增加清洁能源的使用,是中国调整能源结构的重要途经[1]。氢气的热值高且无污染,受到了极大的关注。电解水制氢具有可持续和零碳排放的优点,是最具应用价值的绿氢制备方法[2]。电化学水分解,分为阳极析氧反应(OER)和阴极析氢反应(HER)[3,4]。OER涉及的四电子转移过程使其反应动力学缓慢和在电催化过程中产生较大的过电位,显著影响电解水的效率[5]。贵金属电催化剂的成本过高,使其应用受到限制[6]。在碱性电解质中可使用廉价的非贵金属(如Ni、Co、Fe和Mn等过渡金属族元素)电催化电极材料[7,8],替代商用贵金属OER电催化剂,如IrO2和RuO2[9~11]。因此,开发非贵金属低成本、高活性和高稳定性的析氧催化剂,意义重大。
高熵合金由五种或五种以上等原子比或近等原子比的组元组成,生成简单的固溶体相。这种独特的结构,具有热力学上的高熵效应、动力学上的扩散迟滞效应、性能上的鸡尾酒效应和结构上的晶格畸变效应[12~17]。与传统的合金材料相比,高熵合金具有制备优异电催化电极材料的潜力,其催化剂成分连续可调、力学性能优异、具有耐腐蚀性和机械加工性能。这些特性,使得在很大程度上可调控其电子结构和几何结构[18,19]。Hu等[20]根据Co-Mo双金属相图合成具有不混溶Co/Mo原子比的CoMoFeCoNi高熵纳米颗粒,该方法比使用贵金属Ru提高了氨分解的催化活性和稳定性。Guo等[21]的研究表明,与FeCoNiCrAl高熵催化剂相比,FeNiMnCrCu高熵合金只需要更小的活化能即可引发OER反应,因为其d空位数和晶格空间比较大。Biswas等[22]发现,Au-Ag-Pt-Pd-Cu高熵合金表现出比纯铜更高的催化活性,因为高熵合金里的铜原子受益于其他金属提供的协同效应。Yu等[23]在合金中添加少量O制备的块状(CrFeCoNi)97O3高熵合金,电流密度为10 mA·cm-2时过电位只有196 mV,Tafel斜率为29 mV·dec-1,优于目前报道的块体OER材料的催化性能。研究结果表明,Cr2O3在提高OER催化活性方面有关键作用。块体高熵合金的氧微合金化诱导氧化物的形成,不仅提高了大块材料的催化活性和长期稳定性,还可用简单的铸造冶金工艺大量生产,避免了复杂的湿化学工艺,有利于工业化生产。这些结果均表明,高熵合金在电催化领域有广阔的应用前景。
1 实验方法
1.1 高熵合金的制备
先用真空电弧炉制备富锰高熵合金纽扣锭,然后用铜模浇铸法制备尺寸为15 mm × 5 mm × 80 mm的合金板,其名义成分为Mn50Fe12.5Co12.5Ni12.5Cr12.5(记作M50)。所用的合金原材料有Mn、Ni、Fe、Co和Cr,均为纯度高于99.99%的片状或者块状纯金属。
1.2 电极的制备
先用划片切割机将合金板切成尺寸为15 mm×1 mm×10 mm的电催化剂前驱体合金片,然后将其表面用2000 #砂纸打磨以去除表面的氧化层,接着用硅橡胶将部分合金片密封,将面积为10 mm×10 mm的裸露面作为工作电极。将密封好的合金片放入3 mol/L的盐酸溶液中腐蚀120 s,取出后用去离子水和乙醇将表面清洗干净,干燥后得到电催化电极,记作M50A。
1.3 性能表征
使用型号为Bruker D8 3KW & Bruker AXS(λ=0.154178 nm)的X射线衍射仪分析电极样品的结构,靶材为Cu钯。用配备能量色散X射线光谱仪(EDS)的TESCAN MIRA3场发射扫描电子显微镜(SEM)分析电极材料的表面以及截面的形貌和成分。使用型号为Thermo Fischer ESCALAB 250Xi的光谱仪测量样品的X射线光电子能谱(XPS)。使用Gamry Interface1000电化学工作站测量电极的电化学性能,三电极体系的工作电极为电催化电极M50A,参比电极为Hg/HgO电极,铂片作为对电极,电解液为1 mol/L的KOH溶液。电化学实验前先向电解液中通20 min流量为10 mL·min-1的高纯N2(99.999%)以排除溶液中的O2。测量的电化学曲线包括:(1)线性伏安特性曲线(LSV),评估析氧催化剂的过电位,扫描速率为5 mV·s-1;(2)循环伏安特性曲线(CV),用扫速不同(100~500 mV·s-1)的CV曲线的非法拉第区-0.1~0.2 V(vs.Hg/HgO)评估电极的电化学活性面积(ECSA);(3)电化学阻抗谱(EIS),扫描频率为0.01~105 Hz;(4)用计时电压法评估电极催化性能的稳定性,电流分别取10和50 mA·cm-2,时间为50 h。对所有的电化学参数进行溶液电阻补偿(iR=85%)。使用ZSimpWin软件拟合EIS曲线。
2 结果和讨论
2.1 Mn50Fe12.5Co12.5Ni12.5Cr12.5 合金和多孔电催化电极的结构
图1
图1
M50高熵合金和M50A高熵催化剂的X射线衍射谱
Fig.1
XRD patterns of M50 HEA alloy and M50A HEA catalyst
2.2 多孔电催化电极表面的形貌
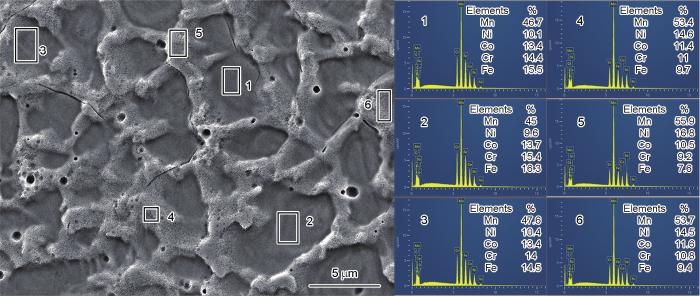
图2a给出了M50合金光滑表面的形貌。M50合金是单相FCC固溶体。为了确定表面的元素分布,对M50合金金相腐蚀后进行EDS分析,结果如图2b所示,可见M50合金表面的Mn和Ni有不同程度的偏析。对不同区域进一步进行EDS统计,得到枝晶和枝晶间的成分(图3)。从表1可见,枝晶的成分富Fe和Cr,枝晶间的成分富Mn和Ni。由于Mn和Cr、Fe的混合焓较大(分别为2和0 kJ/mol),在M50合金的快冷过程中容易被排挤出富含Cr、Fe的枝晶干区域;而Mn和Ni的混合焓为-8 kJ/mol,容易偏聚在枝晶间区域[33,34]。图4a给出了腐蚀后M50A催化剂的表面形貌,图4b~c给出了图4a中对应区域的放大图。可以看出,M50A的表面呈现多孔结构。图4d、e中的EDS面扫和能谱给出了M50A的成分,与表1中M50合金的枝晶成分相同。其原因是,在对M50化学腐蚀的过程中,枝晶内的成分富Cr和枝晶间成分富Mn使晶间偏析成分比枝晶内成分更容易腐蚀掉,因此在表面形成多孔结构。孔的直径约为5 μm,形成通道增大了比表面积,从而有利于提高电催化性能。
图2
图2
M50合金表面的SEM照片和金相腐蚀后的EDS面扫描图
Fig.2
SEM images of the surface of M50 (a) and EDS mappings of M50 after metallographic corrosion (b)
图3
图3
M50枝晶和枝晶间成分的点扫描能谱图
Fig.3
EDS spectrums of dendrite and interdendritic components of M50 (atomic fraction, %)
表1 M50枝晶和枝晶间各元素含量的分布
Table 1
| Mn | Ni | Co | Cr | Fe | |
|---|---|---|---|---|---|
| Dendritic regions | 46.43 | 10.03 | 13.50 | 14.60 | 15.44 |
| Interdendrite regions | 54.33 | 15.30 | 11.14 | 10.33 | 8.90 |
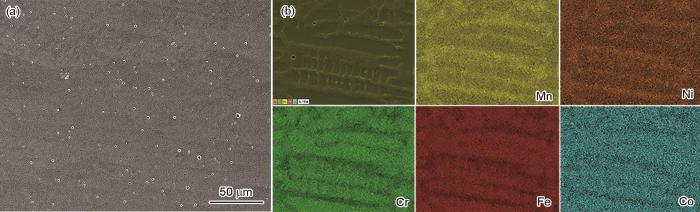
图4
图4
M50A催化剂表面的SEM照片和EDS能谱
Fig.4
SEM (a~c) and EDS mapping (d, e) images of the surface of M50A eletrocatalysts
2.3 多孔电催化电极的析氧性能
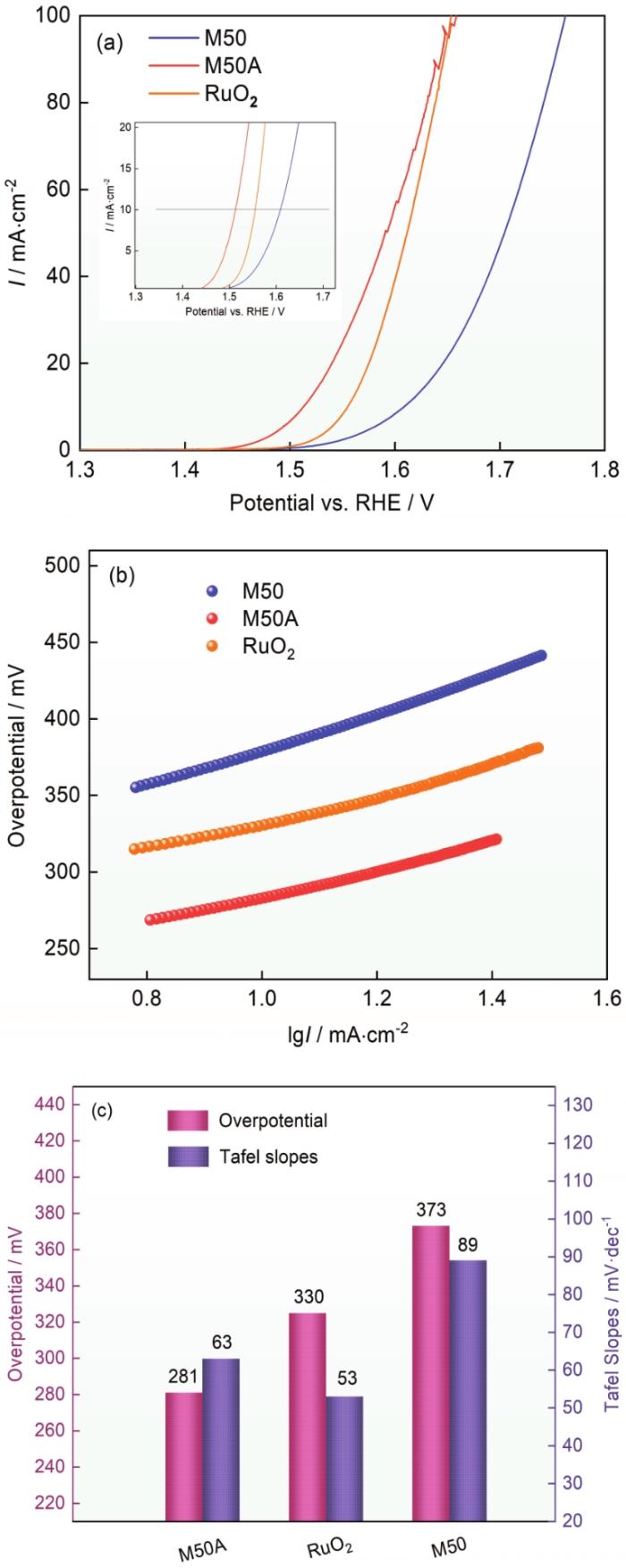
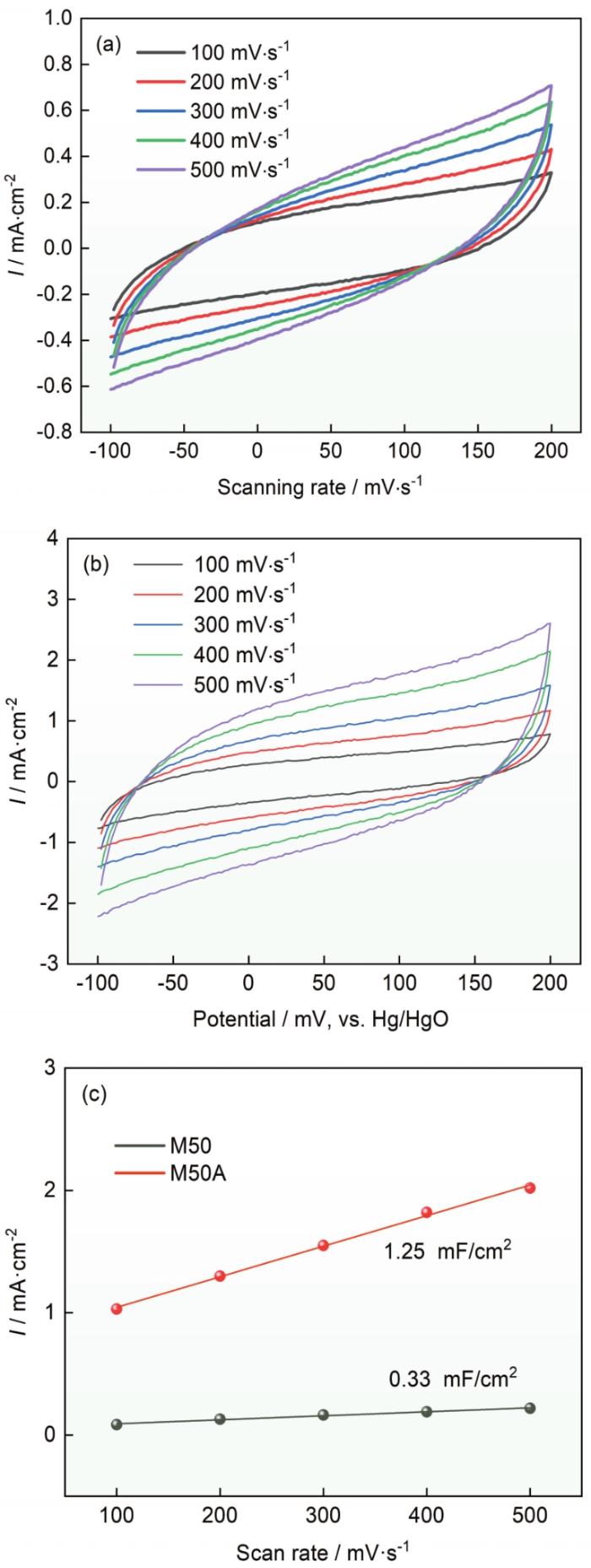
测试了制备出的电催化电极M50A的析氧性能,为了对比同时测试了M50合金和商业RuO2催化剂的析氧性能。图5a给出了不同电极材料的析氧性能。可以看出,M50A催化剂在电流密度为10 mA·cm-2时的析氧过电位为281 mV,低于商业RuO2的325 mV和M50合金的373 mV。Tafel斜率反映了电催化电极的催化动力学过程进行得更快,不同的数值反映了催化过程不同的速率决定性步骤。该数值越小,说明催化反应速率决定性步骤在多电子转移反应的末端,即催化性能越好。如图5b所示,M50A的Tafel斜率为63 mV·dec-1,而M50合金和商业RuO2的Tafel斜率分别为53和89 mV·dec-1。这表明,与M50合金相比,M50A催化剂的析氧动力学过程更快。为了进一步评估M50A电极的电化学性能,测量了M50和M50A的电催化活性面积。催化材料表面的电化学双层电容(Cdl)与ECSA之间存在正相关的关系,因此用CV曲线的非法拉第区进行Cdl值的测量。图6a、b给出了不同扫描速率的CV曲线,分析并计算出M50和M50A的Cdl分别为0.33和1.25 mF·cm-2 (图6c)。这表明,化学腐蚀后的M50A电催化电极表面的电催化活性面积更大,催化活性位点更多,因此电催化析氧性能更好。采用多步计时电位方法评估了M50A电催化性能。电流从10 mA·cm-2增加到50 mA·cm-2,每300 s增加10 mA·cm-2,达到50 mA·cm-2后再依次减小至10 mA·cm-2,并记录相应的电位变化。从50 mA·cm-2开始时电位立即稳定并在300 s内保持恒定,且在结束回到10 mA·cm-2的电压与对应电流的电压相等。这种计时电位响应的稳定性可以说明,M50A催化剂电极具有优异的质量传输特性(OH-向内扩散和氧气泡向外扩散)、导电性、机械稳定性和出色的耐腐蚀性。
图5
图5
M50、M50A 和RuO2的电催化析氧性能
Fig.5
Electrocatalytic OER performance of M50, M50A and RuO2 HEA electrocatalysts in 1 mol/L KOH electrolyte (a) Polarization curves, (b) Tafel curves, (c) corresponding overpotential at 10 mA·cm-2 and Tafel slopes
图6
图6
M50合金不同扫描速率的循环伏安曲线、M50A催化剂不同扫描速率的循环伏安曲线以及M50和M50A的Cdl图
Fig.6
CV curves at various scan rates of M50 (a), CV curves at various scan rates of M50A (b) and Cdl values of M50 and M50A (c)
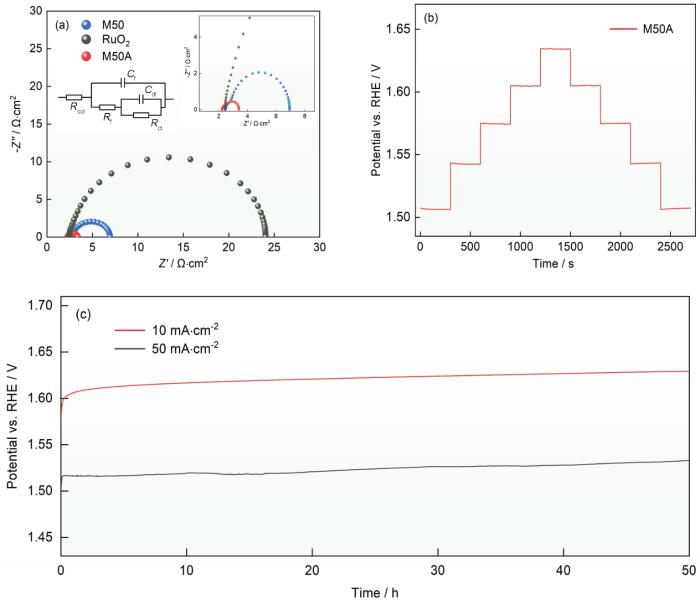
电极的导电性影响电极材料在催化过程中的电荷转移能力。为了更好地分析M50和M50A电催化电极的电荷转移能力,在105~0.01 Hz的频率范围内对M50、RuO2和M50A在1.58 V (vs. RHE)下进行电化学阻抗谱(EIS)测试,结果如图7d所示。在低频处没有观察到Warburg 阻抗,表明在此电位下的OER过程只由电荷转移过程控制。图7d中的插图给出了一个简单的等效电路模型(EET)用于拟合EIS数据,其中Rsol、Rf、Cf、Rct和Cdl分别表示电解液的电阻、催化剂电阻、催化剂电容、电荷转移电阻和双电层电容,其中催化剂电阻和电容是电极表面粗糙度、自身导电性和氧化膜等因素使电场不均匀引起的。所有这些电路元件都是将等效电路拟合到阻抗图估算的,并在表2中列出。如表2所示,M50、RuO2和M50A的电荷转移电阻分别为3.696、14.74和0.6557 Ω·cm2。这表明,不同电极双电层电容Cdl大小的规律与非法拉第区测量循环伏安曲线得到的双电层电容大小规律一致。由于电极表面较为粗糙,这一结果误差较大,因此本文不采用这一拟合数据评判电极的电催化活性面积。对于M50和M50A电催化电极,无论内部催化剂电阻还是界面处的电荷转移电阻,其数量级相同,表明具有良好的电荷转移能力。这一结果,与负载型催化剂或者复合电极相比,电荷转移能力的优势较为明显。χ2表示EIS图中EET电路的拟合优度,其数量级为10-4,表明选择的拟合电路具有良好且合理的电荷转移能力。催化剂的长时间稳定性表征其是否具有实用性。在电流密度分别为10和50 mA·cm-2的条件下测试了M50A进行长达50 h的稳定性,结果表明,工作电压没有明显的上升,表明M50A电催化电极在碱性溶液环境下具有优异的析氧性能稳定性。
图7
图7
M50、M50A和RuO2的阻抗谱(插图分别为拟合等效电路图和局部放大图)、M50催化剂多级计时电位响应以及在恒电流密度10和50 mA·cm-2条件下M50A的稳定性
Fig.7
Nyquist plots of M50, M50A and RuO2 (a), multi-current chronopotentiometry responses (b) and stability test of M50A electrocatalyst at 10 and 50 mA·cm-2 for 50 h (c)
表2 不同催化剂EET的拟合参数
Table 2
| Catalysts | Rs / Ω·cm2 | Cf / mF·cm2 | Rf / Ω·cm2 | Cdl / mF·cm-2 | Rct / Ω·cm2 | χ2 |
|---|---|---|---|---|---|---|
| M50 | 2.457 | 0.3914 | 0.7818 | 1.055 | 3.696 | 3.68×10-4 |
| RuO2 | 2.428 | 0.05694 | 6.919 | 0.03428 | 14.74 | 5.87×10-4 |
| M50A | 2.225 | 2.937 | 0.5119 | 14.17 | 0.6557 | 1.93×10-4 |
2.4 多孔电催化电极OER反应后的表面XPS谱
对电催化析氧反应后的M50A电极表面进行XPS分析,以进一步了解电催化析氧过程。图8a给出了电极表面的XPS全谱,可见存在Mn、Fe、Co、Cr、Ni和O。(图8b)中的Mn 2p谱对应结合能624.7和654.7 eV的峰,表明存在MnO2 [35]。Ni的高分辨XPS谱中的各峰分别为结合能855.8、863和873.1 eV的特征峰,表明存在Ni3+,与文献中的NiOOH的特征峰位置一致[36,37]。在Co的2p谱图中出现了位于781和797.5 eV的特征峰并伴有分别位于787.5 和803 eV的Co2p3/2和Co2p1/2卫星峰。这表明,Co在反应后以CoOOH形式的存在,而不是以金属氧化物形式[38]。对Cr 2p谱图的分析结果证实,位于576.8和586.7 eV的特征峰属于Cr2O3[39, 40]。在图8f的Fe2p谱图中Fe2p3/2和Fe2p1/2峰的结合能分别位于711.5和727 eV,表明Fe在催化剂反应过程中以FeOOH形式存在[39]。对图8g中的O 1s的谱图分析表明,催化剂反应后其表面主要由的金属氧化物(M-O)和金属氢氧化物(M-OH)组成,其中氢氧化物的含量高于金属氧化物的含量,说明M50A催化剂在催化过程中的活性物质为金属的氢氧化物。
图8
图8
M50A电极OER反应后表面的高分辨XPS谱
Fig.8
High-resolution XPS spectra of the M50A electrocatalyst after OER process (a) full survey, (b) Mn 2p, (c) Ni 2p, (d) Co 2p, (e) Cr 2p, (f) Fe 2p and (g) O 1s
2.5 讨论
与M50合金相比,M50A的电催化析氧性能优异,甚至高于商业电催化剂RuO2。其原因有:
(1) 化学腐蚀后,M50A电极比表面积的增大而暴露出更多的活性位点,孔道的形成有利于析氧过程中产生的气体及时脱附进入电解液,使电催化产物及时脱离反应界,避免了催化势垒的提高而使过电位增大。
(2)与负载型析氧催化剂相比,在自支撑型电极结构的M50A电极材料中不存在因负载催化剂与载体之间功函数的差异而使界面电子转移受阻,从而提高了M50A电极材料在电催化过程中导电性,电荷转移能力更强有利于提高电极的电催化性能。
(3) 在碱性电解液中块体高熵催化剂表面的结构能保持长时间的稳定。
3 结论
(1) 用真空熔炼、快速冷却和化学腐蚀方法制备的3D多孔富锰高熵析氧催化剂,在碱性1 mol/L KOH溶液中电流密度和过电位优于商业RuO2析氧催化剂。
(2) 自支撑型电极结构的富锰高熵电极材料优异的导电性提高了电荷转移能力和电催化析氧过程中反应界面的电子转移速率,有利于降低析氧过电位。
(3) 富锰高熵电催化电极材料的优异电催化性能的稳定性,源于高熵结构的耐腐蚀性。
参考文献
Challenges toward carbon neutrality in china: strategies and countermeasures
[J].
Decoupled electrochemical water splitting: from fundamentals to applications
[J].
Preparation, electrical and electrochemical characterizations of CuCoNiFeMn high-entropy-alloy for overall water splitting at neutral-pH
[J].
Mn-doped Co-Al LDHs and its potential use for overall water splitting
[J].
Mn掺杂Co-Al金属氢氧化物的制备及其全解水电化学性能
[J].以Co基氢氧化物为基础用异质元素掺杂方式引入Mn并与Co协同,制备出Mn掺杂Co-Al层状双金属氢氧化物 (Mn-CoAl LDH)。在1 mol/L的KOH碱性电解质中,电流密度达到10 mA·cm<sup>-2</sup>时Mn-CoAl LDH的全解水电势为1.66 V,其性能远优于Co-Al层状双金属氢氧化物(CoAl LDH)、Ni<sub>2/3</sub>S<sub>1/3 </sub>/Nickel Foam (1.76 V)和已经商业化的Pt/C (1.75 V)。这表明,Mn-CoAl LDH催化剂在碱性环境下具有较高的析氢和析氧活性,是一种低成本高性能的双功能电催化剂。
Clean and affordable hydrogen fuel from alkaline water splitting: past, recent progress, and future prospects
[J].
Impact of intermittent operation on lifetime and performance of a PEM water electrolyzer
[J].
Transition metal-based layered double hydroxides for photo(electro)chemical water splitting: a mini review
[J].The conversion of solar energy into usable chemical fuels, such as hydrogen gas, via photo(electro)chemical water splitting is a promising approach for creating a carbon neutral energy ecosystem. The deployment of this technology industrially and at scale requires photoelectrodes that are highly active, cost-effective, and stable. To create these new photoelectrodes, transition metal-based electrocatalysts have been proposed as potential cocatalysts for improving the performance of water splitting catalysts. Layered double hydroxides (LDHs) are a class of clays with brucite like layers and intercalated anions. Transition metal-based LDHs are increasingly popular in the field of photo(electro)chemical water splitting due to their unique physicochemical properties. This article aims to review recent advances in transition metal-based LDHs for photo(electro)chemical water splitting. This article provides a brief overview of the research in a format approachable for the general scientific audience. Specifically, this review examines the following areas: (i) routes for synthesis of transition metal-based LDHs, (ii) recent developments in transition metal-based LDHs for photo(electro)chemical water splitting, and (iii) an overview of the structure-property relationships therein.
Recent advances in transition-metal-sulfide-based bifunctional electrocatalysts for overall water splitting
[J].
Ultralow Ru doping induced interface engineering in MOF derived ruthenium-cobalt oxide hollow nanobox for efficient water oxidation electrocatalysis
[J].
Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: a comparative study of nanoparticles and bulk materials
[J].
Advances in noble metal (Ru, Rh, and Ir) doping for boosting water splitting electrocatalysis
[J].
Nanostructured high-entropy alloys with multiple principal elements: novel alloy design concepts and outcomes
[J].
A critical review of high entropy alloys and related concepts
[J].
High-entropy alloys
[J].Alloying has long been used to confer desirable properties to materials. Typically, it involves the addition of relatively small amounts of secondary elements to a primary element. For the past decade and a half, however, a new alloying strategy that involves the combination of multiple principal elements in high concentrations to create new materials called high-entropy alloys has been in vogue. The multi-dimensional compositional space that can be tackled with this approach is practically limitless, and only tiny regions have been investigated so far. Nevertheless, a few high-entropy alloys have already been shown to possess exceptional properties, exceeding those of conventional alloys, and other outstanding high-entropy alloys are likely to be discovered in the future. Here, we review recent progress in understanding the salient features of high-entropy alloys. Model alloys whose behaviour has been carefully investigated are highlighted and their fundamental properties and underlying elementary mechanisms discussed. We also address the vast compositional space that remains to be explored and outline fruitful ways to identify regions within this space where high-entropy alloys with potentially interesting properties may be lurking.
Understanding chemical short-range ordering/demixing coupled with lattice distortion in solid solution high entropy alloys
[J].
Microstructures and properties of high-entropy alloys
[J].
Electrocatalytic oxygen evolution performance of high entropy FeCoNiMoCr alloy thin film electrode
[J].
FeCoNiMoCr高熵合金薄膜电极的电催化析氧性能
[J].
Novel and promising electrocatalyst for oxygen evolution reaction based on MnFeCoNi high entropy alloy
[J].
A perspective on the catalysis using the high entropy alloys
[J].
Highly efficient decomposition of ammonia using high-entropy alloy catalysts
[J].Ammonia represents a promising liquid fuel for hydrogen storage, but its large-scale application is limited by the need for precious metal ruthenium (Ru) as catalyst. Here we report on highly efficient ammonia decomposition using novel high-entropy alloy (HEA) catalysts made of earth abundant elements. Quinary CoMoFeNiCu nanoparticles are synthesized in a single solid-solution phase with robust control over the Co/Mo atomic ratio, including those ratios considered to be immiscible according to the Co-Mo bimetallic phase diagram. These HEA nanoparticles demonstrate substantially enhanced catalytic activity and stability for ammonia decomposition, with improvement factors achieving >20 versus Ru catalysts. Catalytic activity of HEA nanoparticles is robustly tunable by varying the Co/Mo ratio, allowing for the optimization of surface property to maximize the reactivity under different reaction conditions. Our work highlights the great potential of HEAs for catalyzing chemical transformation and energy conversion reactions.
Electrocatalytic activity of high-entropy alloys toward oxygen evolution reaction
[J].
High-entropy alloys as catalysts for the CO2 and CO reduction reactions: experimental realization
[J].
Engineering microdomains of oxides in high-entropy alloy electrodes toward efficient oxygen evolution
[J].
A self-supporting electrode with in-situ partial transformation of Fe-MOF into amorphous NiFe-LDH for efficient oxygen evolution reaction
[J].
Oxygen reduction on high surface area Pt-based alloy catalysts in comparison to well defined smooth bulk alloy electrodes
[J].
In-situ transformational mycelium-like metal phosphides-encapsulated carbon nanotubes coating on the stainless steel mesh as robust self-supporting electrocatalyst for water splitting
[J].
High entropy alloy as a highly active and stable electrocatalyst for hydrogen evolution reaction
[J].
Corrosion and alloy engineering in rational design of high current density electrodes for efficient water splitting
[J].
Self-supported transition metal phosphide based electrodes as high-efficient water splitting cathodes
[J].Electrolytic water splitting has been considered as a promising technology to produce highly pure H2 by using electrical power produced from wind, solar energy or other fitful renewable energy resources. Combining novel self-supporting structure and high-performance transition metal phosphides (TMP) shows substantial promise for practical application in water splitting. In this review, we try to provide a comprehensive analysis of the design and fabrication of various self-supported TMP electrodes for hydrogen evolution reaction, which are divided into three categories: catalysts growing on carbon-based substrates, catalysts growing on metal-based substrates and freestanding catalyst films. The material structures together with catalytic performances of self-supported electrodes are presented and discussed. We also show the specific strategies to further improve the catalytic performance by elemental doping or incorporation of nanocarbons. The simple and one-step methods to fabricate self-supported TMP electrodes are also highlighted. Finally, the challenges and perspectives for self-supported TMP electrodes in water splitting application are briefly discussed.
Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution
[J].
Understanding crystallographic orientation dependent dissolution rates of 90Cu-10Ni alloy: New insights based on AFM/SKPFM measurements and coordination number/electronic structure calculations
[J].
Crystallographic anisotropy of corrosion rate and surface faceting of polycrystalline 90Cu-10Ni in acidic NaCl solution
[J].
Effects of milling time, sintering temperature, Al content on the chemical nature, microhardness and microstructure of mechanochemically synthesized FeCoNiCrMn high entropy alloy
[J].
Microstructure evolution and composition redistribution of FeCoNiCrMn high entropy alloy under extreme plastic deformation
[J].
The evaluation of the long-term stability of α-MnO2 based OER electrocatalyst in neutral medium by using data processing approach
[J].alpha-MnO2 has attracted specific interest as one of the most promising candidates for the Oxygen Evolution Reaction (OER) electrocatalyst owing to its low-cost, non-toxicity, earth-abundancy and high activity/stability properties. Long-term operating stability and activity of candidate electrocatalysts are important parameters for evaluating their commercial potential. However, long-term stability and activity parameters in electrocatalysis depend on various chemical factors (such as pH, composition and oxidation state of catalyst, etc.) and physical factors (such as particle size, morphology, conductivity, etc.). It is extremely difficult to evaluate and estimate which factor dominates the electrocatalytic stability, because electrocatalytic reactions occur via multistep processes, which contain chemical, electrochemical and mechanical treatments. In addition to advanced research methods that can provide accurate information about electrochemical systems, it is essential to develop data analysis methods in order to make significant progress. A new and powerful systematic approach is needed to determine the long-term stability of active and stable electrocatalysts. In this study, in order to evaluate and estimate to long term stability and activation loss of electrocatalysts, we propose data processing approach using a typical electrochemical measurement. A scientific software tool based on C++; ROOT, was used for the analysis and visualisation of data processing. Various statistical and mathematical functions are well integrated into the framework and this allows to operate data with a few simple commands. (C) 2019 Elsevier B.V.
Effects of Fe electrolyte impurities on Ni(OH)2/NiOOH structure and oxygen evolution activity
[J].
Morphology dynamics of single-layered Ni(OH)2/NiOOH nanosheets and subsequent Fe incorporation studied by in situ electrochemical atomic force microscopy
[J].
The latest development of CoOOH two-dimensional materials used as OER catalysts
[J].
Coupling interface constructions of NiO-Cr2O3 heterostructures for efficient electrocatalytic oxygen evolution
[J].
Cr-doped CoFe layered double hydroxides: highly efficient and robust bifunctional electrocatalyst for the oxidation of water and urea
[J].