掺杂金属离子可进一步提高MOFs的气体吸附能力[8~11]。Han等[12]研究了Li掺杂的Li·Cu3(BTC)2和Li·MIL-101(Cr)的H2吸附能力,发现在77 K和100 kPa下其储氢能力分别提高到145%、143%。Yang等[13]将NOTT-200浸渍在锂的氯盐溶液中,发现其储氢量大大提高。Cao等[14]研究了Li+、Na+和K+掺杂对HKUST-l的CO2吸附性能的影响,发现在298 K和1800 kPa下其CO2吸附量最高可达8.64 mmol/g(1K-HKUST-l),比纯HKUST-l提高11%。Poloni等[15]基于密度泛函理论(DFT)计算了Ca、Mg和9个二价过渡金属阳离子对M-BTT和M-MOF-74的CO2吸附行为的影响,发现二价阳离子的特定电子结构和金属配位点在CO2结合时产生的对称性有利于对气体的吸附。
本文以对苯二甲酸为配体,钛酸异丙酯为金属源用溶剂热法制备金属有机骨架材料MIL125,然后用盐溶液浸渍进行后功能化改性,制备一系列Li+、Na+和K+掺杂的M@MIL125-t(M代表碱金属:Li,Na和K;t代表氯盐溶液浸渍处理时间:6,9,12 h),研究其在293 K和101.325 kPa条件下对CO2吸附性能。
1 实验方法
1.1 实验用试剂和仪器
实验用试剂:对苯二甲酸(CAS:100-21-0,98%);钛酸异丙酯(CAS:546-68-9,97%);N,N-二甲基甲酰胺(DMF,CAS:68-12-2,分析纯);甲醇(CAS:67-56-1,分析纯);氯化锂(CAS:7447-41-8,97%);氯化钠(CAS:7647-14-5,99.5%);氯化钾(CAS:7447-40-7,99.5%)。
实验用仪器:傅里叶变换红外光谱仪(FT-IR,Bruker AXS TENSOR-27);X射线衍射仪(XRD,Bruker-D8 Advance);热重分析仪(TG,TA-SDT Q600);扫描电子显微镜(SEM,SU8020场发射扫描电子显微镜);气体吸附(Quantachrome Instruments Autosorb-1物理吸附仪),在77 K下进行氮气等温吸脱附测试,在293 K下进行CO2吸附测试。
1.2 MIL125和M@MIL125-t 样品的制备
MIL125的制备:将1.3108 g的对苯二甲酸,加入到25 mL的N,N-二甲基甲酰胺/甲醇(体积比4∶1)混合溶剂中,得到均匀混合液;将1.440 mL钛酸异丙酯在0℃冰水浴条件下缓慢加入到混合液中,得到白色悬浊液;然后将悬浊液移入100 mL内含聚四氟乙烯的高压反应釜中,在110℃下晶化72 h,冷却后用DMF浸泡活化处理过夜后离心分离,最后将样品在60℃真空干燥6 h,然后甲醇用相同的步骤浸泡活化处理,得到MIL125。
M@MIL125-t的制备:用碱金属氯盐配置成摩尔浓度为0.02 mol/L的浸渍液。将0.0424 g氯化锂、0.0585 g氯化钠和0.0745 g氯化钾分别溶解在50 mL无水乙醇/DMF混合溶剂(体积比4∶1)中。将9份质量为1 g的MIL125粉末分别浸渍在上述三种浸渍液中,浸渍时间分别为6 h、9 h和12 h。浸渍完成后将产物用无水乙醇洗涤三次,抽滤后在60℃真空干燥8 h,将得到的九组掺杂样品记为M@MIL125-t。
2 结果和讨论
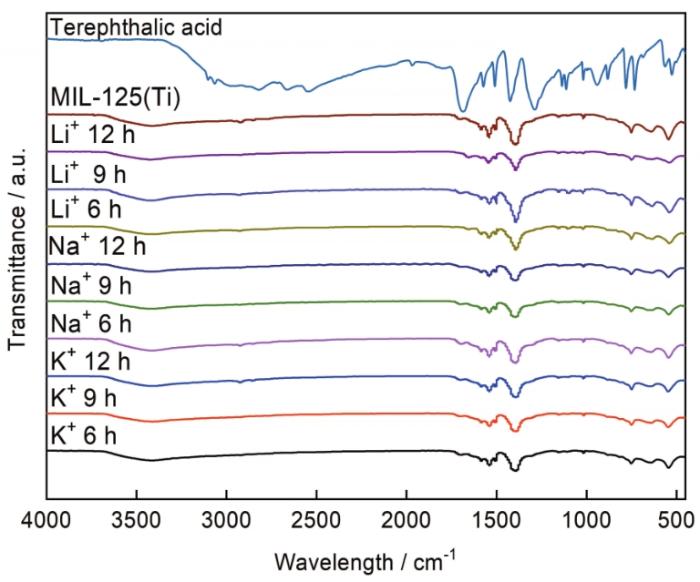
2.1 样品的傅里叶红外光谱
图1
红外光谱中出现在1400-1590 cm-1范围内的吸收峰对应COO-的对称伸缩振动和O-C-O的不对称伸缩振动,出现在745 cm-1附近的吸收峰对应Ti-O的伸缩振动,出现在505~760 cm-1范围内的吸收峰对应Ti-O-Ti的对称伸缩振动[17]。光谱中出现在3200~3600 cm-1范围内的吸收峰来自样品吸附的水,这种低强度的宽峰表明水的含量很低。
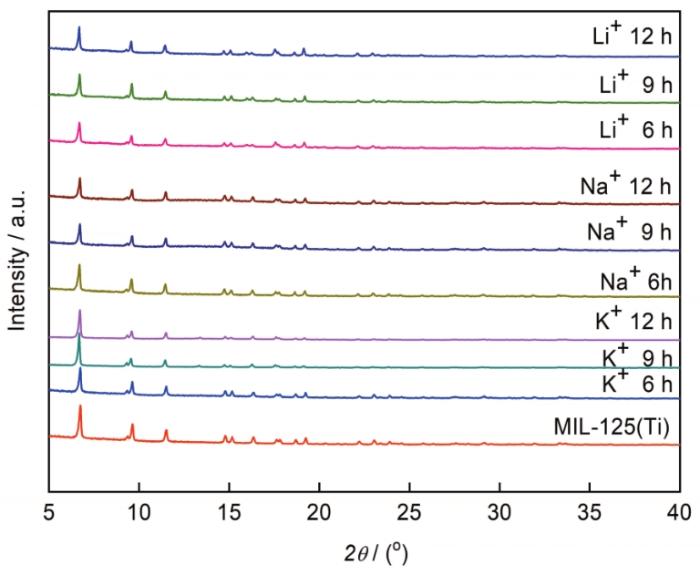
2.2 样品的物相组成
图2
对比不同浸渍金属Li+、Na+和K+和浸渍时间6h、9 h和12 h后MIL125的晶体结构,发现在每种碱金属的氯盐溶液中浸渍一定时间后MIL125的衍射峰强度均稍有减弱。其原因是,盐溶液浸渍处理并干燥后,不同尺寸的碱金属离子沉积在MIL125的孔道内外,特别是滞留在孔道内的碱金属离子堵塞了孔道,使晶格尺寸发生变化,引起MIL125晶体结构的细微变形。
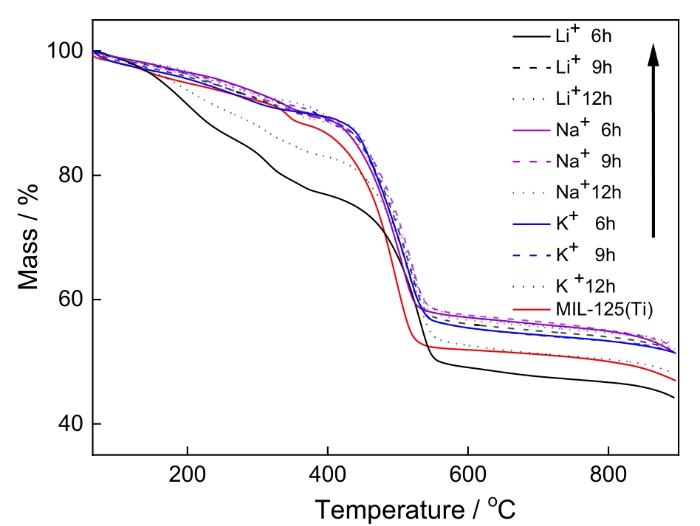
2.3 热稳定性
图3给出了碱金属掺杂改性MIL125前后(在空气中)的热失重曲线。可以看出,掺杂Na+和K+的MIL125其失重曲线基本相同,后者的质量损失略有减小。掺杂Li+的MIL125其质量损失十分明显。其原因是,用于掺杂的Li+半径较小,洗涤干燥后掺杂的比例降低。此外,随着浸渍时间的延长MIL125的总质量损失有所降低,因为掺杂时间的延长有利于Li+进入孔道和稳定晶体骨架。
图3
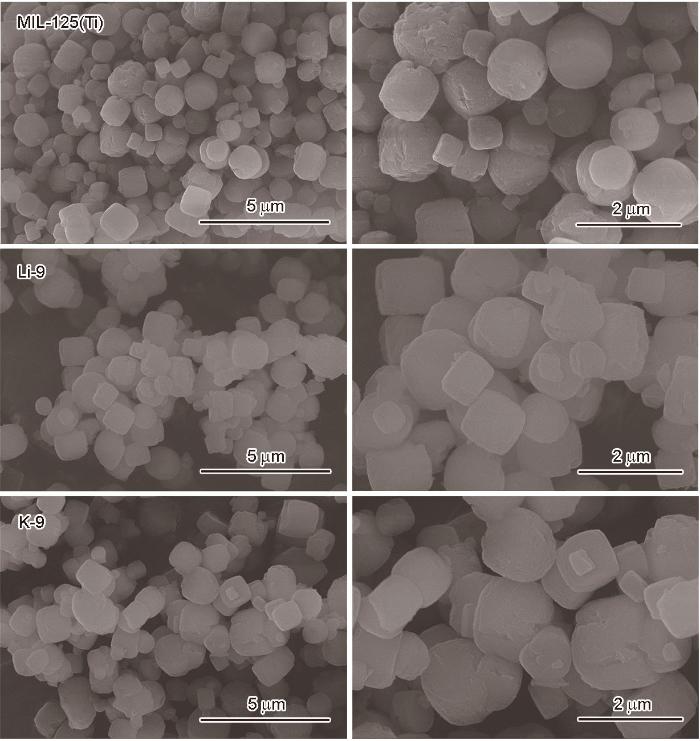
2.4 MIL125,Li@MIL125-9和K@MIL125-9的形貌
图4
图4
MIL125,Li@MIL125-9和K@MIL125-9的SEM照片
Fig.4
SEM images of MIL125, Li@MIL125-9 and K@MIL125-9
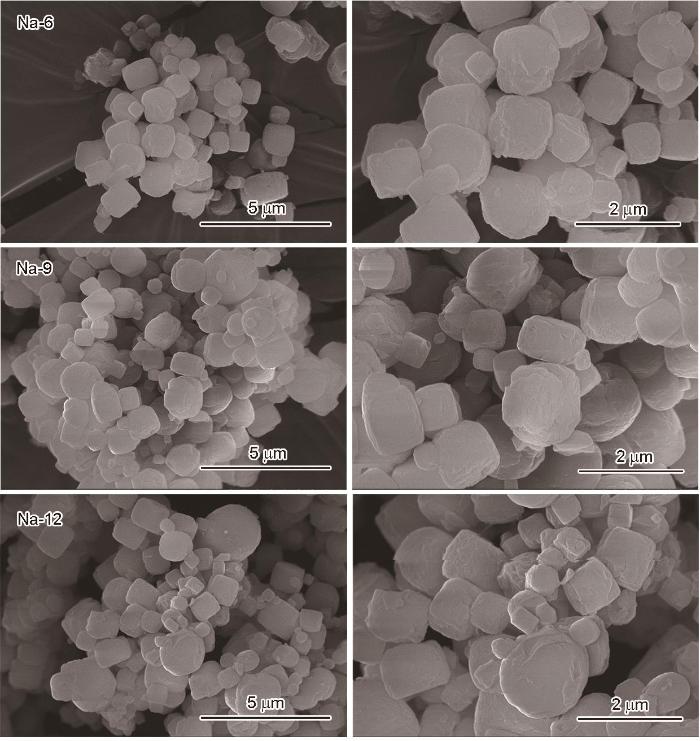
图5
以Na@MIL125-t为例,研究了Na+浸渍掺杂时间对MIL125微观形貌的影响。浸渍液的摩尔浓度均为0.02 mol/L,浸渍时间分别为6 h、9 h、12 h,结果如图5所示。可以看出,随着浸渍时间的延长Na@MIL125-t均为方盘状。腐蚀的强度与电解质溶液浓度和浸渍时间有关。随着浸渍时间的延长腐蚀程度提高,粒径变小的颗粒数量逐渐增多。
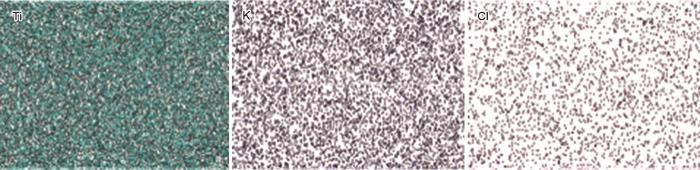
为了验证碱金属盐溶液后浸渍掺杂的有效性和碱金属在MIL125骨架中的存在情况,以K@MIL125-9为例,进行SEM-EDS面扫描研究了Ti、K和Cl元素的分布。从图6可见,K+均匀地分布在K@MIL125-9中。这表明,碱金属离子浸渍及活化处理后仍能很好的结合在MIL125的骨架结构当中,表明这种后掺杂简单有效。Na@MIL125-9的EDS结果也表明,Na+的分布均匀。
图6
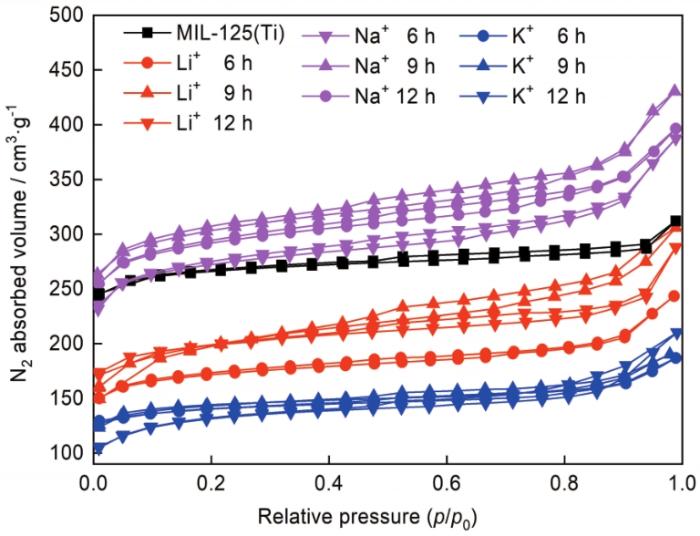
2.5 样品的N2 等温吸脱附
图7
图7
MIL125和M@MIL125-t的N2等温吸脱附曲线
Fig.7
N2 adsorption isotherms of MIL125 and M@MIL125-t at 77 K
图8
掺杂后MIL125的比表面积均发生了变化,主要是碱金属盐溶液浸渍腐蚀了MIL125的骨架结构。掺杂不同碱金属时,M@MIL125-t的最佳浸渍时间均为9 h。掺杂Li+时,浸渍时间较短时大量Li+因半径较小而聚集在孔道中,使孔道堵塞而减小了比表面积。随着浸渍时间的增加骨架和溶液中的Li+达到动态平衡,浸渍液置换孔道中未配位的有机配体,使MIL125-t的结构发生了变化。
2.6 样品的CO2 吸附性能
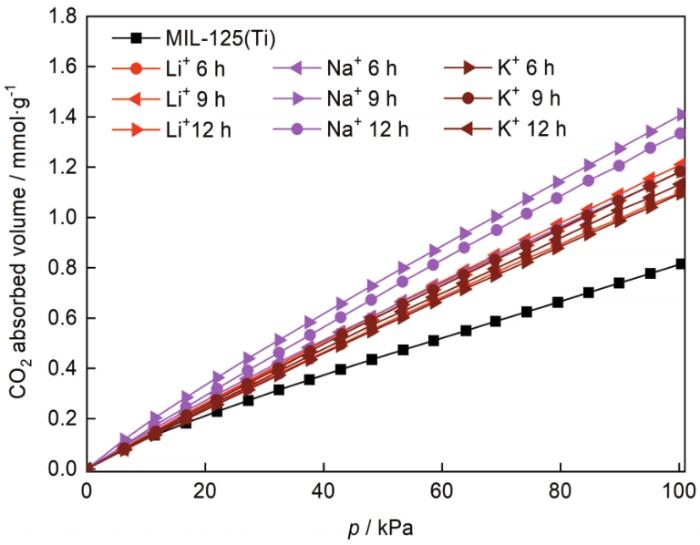
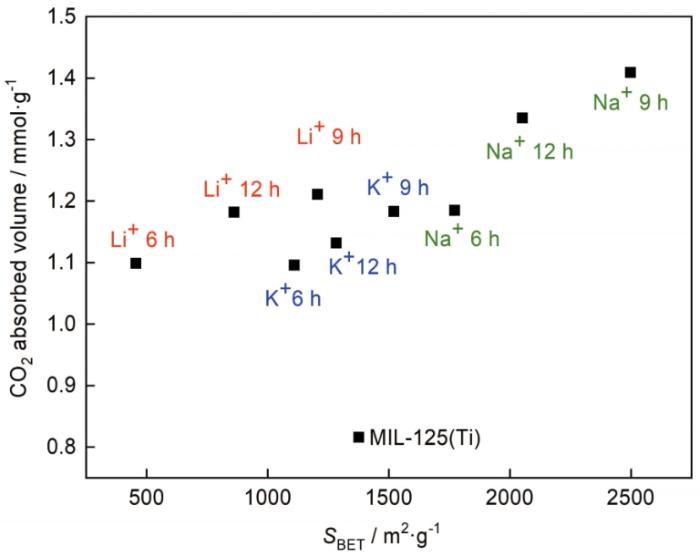
图9给出了MIL125和M@MIL125-t在293 K和101.325 kPa条件下的CO2吸附曲线,图10给出了掺杂不同种类碱金属离子时CO2吸附量与比表面积的关系。可以看出,MIL125的CO2吸附量为0.82 mmol/g,掺杂Li+、Na+、K+后CO2吸附量均有提高,其原因可能是CO2优先吸附在碱金属离子的周围。浸渍处理时间为9 h时,Li@MIL125-t、Na@MIL125-t和K@MIL125-t的比表面积和CO2吸附量达到最大。Na@MIL125-9的CO2吸附量最高为1.41 mmol/g,提高了约72.0%;Li@MIL125-9的CO2吸附量为1.21 mmol/g,提高了47.6%;K@MIL125-9的CO2吸附量为1.18 mmol/g,提高了43.9%。
图9
图9
MIL125和M@MIL125-t的CO2吸附曲线
Fig.9
CO2 adsorption isotherms of MIL125 and M@MIL125-t (293 K, 101.325 kPa)
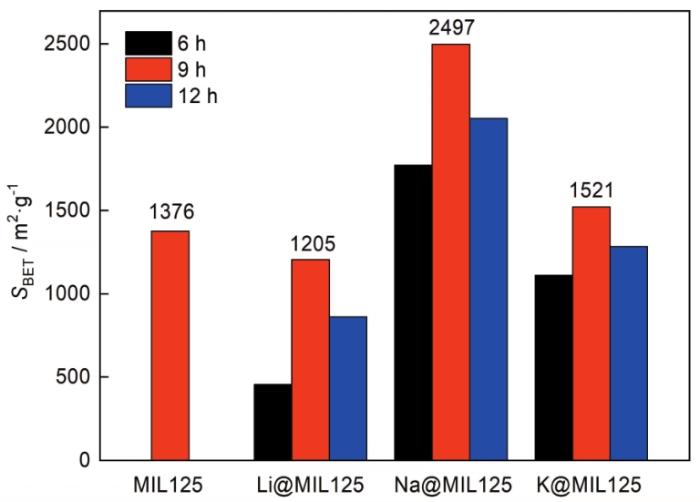
图10
图10
CO2吸附量与MIL125、M@MIL125-t中SBET的关系
Fig.10
Relationship between CO2 uptake and SBET in M@MIL125-t(293 K and 101.325 kPa)
可以看出,碱金属掺杂影响MIL125的CO2吸附性能,CO2的吸附量与比表面积的关系很大。Na@MIL125-9的比表面积和CO2吸附量都最大,Li@MIL125-9的比表面积变小,但CO2吸附量增大。值得注意的是,Li@MIL125-12和K@MIL125-9的CO2吸附量接近,但是其比表面积分别不同(分别为861 m2/g和1521 m2/g)。其原因可能是,Li+的第一电离能和Lennard-Jones势更低,在101.325 kPa条件下对CO2的吸附性能更显著[19]。
3 结论
(1) 用不同种类的碱金属和浸渍时间对MIL125掺杂,对其成键情况、物相结构和热稳定性没有明显的影响。碱金属浸渍液的腐蚀,使MIL125的形貌逐渐由圆饼状向方饼状转变,晶粒尺寸显著减小。
(2) 用Li+、Na+和K+三种碱金属掺杂,碱金属盐溶液浸渍9 h后M@MIL125-t的比表面积最大,Na@MIL125-t系列的比表面积最高。
(3) 用碱金属掺杂后,M@MIL125-t的CO2吸附量比MIL125均有不同程度的提高,因为CO2优先吸附在新引入的Li+、Na+和K+周围。
参考文献
Bimetallic metal-organic frameworks (MOFs) for gas storage and separation
[J].
Improving the mechanical stability of metal-organic frameworks using chemical caryatids
[J].Metal-organic frameworks (MOFs) have emerged as versatile materials for applications ranging from gas separation and storage, catalysis, and sensing. The attractive feature of MOFs is that, by changing the ligand and/or metal, they can be chemically tuned to perform optimally for a given application. In most, if not all, of these applications one also needs a material that has a sufficient mechanical stability, but our understanding of how changes in the chemical structure influence mechanical stability is limited. In this work, we rationalize how the mechanical properties of MOFs are related to framework bonding topology and ligand structure. We illustrate that the functional groups on the organic ligands can either enhance the mechanical stability through formation of a secondary network of nonbonded interactions or soften the material by destabilizing the bonded network of a MOF. In addition, we show that synergistic effect of the bonding network of the material and the secondary network is required to achieve optimal mechanical stability of a MOF. The developed molecular insights in this work can be used for systematic improvement of the mechanical stability of the materials by careful selection of the functional groups.
Ordered macro-microporous metal-organic framework single crystals
[J].We constructed highly oriented and ordered macropores within metal-organic framework (MOF) single crystals, opening up the area of three-dimensional-ordered macro-microporous materials (that is, materials containing both macro- and micropores) in single-crystalline form. Our methodology relies on the strong shaping effects of a polystyrene nanosphere monolith template and a double-solvent-induced heterogeneous nucleation approach. This process synergistically enabled the in situ growth of MOFs within ordered voids, rendering a single crystal with oriented and ordered macro-microporous structure. The improved mass diffusion properties of such hierarchical frameworks, together with their robust single-crystalline nature, endow them with superior catalytic activity and recyclability for bulky-molecule reactions, as compared with conventional, polycrystalline hollow, and disordered macroporous ZIF-8.Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Energetic evaluation of swing adsorption processes for CO2, capture in selected MOFs and zeolites: Effect of impurities
[J].
Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature
[J].Metal-organic frameworks (MOFs) show high CO2 storage capacity at room temperature. Gravimetric CO2 isotherms for MOF-2, MOF-505, Cu3(BTC)2, MOF-74, IRMOFs-11, -3, -6, and -1, and MOF-177 are reported up to 42 bar. Type I isotherms are found in all cases except for MOFs based on Zn4O(O2C)6 clusters, which reveal a sigmoidal isotherm (having a step). The various pressures of the isotherm steps correlate with increasing pore size, which indicates potential for gas separations. The amine functionality of the IRMOF-3 pore shows evidence of relatively increased affinity for CO2. Capacities qualitatively scale with surface area and range from 3.2 mmol/g for MOF-2 to 33.5 mmol/g (320 cm3(STP)/cm3, 147 wt %) for MOF-177, the highest CO2 capacity of any porous material reported.
Adsorption and diffusion of carbon dioxide on metal-organic framework (MOF-5)
[J].
CO2 capture and conversion using Mg-MOF-74 prepared by a sonochemical method
[J].
Effects of amino functionality on uptake of CO2, CH4, and selectivity of CO2/CH4 on titanium based MOFs
[J].
Effect of NH2 and CF3 functionalization on the hydrogen sorption properties of MOFs
[J].
Lithium doping on metal-organic frameworks for enhancing H2 Storage
[J].
Lithium-doped metal-organic frameworks for reversible H2 storage at ambient temperature
[J].
Cation-induced kinetic trapping and enhanced hydrogen adsorption in a modulated anionic metal-organic framework
[J].
Alkali metal cation doping of metal-organic framework for enhancing carbon dioxide adsorption capacity
[J].Metal-organic frameworks (MOFs) have attracted much attention as adsorbents for the separation of CO<sub>2</sub> from flue gas or natural gas. Here, a typical metal-organic framework HKUST-1(also named Cu-BTC or MOF-199) was chemically reduced by doping it with alkali metals (Li, Na and K) and they were further used to investigate their CO<sub>2</sub> adsorption capacities. The structural information, surface chemistry and thermal behavior of the prepared adsorbent samples were characterized by X-ray powder diffraction (XRD), thermo-gravimetric analysis (TGA) and nitrogen adsorption-desorption isotherm analysis. The results showed that the CO<sub>2</sub> storage capacity of HKUST-1 doped with moderate quantities of Li<sup>+</sup>, Na<sup>+</sup> and K<sup>+</sup>, individually, was greater than that of unmodified HKUST-1. The highest CO<sub>2</sub> adsorption uptake of 8.64 mmol/g was obtained with 1K-HKUST-1, and it was ca. 11% increase in adsorption capacity at 298 K and 18 bar as compared with HKUST-1. Moreover, adsorption tests showed that HKUST-1 and 1K-HKUST-1 displayed much higher adsorption capacities of CO<sub>2</sub> than those of N<sub>2</sub>. Finally, the adsorption/desorption cycle experiment revealed that the adsorption performance of 1K-HKUST-1 was fairly stable, without obvious deterioration in the adsorption capacity of CO<sub>2</sub> after 10 cycles.
Understanding trends in CO2 adsorption in metal-organic frameworks with open-metal sites
[J].
Adsorption/catalytic properties of MIL125 and NH2-MIL125
[J].
Synthesis of highly crystalline NH2-MIL125 (Ti) with s-shaped water isotherms for adsorption heat transformation
[J].
Highly selective H2O2-based oxidation of alkylphenols to p-benzoquinones over MIL125 metal-organic frameworks
[J].
The fixation of carbon dioxide with epoxides catalyzed by cation-exchanged metal-organic framework
[J].
Surfactant-assisted synthesis of hierarchical NH2-MIL125 for the removal of organic dyes
[J].