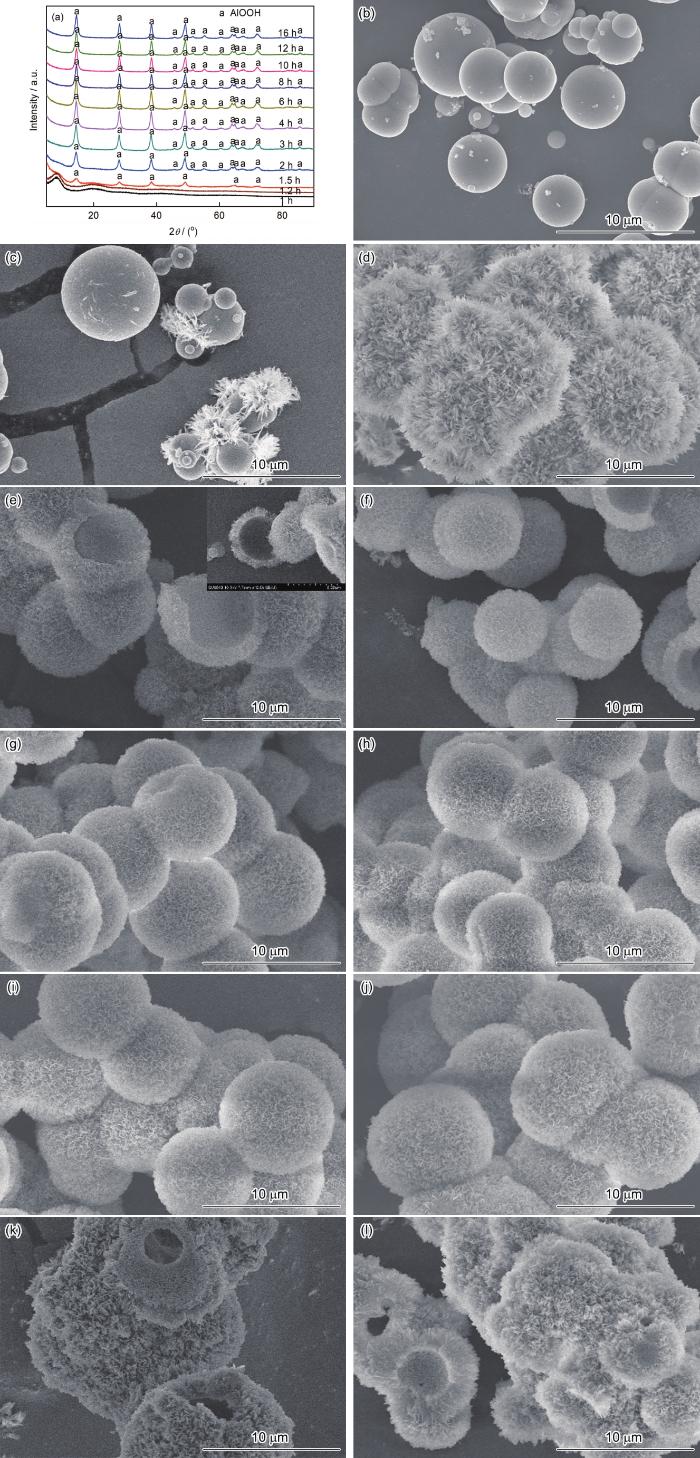
图3给出了水热反应时间对样品结构和形貌的影响,Al3+的浓度为0.2 mol·L-1、Al3+/尿素摩尔比为1∶2.5、水热温度为180℃。由图3可见,水热反应1 h得到的非晶态Al(OH)3球表面光滑,且因表面能的作用有融合的趋势[14,15]。反应1.2 h得到非晶态Al(OH)3球,在其表面长出了花状晶须。反应1.5 h得到的Al(OH)3晶体球,其表面完全被纤维状颗粒包覆(图3d)。反应时间超过2 h的样品,均为勃姆石AlOOH,且其纤维状颗粒转变为片状颗粒并生成空心核壳结构(图3e),还可观测到非晶态Al(OH)3球核。反应3 h得到的样品其形貌与反应时间为2 h的样品相比没有显著的变化,但是非晶态Al(OH)3球核基本上全部消耗,表明已形成空心球形结构。延长反应时间到4~6 h,空心球结构没有明显的变化,但是不规则的小颗粒逐渐消失,可能是Ostwald熟化作用所致,此时高表面能的小颗粒溶解以补充非晶态Al(OH)3球核的消耗[14]。反应8 h得到的空心球形颗粒形貌规则,粒径约为7 μm,表面由规则的片状颗粒组成。反应时间为10 h,空心球有长大趋势。进一步延长反应时间至14~16 h,则片状颗粒的过度生长使空心球形结构受到破坏(图3h)。因此,选择水热反应时间为8 h。
图3
图3
反应时间不同的样品的XRD谱和SEM形貌
Fig.3
XRD patterns and SEM images of specimens obtained by different reaction time (a) XRD patterns, (b) 1 h, (c) 1.2 h, (d) 1.5 h, (e) 2 h, (f) 3 h, (g) 4 h, (h) 6 h, (i) 8 h, (j) 10 h, (k) 14 h, (l) 16 h
2.4 Al3+ 浓度的影响
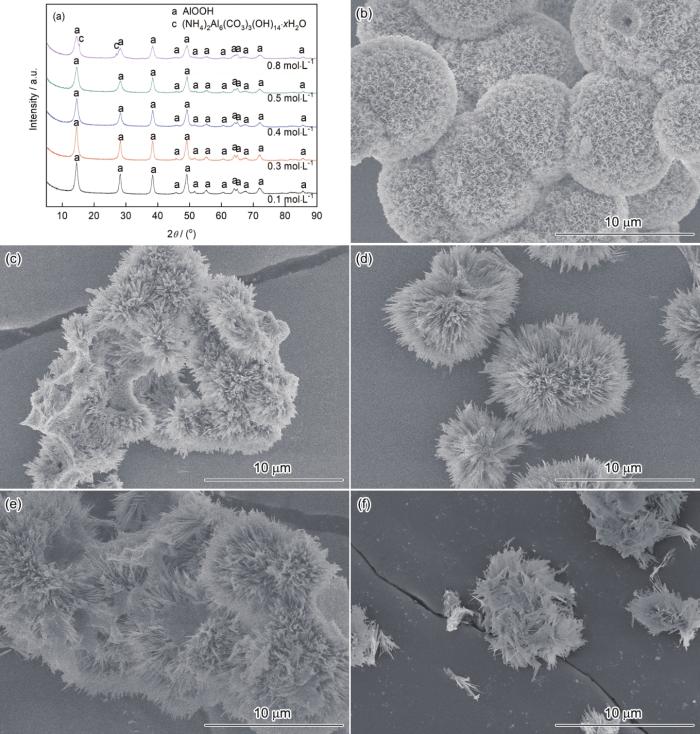
图4给出了Al3+浓度对样品结构和形貌的影响,Al3+/尿素摩尔比为1∶2.5、水热温度为180℃、反应时间为8 h。由图4可见,所得样品均为勃姆石AlOOH。Al3+浓度的变化对样品的形貌有显著的影响。Al3+浓度为0.1 mol·L-1得到空心球形结构样品,粒径约为10 μm,比Al3+浓度为0.2 mol·L-1时的样品粒径大(图3i)。Al3+浓度的提高使花状AlOOH生成(图4c和4d),Al3+浓度为0.4 mol·L-1时花状结构更为规则。体系碱度的提高促进了AlOOH晶体的极性生长,使AlOOH片状颗粒生长为针状颗粒。进一步提高Al3+浓度使花状结构继续长大,且出现明显的团聚。Al3+浓度为0.8 mol·L-1时花状结构破坏,得到黏连的片状颗粒。因此,实验中选择Al3+浓度为0.2 mol·L-1。
图4
图4
Al3+浓度不同的AlOOH的XRD谱和SEM形貌
Fig.4
XRD patterns and SEM images of AlOOH obtained by varied Al3+ concentrations (a) XRD patterns, (b) 0.1, (c) 0.3, (d) 0.4, (e) 0.5 and (f) 0.8 mol·L-1
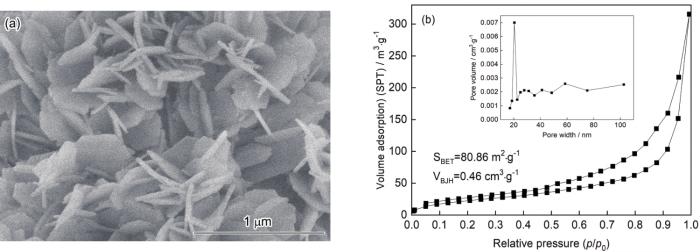
2.5 空心球形AlOOH的生长机理
图5
图5
AlOOH的SEM形貌和N2吸附-解吸曲线
Fig.5
SEM zoom image (a) and N2 adsorption-desorption curves (b) of AlOOH
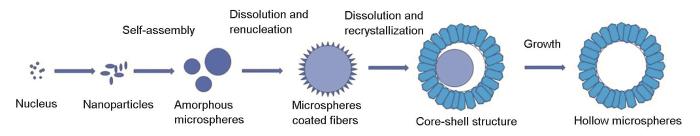
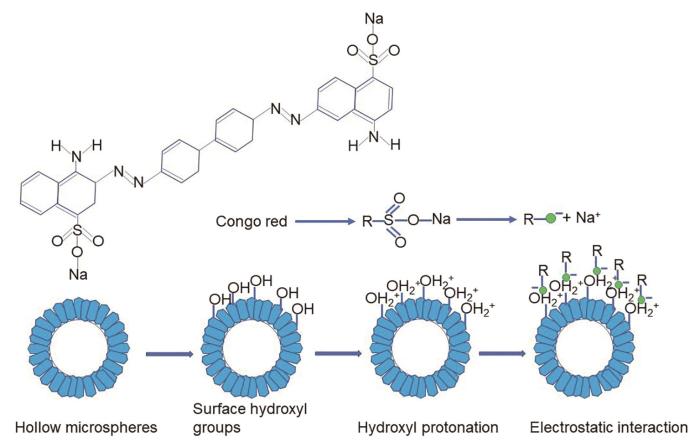
空心球形AlOOH的生成是一个沉淀、溶解再结晶的过程。尿素水解生成的OH–,与溶液中的Al3+反应生成Al(OH)3晶核。SO
图6
图6
空心球形AlOOH的形成过程示意图
Fig.6
Schematic formation evolution diagram of hollow spherical AlOOH
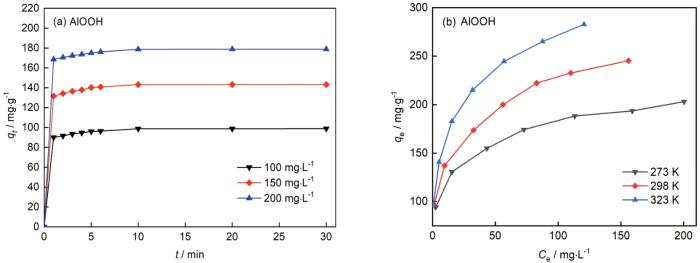
2.6 对刚果红的吸附性能
图7
图7
空心球形AlOOH的吸附容量与时间和刚果红平衡浓度的关系
Fig.7
Adsorption capacities versus contact time (a) and equilibrium concentration of Congo red (b) of hollow spherical AlOOH
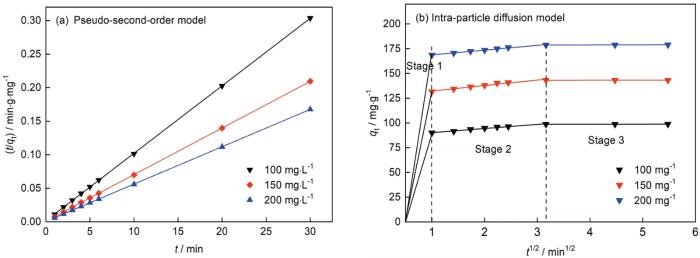
可用拟二阶动力学模型
和粒子内扩散模型
拟合实验数据。式中t(min)为反应时间,qe和qt(mg·g-1)为吸附平衡时和吸附时间为t时的吸附容量,k2(g·mg-1·min-1)为吸附速率常数,k(mg·g-1·min1/2)为粒子内扩散速率常数。拟合曲线在图8中给出,相关参数列于表1。由表1可见,拟二阶动力学模型的R2高达0.9999,且平衡吸附容量与实验值极为接近,表明吸附过程符合拟二阶动力学模型。这个结果,与ZnAl-LDH/Al(OH)3纳米片[19]和HAM@γ-AlOOH/Fe(OH)3微球[20]吸附刚果红的结果一致。吸附的第一阶段为初始时间1 min,瞬时外扩散速率很高,与刚果红的浓度差有关[14]。第二阶段为平稳吸附阶段,吸附由外扩散经离子内通道扩散到纳米片表面。在最后阶段吸附达到平衡。可使用Langmuir
图8
图8
拟二阶动力学和粒子内扩散模型的拟合曲线
Fig.8
Plots of the Pseudo-second-order model (a) and intra-particle diffusion model (b)
表1 吸附刚果红的拟二阶和粒子内扩散模型动力学参数
Table 1
C0 / mg·g-1 | Pseudo-second-order model | Intra-particle-diffusion model | |||||||
|---|---|---|---|---|---|---|---|---|---|
| qe | k2 | R2 | ks1 | ks2 | ks3 | R12 | R22 | R32 | |
| / mg·g-1 | / g·mg-1·min-1 | mg·g-1·min1/2 | |||||||
| 100 | 99.38 | 0.0636 | 0.9999 | 90.02 | 4.18 | 0.03 | 1 | 0.9787 | 0.9996 |
| 150 | 143.67 | 0.0537 | 0.9999 | 131.60 | 5.50 | 0.03 | 1 | 0.9566 | 0.9415 |
| 200 | 179.23 | 0.0525 | 0.9999 | 168.64 | 4.80 | 0.06 | 1 | 0.9929 | 0.9926 |
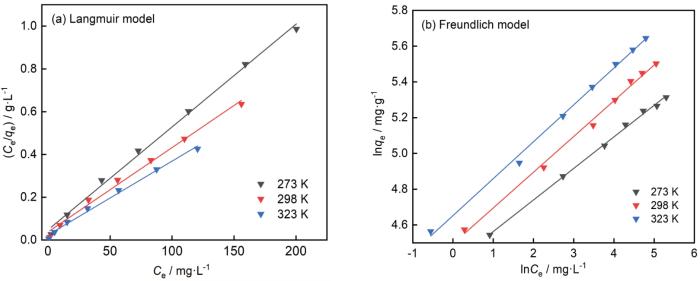
和Freundlich模型
拟合实验数据。式中Ce(mg·L-1)为吸附平衡浓度,qe(mg·g-1)为平衡吸附容量,Qmax(mg·g-1)为最大吸附容量,k(L·mg-1)为Langmuir吸附常数,K和n为吸附容量和吸附强度的特征参数。结果在图9中给出,相关系数列于表2。Langmuir模型和Freundlich模型的拟合系数均高于0.99,表明吸附为单层或多层表面的均相吸附[21]。1/n小于1,吸附容易进行。K值随着温度的升高而增大,表明升温有利吸附。用Langmuir模型拟合得到25℃时空心球形AlOOH吸附刚果红的最大容量为253.81 mg·g-1,略高于HAM@γ-AlOOH/Fe(OH)3微球(252.53 mg·g-1)[20]和γ-AlOOH微球(224.72 mg·g-1)[22]。
图9
图9
吸附刚果红的等温线模型
Fig.9
Langmuir (a) and Freundlich (b) isotherms plots of adsorption of CR
表2 Langmuir和Freundlich模型的拟合参数
Table 2
| T/K | Langmuir isotherm model | Freundlich isotherm model | ||||
|---|---|---|---|---|---|---|
| qmax/ | k/ | R2 | K/ | 1/n | R2 | |
| mg·g-1 | L·mg-1 | (mg·g-1)(L·mg-1)1/n | ||||
| 273 | 207.90 | 0.1001 | 0.9956 | 80.3820 | 0.176 | 0.9974 |
| 298 | 253.81 | 0.1038 | 0.9912 | 89.5888 | 0.200 | 0.9944 |
| 323 | 289.86 | 0.1443 | 0.9916 | 104.8636 | 0.206 | 0.9960 |
可用
估算吸附过程的热力学参数,结果列于表3。式中
表3 吸附刚果红过程的热力学参数
Table 3
| T/K | ΔGΘ/kJ·mol-1 | ΔHΘ/kJ·mol-1 | ΔSΘ/J·mol-1·K-1 |
|---|---|---|---|
| 273 | -9.97 | 3.869 | 50.55 |
| 298 | -11.14 | ||
| 323 | -12.49 |
图10
图10
吸附前后AlOOH的红外光谱
Fig.10
FT-IR spectra of AlOOH before (a) and after (b) adsorption
图11
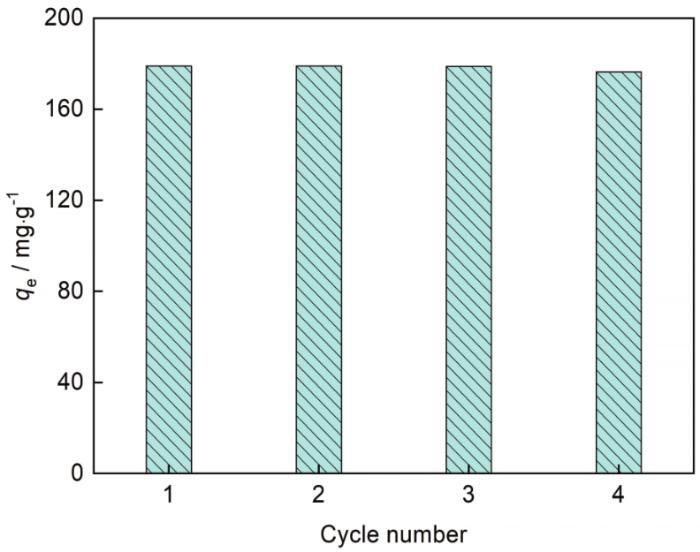
将吸附刚果红的AlOOH在350℃煅烧2 h后,其在25℃、吸附时间10 min、刚果红浓度200 mg·g-1条件下的吸附实验结果,如图12所示。可以看出,四个循环周期后AlOOH的吸附容量仍为97%,表明其为可再生的吸附剂。
图12
图12
循环测试中AlOOH吸附刚果红容量的变化
Fig.12
Equilibrium adsorption capacities variation of AlOOH for CR in recycle tests
3 结论
(1) 将铝渣资源化处理后,可用无模板水热制备由纳米薄片组成的空心球形AlOOH。用空心球形AlOOH吸附刚果红,10 min即达吸附平衡,吸附容量达253.81 mg·g-1。吸附过程符合拟二阶动力学模型,Langmuir和Freundlich模型都能很好的描述吸附过程。
(2) 空心球形AlOOH吸附剂价廉、制备过程简单、对有机染料的吸附快、吸附容量高且可再生。
参考文献
Preparation of magnetic amino acid-functionalized aluminum alginate gel polymer and its super adsorption on azo dyes
[J].A novel magnetic amino acid functionalized aluminum alginate gel polymer Gly/Al/SA@Fe3O4 was prepared via droplet polymerization technique with sodium alginate (SA) as raw material, which was crosslinked with Al(Ⅲ) ions, while glycine (Gly) and Fe3O4 were simultaneously added. The prepared Gly/Al/SA@Fe3O4 was characterized by means of scanning electron microscopy (SEM), X-ray energy dispersive spectroscopy (EDS), Fourier infrared spectroscopy (FT-IR), X-ray diffractometer (XRD) and vibrating sample magnetometer (VSM). Its absorption performance for azo dyes was also investigated. The results show that Gly/Al/SA@Fe3O4 is a kind of three-dimensional network-like polymer particles with a fancy fold structure on the surface, and its magnetic response ability is good. Gly/Al/SA@Fe3O4 shows strong adsorption performance with high adsorption rate for Direct Black 19(DB 19) and Direct Brown 2(DB 2) dyes in water. The dynamic equilibrium adsorption capacities reached 2500 mg/L and 3126 mg/L, respectively for the above two dyes in 15min and 60 min. The adsorption process can be described by a quasi-second-order rate equation, and the isothermal adsorption data conform to Langmuir model. The interaction between the adsorbents and dye molecules was synergistic through electrostatic adsorption, hydrogen bonding, ligand exchange and chemisorption. Gly/Al/SA@Fe3O4 particles are green and environment-friendly, and have strong water purification ability for wastewater containing high concentration azo dye, therefore, it can be used for rapid solid-liquid separation with magnetic field.
磁性氨基酸功能化海藻酸铝凝胶聚合物的制备及对偶氮染料的超强吸附
[J].以海藻酸钠为原料,采用液滴聚合法将其与Al(Ⅲ)离子交联并引入甘氨酸和Fe<sub>3</sub>O<sub>4</sub>,制备出磁性氨基酸功能化海藻酸铝凝胶聚合物(Gly/Al/SA@Fe<sub>3</sub>O<sub>4</sub>),使用扫描电镜(SEM)、X射线能谱仪(EDS)、傅里叶红外光谱仪(FT-IR)、X射线衍射仪(XRD)和振动样品磁强计(VSM)等手段对其表征,研究了这种凝胶聚合物对偶氮染料的吸附性能。结果表明,Gly/Al/SA@Fe<sub>3</sub>O<sub>4</sub>是一种表面具有花式褶皱结构的三维网状聚合物颗粒,其磁响应能力良好。Gly/Al/SA@Fe<sub>3</sub>O<sub>4</sub>对水体中直接黑19(DB 19)和直接棕2(DB 2)染料的吸附性能超强,吸附速率极高,吸附15 min和60 min达到动态平衡的吸附量分别为2500和3126 mg/L。吸附过程可用拟二级速率方程描述,等温吸附数据符合Langmuir模型。吸附剂与染料分子间的相互作用通过静电吸附、氢键作用、配体交换和化学吸附协同实现。Gly/Al/SA@Fe<sub>3</sub>O<sub>4</sub>颗粒绿色环保,对高浓度偶氮染料废水有超强的净水性能,并可用磁场进行快速固液分离。
Influence of PVP surfactant on the morphology and properties of ZnO micro/nanoflowers for dye mixtures and textile wastewater degradation
[J].
Highly efficient removal of toxic organic dyes, chemical solvents and oils by mesoporous exfoliated graphite: Synthesis and mechanism
[J].
Ferrocene-functionalized silsesquioxane-based porous polymer for efficient removal of dyes and heavy metal ions
[J].A novel ferrocene-linked organic-inorganic hybrid porous polymer has been successfully prepared by Friedel-Crafts reaction of octavinylsilsesquioxane and ferrocene. The relationship of structure/property was investigated by FTIR, NMR, XRD, Brunauer-Emmett-Teller (BET) etc. The obtained porous polymer exhibited a high surface area of 1015 m g and a hierarchical pore structure. It could be applied to wastewater treatment with the absorption capacity of up to 1683 mg g for Congo red (CR), 1083 mg g for crystal violet (CV), 1003 mg g for rhodamine B (RB), 441 mg g for methylene blue (MB), 191 mg g for Hg, and 328 mg g for Pb. Remarkably, it could be easily regenerated and the removal efficiency remains almost constant even after six cycles.© 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Preparation of hollow magnetic graphene oxide and its adsorption performance for methylene blue
[J].The Fe3O4 coated polystyrene microsphere (PS), namely Fe3O4@PSwas firstly fabricated by co-precipitation method with FeCl2·6H2O and FeCl3 as raw material, and PS microsphere as tempelate. Then Fe3O4@PS was immersed in toluene solution for removing the PS template. Next, the hollow Fe3O4 microsphere was coated with graphene oxide sheets under sonication to produce the hollow magnetic graphene oxide (HMGO). Subsequently, the absorption performance of the HMGO for methylene blue (MB) was assessed in an artificial waste MB solution. Results verified that the adsorption process reach to equilibrium at 55℃ after 60 min. The maximum adsorption capacity of MB on HMGO is 349.85 mg·g-1. The adsorbent shows good stability and reusability, after 8 times recycling the adsorption rate is still higher than 80%. The adsorption process of MB on HMGO can be well fitted by Pseudo-second-order kinetic model and the adsorption rate is sensitive to the initial concentration. The adsorption isotherm conforms to the Langmuir isotherm model, and the adsorption process is a single-layer surface adsorption.
中空磁性氧化石墨烯的制备及其对亚甲基蓝吸附性能
[J].用共沉淀法将Fe<sub>3</sub>O<sub>4</sub>沉淀在PS微球上并用甲苯去除PS制备出Fe<sub>3</sub>O<sub>4</sub>@PS,再用超声将用Hummers法制备的氧化石墨烯包裹在Fe<sub>3</sub>O<sub>4</sub>@PS表面制备出中空磁性氧化石墨烯,研究了这种复合材料对模拟亚甲基蓝废水的吸附。结果表明:在55℃,用中空磁性氧化石墨烯对亚甲基蓝染料吸附60 min达到平衡,最大吸附量为349.85 mg·g<sup>-1</sup>。吸附剂循环8次,吸附效率仍高于80%。用准二级动力学模型可很好地拟合中空磁性氧化石墨烯对亚甲基蓝的吸附。结果表明,吸附速率对亚甲基蓝染料的初始浓度较为敏感,主要为化学吸附。吸附过程符合Langmuir等温吸附模型,说明这种吸附为单层表面吸附。
Synthesis and photocatalytic performance of ZnO with flower-like structure from zinc oxide ore
[J].AAAAAEmploying zinc sulfate solution obtained from zinc oxide ore as raw material, sodium hydroxide as precipitant and PEG20000 as dispersant, ultrafine ZnO powders with different morphologies were successfully synthesized through hydrothermal method. The influences of the dosage of PEG20000 solution, molar ratio of OH -/Zn 2+, reaction temperature, reaction time and Zn 2+ concentration on the structures and morphologies of the ZnO powders were discussed in detail. The reaction conditions of synthesizing ZnO powders with flower-like structure were obtained as below: dosage of PEG20000 with 10% mass fraction 5 mL, molar ratio of OH - to Zn 2+ 5, reaction temperature 150 °C, reaction time 8 h at Zn 2+ concentration 1 mol L -1. The growth mechanism of ZnO particles with different morphologies was proposed. The ZnO powder with flower-like structure are composed of multiple micro-rods with hexagon morphology and has good photocatalytic degradation ability to degrade RhB. 20 mL RhB solution with 15 mg L -1 could be completely degraded over flower-like ZnO powder 300 mg within 3 h.
Mesoporous magnesia: synthesis, characteri- zation, adsorption behavior and cytotoxic activity
[J].
Synthesis, characterization, and catalytic property of nanosized MgO flakes with different shapes
[J].
Hydrothermal synthesis and photocatalytic activity of CdO2 nanocrystals
[J].
Facile synthesis and characterization of γ-AlOOH/PVA composite granules for Cr(VI) adsorption
[J].
Adsorption and on-site transformation of transition metal cations on Ni-doped AlOOH nanoflowers for OER electrocatalysis
[J].
Removal of fluoride from wastewater solution using Ce-AlOOH with oxalic acid as modification
[J].
Total recycle strategy of phosphorus recovery from wastewater using granule chitosan inlaid with γ-AlOOH
[J].
Additive-free and time-saving microwave hydrothermal synthesis of hollow microspheres structured boehmite
[J].
Template-free synthesis of hierarchical spindle-like γ-Al2O3 materials and their adsorption affinity towards organic and inorganic pollutants in water
[J].
Synthesis and application of Fe3O4/FeWO4 composite as an efficient and magnetically recoverable visible light-driven photocatalyst for the reduction of Cr(VI)
[J].
Morphology-controlled hydrothermal synthesis of boehmite via an anions competition method
[J].
Template-free hydrothermal fabrication of hierarchically organized γ-AlOOH hollow microspheres
[J].
Superb adsorption of organic dyes from aqueous solution on hierarchically porous composites constructed by ZnAl-LDH/Al(OH)3 nanosheets
[J].
Monodispersed hollow aluminosilica microsphere @hierarchical γ-AlOOH deposited with or without Fe(OH)3 nanoparticles for efficient adsorption of organic pollutants
[J].
Layered mesoporous Mg(OH)2/GO nanosheet composite for efficient removal of water contaminants
[J].
Synthesis and characterization of hierarchical γ-AlOOH and γ-Al2O3 microspheres with high adsorption performance for organic dyes
[J].
Space-confined effect one-pot synthesis of γ-AlO(OH)/MgAl-LDH heterostructures with excellent adsorption performance
[J].
Synthesis of flower-like boehmite (γ-AlOOH) via a one-step ionic liquid-assisted hydrothermal route
[J].
Amino-functionalized hierarchical porous SiO2-AlOOH composite nanosheets with enhanced adsorption performance
[J].
Solvent-free hydrothermal synthesis of gamma-aluminum oxide nanoparticles with selective adsorption of Congo red
[J].Aluminum hydroxide and oxide have been widely used for decontamination due to their environmentally friendly nature and cost effectiveness. Aluminum (hydro) oxides are the main phases of aluminum-derived environment materials. Herein, the solvent-free hydrothermal synthesis of gamma-aluminum oxide (γ-AlO) nanoparticles and phase transformation of AlOOH into γ-AlO are reported. Hydrothermal treatment of NH·HO-induced aluminum precipitate resulted in the formation of AlOOH, which was an intermediate product of γ-AlO. AlOOH was transformed into highly crystalline 20-nm γ-AlO particles through calcination at 500 °C due to dehydration. The transformation was confirmed through X-ray diffraction (XRD) and thermogravimetric (TG) analyses. The resulting γ-AlO had superior adsorption ability for the anionic Congo red (CR) dye than for the cationic methylene blue (MB) and malachite green (MG) dyes. The selective adsorption ability of CR instead of MB was attributed to the electrostatic attraction and hydrogen bonds between the amino group and azo double bond of CR, and between the amino group and hydroxyl group in γ-AlO. Thus, this study investigated crystalline phase transformation into γ-AlO and selective adsorption capacity of CR, which provides important information regarding the synthesis of crystalline γ-AlO adsorbent, with selective adsorption ability for decontamination applications.Copyright © 2018 Elsevier Inc. All rights reserved.
Fabrication of hierarchical porous ZnO-Al2O3 microspheres with enhanced adsorption performance
[J].