钴是一种剧毒重金属[1~3]。目前去除废水中Co2+的方法,有化学沉淀、混凝、离子交换、吸附、电化学和膜分离等[4]。吸附法操作简易、成本低且效率高[5]。吸附法使用的吸附剂,最常见的有沸石[6]、离子交换树脂[7]、壳聚糖[8]、活性炭[9]等。活性炭的孔隙率高、孔容大、官能团丰富,且易改性和再生[10]。传统的活性炭是用煤沥青等工业原料制造的,成本高且性能不高[11]。生物质原料的来源广泛,适合用作制造生物炭的原料[12]。Qiang和Yang等[13]研究了稻草在不同温度下热解吸附Ni2+、Co2+的性能和机理,发现稻草生物炭对Co2+和Ni2+的最高吸附量分别高达39.92和33.12 mg·g-1。余建星等[14]以核桃青皮为原料用水热法制备炭前驱体,然后以终温800℃活化制备出生物质炭(HBC800)。这种生物质炭对Ni2+的最大理论吸附量高达 127.39 mg·g-1。Elham Aboli等[15]以柑橘叶为原料制备的生物炭,对Pb2+、Co2+和Ni2+的最大吸附容量分别为69.82、60.60和58.14 mg·g-1。Arnelli等[16]使用稻壳为原料、以KOH为活性剂,高温热解制备活性炭再使用十二烷基硫酸钠(SLS)为表面活性剂对其进一步改性,在300 min内对40 mg·L-1的Ni2+的吸附效率为95.97%。
1 实验方法
1.1 实验用试剂和仪器设备
CH4N2O,Co(NO3)3·6H2O(分析纯);芦荟叶皮;纯水。
GSL-1600X型高温真空管式炉;Nanosem430型扫描电子显微镜(SEM);3H-2000PS1型自动氮气吸附仪(BET);Nicolet is 50型傅立叶红外光谱仪(FTIR);ESCALAB25型X-射线光电子能谱(XPS);Zetasizer Nano ZS90型Zeta电位仪;ICPS-7510 PLUS电感耦合等离子体原子发射光谱仪。
1.2 N掺杂生物炭的制备
将用纯水洗净的芦荟叶皮在105℃干燥24 h,然后将其磨粉并过80 目筛。在一定质量的芦荟叶皮粉中按2∶1的质量比加入尿素,再加入去离子水(10 mL·3 g-1芦荟叶皮)混合均匀后放入高压反应釜。将高压反应釜置于鼓风干燥箱中(200℃,16 h)进行反应,制备出生物炭前驱体。将生物炭前驱体置于高温真空管式炉中在惰性气体氛围下(N2,60 mL·min-1)程序升温(5℃·min-1)至不同终温(600、700、800℃)热解3 h,然后自然降温至室温得到炭材料。将炭材料研磨至通过80目筛,得到N掺杂生物炭(NBC x,其中x代表热解终温)。
1.3 性能表征
1.3.1 吸附等温线的测定
在50 mL不同初始浓度的Co2+溶液(25、50、100、200、300、400和600 mg·L-1)中分别加入50 mg 的NBC x,在25℃下静态吸附1440 min后,测试溶液中Co2+剩余的浓度,绘制NBC x 吸附等温线。NBC x 对Co2+的吸附量qt (mg·g-1)[22]为
式中qt (mg·g-1)为t时吸附剂已吸附的吸附质的量;C0 (mg·L-1)为吸附质的初始浓度;Ct (mg·L-1)为t时吸附质的浓度;V(L)为吸附质溶液的体积;m(g)为吸附剂的质量。
式中qe (mg·g-1)为平衡吸附量;Q (mg·g-1)为吸附到达平衡时单层最大吸附量;Ce (mg·L-1)为吸附质在平衡时的浓度;KL (L·mg-1)为表面吸附亲和性常数。
1.3.2 吸附动力学的测定
式中t(min)为吸附剂与溶液的接触时间,qt (mg/g)为t时刻的吸附量,qe (mg/g)为平衡时的吸附量K2 (g·mg‒1·min‒1),准二级动力学吸附速率常数。
1.3.3 动态吸附
在25℃测试NBC x 在流速1 mL·min-1条件下的动态吸附过程,为模拟固定床吸附。在玻璃圆管(长10 cm,内径0.8 cm)中加入200 mg 的NBC x 模拟吸附柱,用蠕动泵将Co2+溶液(50 mg·L-1)泵入吸附柱,自吸附柱下进上出流入收集装置,定时(10、30、60、90、120、180、240和300 min)取样测定溶液中Co2+的浓度。Ct达到C0的10%的t时刻为吸附剂NBC x 的穿透时间,Ct达到C0的90%的t时刻为吸附剂NBC x 的饱和时间。
1.3.4 吸附剂质量对吸附的影响
在8份50 mL浓度为1500 mg·L-1的Co2+溶液中分别加入50、100、200、300、400、500、600 mg的NBC x,放入恒温(25℃)振荡摇床(120 r/min)1440 min取样,以检测溶液中Co2+浓度并画出去除率曲线。去除率η(%)[27]为
式中C0 (mg·L-1)为吸附质的初始浓度;Ct (mg·L-1)为t时刻吸附质的浓度。
2 结果和讨论
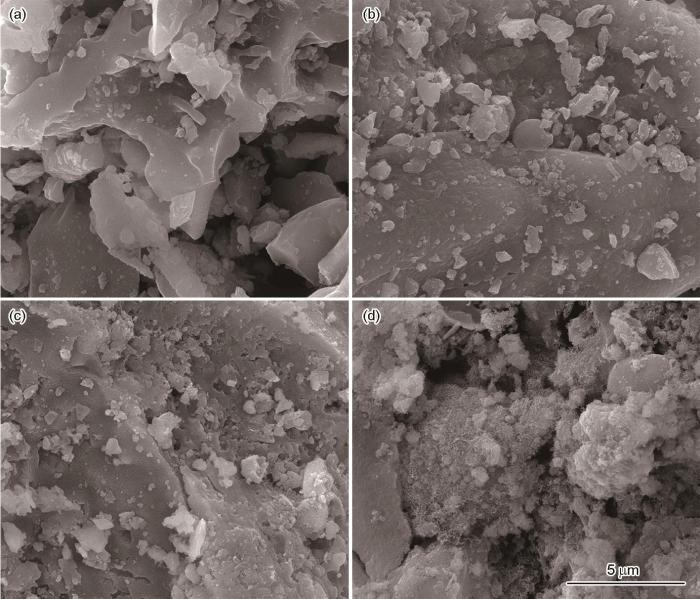
2.1 NBC x 吸附Co2+ 前后的形貌
图1给出了NBC x 吸附Co2+前后的扫描电镜照片。图1a、b、c分别给出了活化终温为600、700、800℃时NBC x 的SEM照片。可以看出,这种生物炭材料具有明显的层块状堆积结构。与NBC600和NBC700相比,NBC800的表面层块堆积最复杂,层块最粗糙和细小,暴露出更多的活性官能团。其原因是,随着热解温度的提高NBC x 中的尿素和活性官能团热解生成气体,这些气体膨胀挥发产生更多的孔道,使得NBC x 表面更为粗糙。这有助于提高生物炭材料的比表面积和孔道的丰富性,为Co2+提供大量的吸附位着点[28]。图1d给出了NBC800吸附Co2+后的SEM照片,可见吸附Co2+后NBC800表面的层块上附着了大量的霜状物。发生这种现象的原因是,大量Co2+进入NBC800的孔道与炭材料表面的官能团发生络合作用或共沉淀作用,使吸附后的Co2+均匀分布在NBC800的表面。
图1
图1
NBC x 吸附Co2+前后的SEM照片
Fig.1
SEM images of the samples NBC600 (a), NBC700 (b), NBC800 (c) and NBC800⁃Co2+ (d)
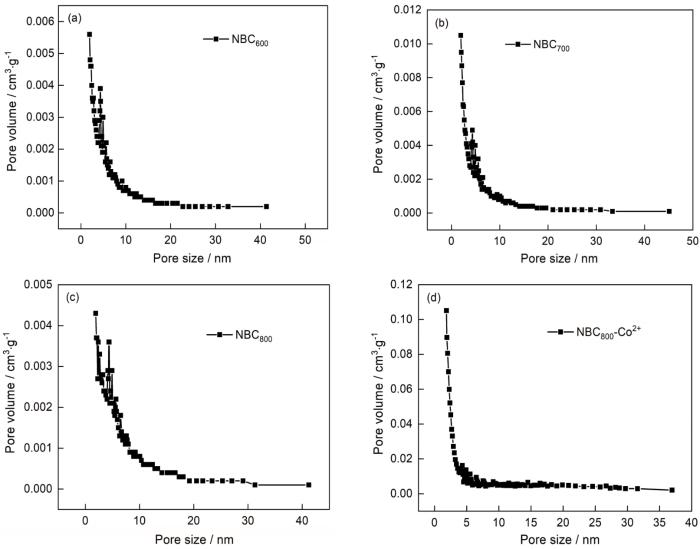
2.2 BET分析
图2
图3
表1列出了不同的NBCx比表面积和孔隙结构参数。从表1可见,随着热解终温由600℃提高到700℃,NBC x 的比表面积和总孔容明显增大,但是平均孔径减小。其原因是,随着温度的升高NBC x 中的活性官能团以有机小分子气体的形式脱除,在气体膨胀挥发的过程中制造了更多的微孔[31]。随着热解终温由700℃提高到800℃,NBC x 的比表面积下降而非微孔的比例明显提高。其原因是,随着温度的提高NBC x 中的活性官能团继续脱除,在气体膨胀挥发的过程中将一部分微孔扩大为中孔。吸附Co2+后NBC800的比表面积和孔结构参数都明显增大,非微孔的比例明显降低。其原因是,Co2+在NBC800表面活性官能团的作用下附着在其表面,使NBC800的孔道大量增加、表面结构更为复杂。这种现象与SEM观察的结果符合。
表1 NBC x 比表面积和孔隙结构参数
Table 1
| Samples | Dap /nm | SBET /m2·g-1 | Smic /m2·g-1 | Vt /cm3·g-1 | Vmic /cm3·g-1 | Non-Vmic/Vt |
|---|---|---|---|---|---|---|
| NBC600 | 4.58 | 33 | 18 | 0.03 | 0.02 | 0.33 |
| NBC700 | 3.42 | 69 | 48 | 0.05 | 0.03 | 0.40 |
| NBC800 | 5.68 | 32 | 15 | 0.04 | 0.01 | 0.75 |
| NBC800-Co2+ | 4.00 | 325 | 249 | 0.30 | 0.15 | 0.50 |
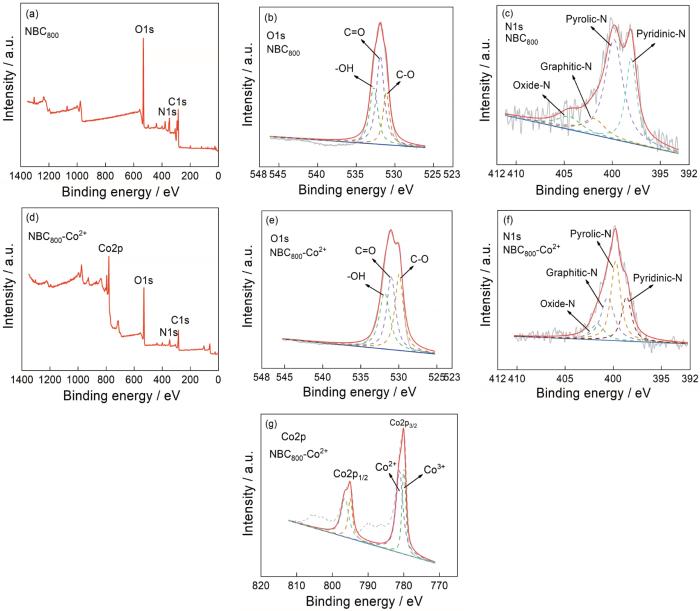
2.3 XPS分析
图4
图4
NBC800吸附Co2+前后的XPS谱图
Fig.4
XPS spectra of the samples (a) survey spectra of NBC800; (b) O1s spectrum of NBC800; (c) N1s spectrum of NBC800; (d) survey spectra of NBC800-Co2+; (e) O1s spectrum of NBC800-Co2+; (f) N1s spectrum of NBC800-Co2+; (g) Co2p spectrum of NBC800-Co2+
表2 XPS分析元素摩尔分数
Table 2
| Samples | C1s | O1s | N1s | Co2p |
|---|---|---|---|---|
| NBC600 | 82.4 | 16.4 | 1.2 | - |
| NBC700 | 80.9 | 17.4 | 1.7 | - |
| NBC800 | 49.76 | 46.35 | 3.89 | - |
| NBC800-Co2+ | 40.54 | 41.61 | 3.92 | 13.93 |
表3 XPS分析中O1s、N1s和Co2p谱图的官能团摩尔分数
Table 3
| Samples | -OH | C=O | C-O | Oxide-N | Graphitic-N | Pyrolic-N | Pyridinic-N | Co3+ | Co2+ |
|---|---|---|---|---|---|---|---|---|---|
| NBC600 | 4.63 | 6.37 | 5.40 | 0.19 | 0.24 | 0.38 | 0.39 | - | - |
| NBC700 | 4.67 | 7.85 | 4.88 | 0.16 | 0.17 | 0.75 | 0.62 | - | - |
| NBC800 | 14.20 | 21.69 | 10.46 | 0.36 | 0.41 | 2.05 | 1.07 | - | - |
| NBC800-Co2+ | 13.25 | 14.28 | 14.08 | 0.42 | 1.12 | 1.51 | 0.87 | 4.97 | 8.96 |
由图4c、f可知,NBC800吸附Co2+前后的N谱可分为4个特征吸收峰Oxide-N、Graphitic-N、Pyrolic-N和Pyridinic-N,其结合能分别约为402.1、400.7、399.8和398.8 eV,吸附Co2+前后4种形式的N元素相对含量变化明显。由表3可知,在吸附前的NBC800中Oxide-N、Graphitic-N、Pyrolic-N和Pyridinic-N的含量分别为0.36%、0.41%、2.05%和1.07%,都明显高于NBC600和NBC700。N元素主要以Pyrolic-N和Pyridinic-N的形式存在。NBC800吸附Co2+后Pyrolic-N(1.51%)和Pyridinic-N (0.87%)的含量明显降低,表明吡咯和吡啶基团参与了吸附过程,Co2+与活性基团的结合影响了Pyrolic-N和Pyridinic-N结合能的信号[33]。由图4g可知,NBC800吸附Co2+后Co2p的谱图可分为两个特征吸收峰Co2+和Co3+,结合能分别约为781.2和779.96 eV。由表3可知,Co2+和Co3+的占比分别为8.96%和4.97%,且部分Co2+被羧基等含氧官能团氧化成Co3+,表明Co2+成功地吸附在NBC800表面[34]。
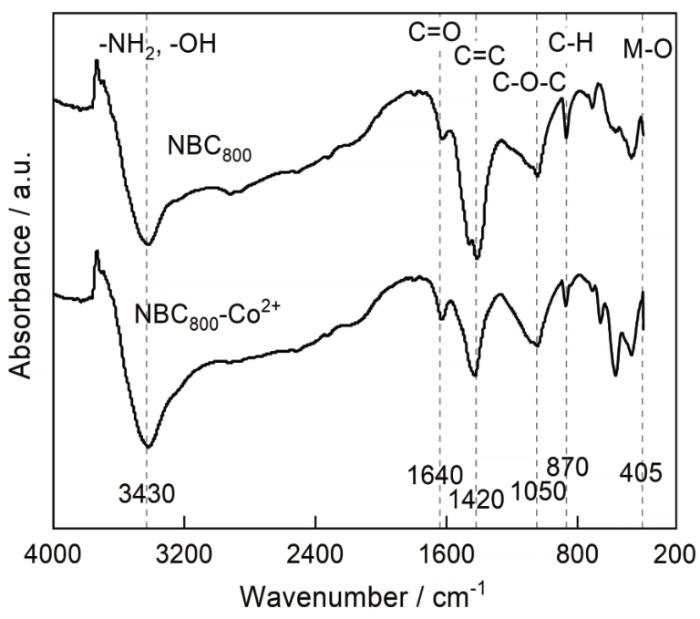
2.4 FTIR分析
图5给出了NBC800吸附Co2+前后的FTIR图,图中3430 cm-1附近的FTIR强吸收带可能是自由氨基和羟基键(-NH2和-OH)伸缩振动引起的。NBC800吸附Co2+后吸收带强度出现明显减弱,其原因是Co2+与-NH2和-OH发生离子交换和络合作用等化学吸附行为削弱了相关基团的吸收峰[35,36]。在1640 cm-1附近的FTIR吸收峰可能是羧基、醛基和酮基中的-C=O键的伸缩振动引起的,NBC800吸附Co2+后吸收带强度出现明显的减弱和偏移,是Co2+与-COOH发生离子交换和络合等化学吸附行为引起的[37]。在1420 cm-1附近的吸收峰可能是芳香族化合物基团的伸缩振动引起的,NBC800吸附Co2+后吸收峰发生了减弱和偏移,因为芳香族化合物的π电子与Co2+的稳定结合降低了键能[38]。870 cm-1附近的吸收峰是吡啶和杂环化合物中C-H键的面外弯曲振动引起的,NBC800吸附Co2+后吸收峰发生了减弱和偏移。NBC800吸附Co2+后在405 cm-1附近出现了明显的吸收峰增强和偏移,表明NBC800成功地吸附了Co2+,吸附后吸收峰出现偏移现象是形成Co-O引起的。
图5
图5
NBC800吸附Co2+前后的FTIR图
Fig.5
FTIR diagram of NBC800 before and after Co2+ adsorption
2.5 Zeta分析
图6给出了NBC x 在不同pH值下的电位。可以看出,pH值低于3时NBC x 都带正电荷。随着pH值的增大NBC x 的表面电荷迅速由正变负。-COOH基团不带正电荷且-OH基团的极性非常弱,因此NBC x 基团仅在低pH值下呈弱正电荷,与Zeta电位的结果一致。随着热解终温的提高NBC x 中的活性官能团进一步以有机小分子气体的形式脱除,NBC x 的零电荷点(pHPZC)应该增大。但是,NBC x 的pHPZC随热解终温升高呈现降低的趋势,NBC600的pHPZC=4.72,NBC700的pHPZC=4.11,NBC800的pHPZC=3.49。随着热解终温的提高NBC x 中的活性基团虽然脱除了一部分,但是这些小分子气体膨胀挥发的同时产生了更多的孔道结构,使更多的活性基团得以暴露,因此NBC x 的pHPZC呈现减小的趋势。NBC800的pHPZC=3.49,表明在pH>3.49时NBC800表面带负电,有利于吸引水体中Co2+等重金属阳离子,也有利于NBC800通过包括静电作用在内的物化作用吸附这些阳离子。
图6
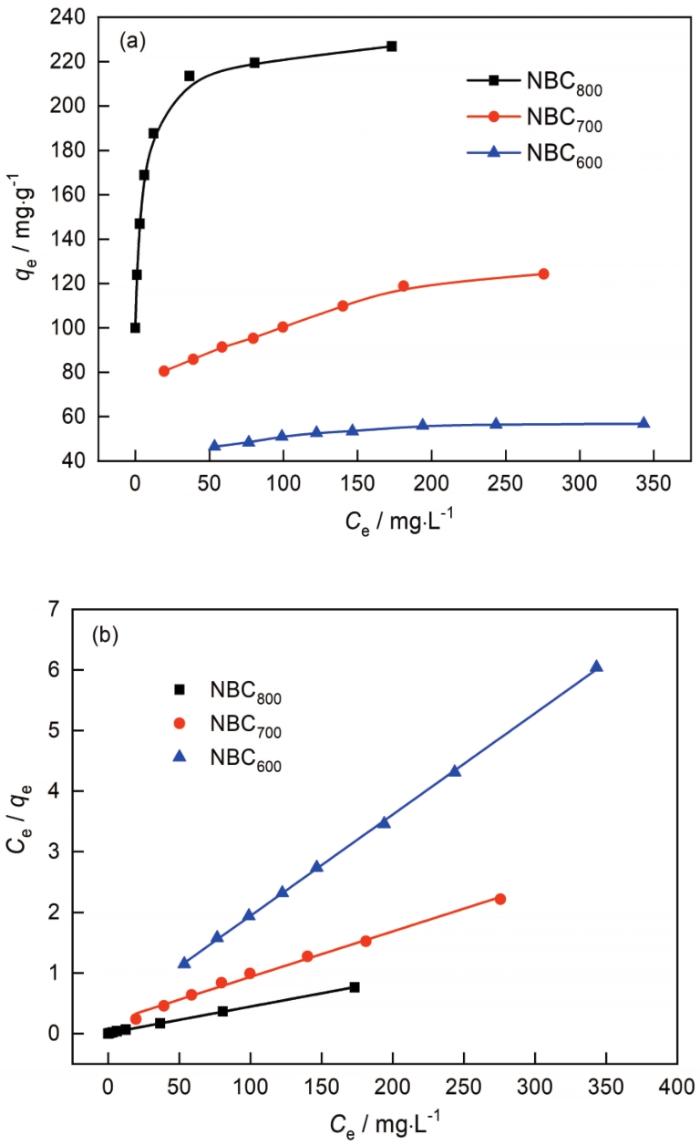
2.6 吸附等温线
图7a给出了NBC x 吸附Co2+的吸附等温曲线,可见NBC x 对Co2+的吸附能力较好。当Co2+溶液的C0较低时Co2+在NBC x 上的平衡吸附量较低,但是对Co2+的去除率较高。随着Co2+溶液的C0的提高固液间金属的离子浓度梯度增大,有利于克服NBC x 与溶液间的界面传质阻力,使Co2+在NBC x 上的吸附量很快增加,NBC x 孔道以外表面的活性基团吸附Co2+达到饱和后Co2+开始进入孔道内,孔道内壁的活性基团开始参与吸附使Co2+的去除率呈现减小的趋势。随着Co2+溶液中的C0继续增加NBC x 对Co2+的吸附达到平衡,对Co2+的去除率最小。NBC800吸附等温线的起始斜率最高,平衡吸附量受金属离子溶液的C0的影响较小。其原因是,NBC800表面丰富的中孔可大量吸附Co2+且孔道不易阻塞。
图7
图7
NBC x 吸附Co2+的吸附等温曲线和Langmuir拟合
Fig.7
Adsorption isotherm of Co2+ by NBC x and Langmuir model fitting (a) adsorption isotherm curve; (b) Langmuir model fitting diagram
由图7b可见,Langmuir模型(R2>0.9923)的拟合效果更接近于NBC x 对Co2+的吸附行为,表明NBC x 吸附Co2+具有单分子层吸附特征,即吸附过程符合化学吸附(静电吸引、离子交换、金属离子和官能团的络合作用等)。根据模型的计算结果表明,NBC800对Co2+的吸附能力最强,最大理论吸附量为228.31 mg·g-1。
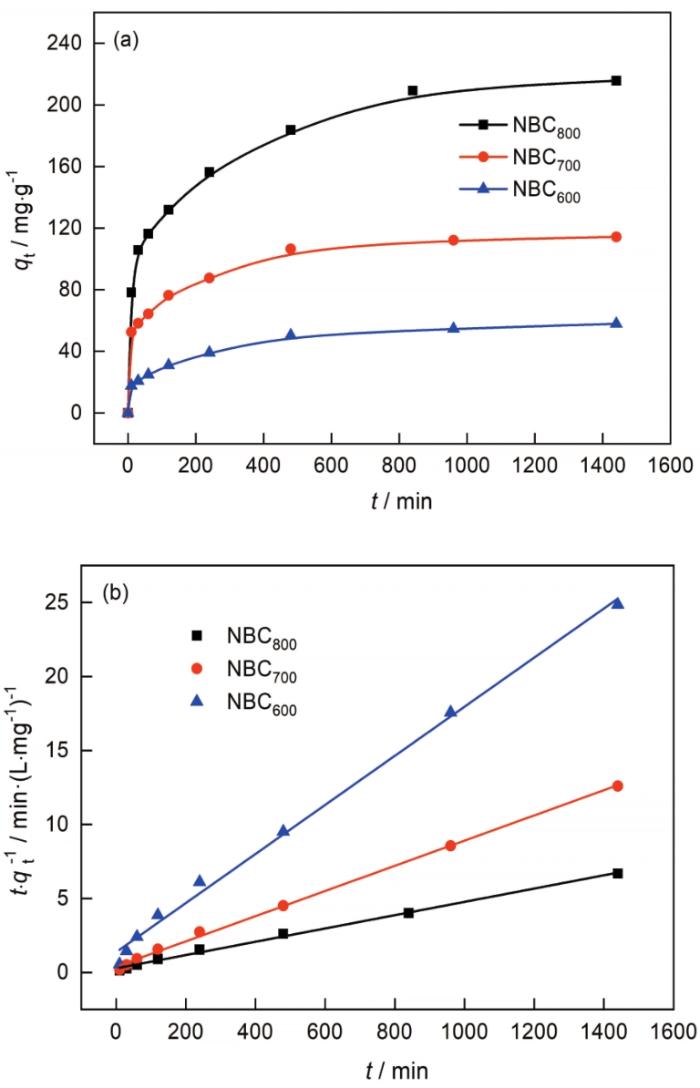
2.7 动力学分析
图8a给出了NBC x 对Co2+的吸附动力学曲线。可以看出,NBC x 对Co2+的吸附过程进行较快,在吸附开始后约300 min趋于平衡。在吸附过程的前期(0~200 min)溶液中Co2+的浓度较高,NBC x 表面仍有较多的活性基团未吸附,此时Co2+在NBC x 上的吸附量较低。随着吸附时间的增加,NBC x 对于Co2+的吸附量逐渐增加。随着NBC x 对Co2+的进一步吸附(200~300 min)溶液中的Co2+浓度降低,NBCx表面的活性基团被Co2+占据并饱和,NBC x 对Co2+的吸附达到平衡,对Co2+的去除率达到最大。
图8
图8
NBC x 对Co2+的吸附动力学曲线和NBC x 的吸附动力学拟合
Fig.8
Adsorption kinetics curve of Co2+ by NBC x and fitting of pseudo-second-order kinetics model (a) adsorption kinetics curve; (b) fitting diagram of pseudo-second-order dynamics model
图8b给出了NBCx的吸附动力学拟合。可以看出,拟二级动力学模型(R2>0.9957)更好地描述了Co2+在NBC x 上的吸附动力学。根据该模型计算的平衡吸附容量NBC800(qe=222.72 mg·g-1)>NBC700(qe=117.51 mg·g-1)>NBC600(qe=60.27 mg·g-1),与实验结果非常接近,表明NBC x 吸附Co2+的方式主要是Co2+与NBC x 表面官能团之间的化学相互作用。NBC x 对Co2+的吸附达到平衡的时间基本相同,但是NBC800具有最大的平衡吸附容量,表明NBC800可能比NBC600和NBC700具有更多的表面活性官能团。这一实验结果与XPS分析的结果一致,符合SEM和BET的预测。
2.8 固定床吸附
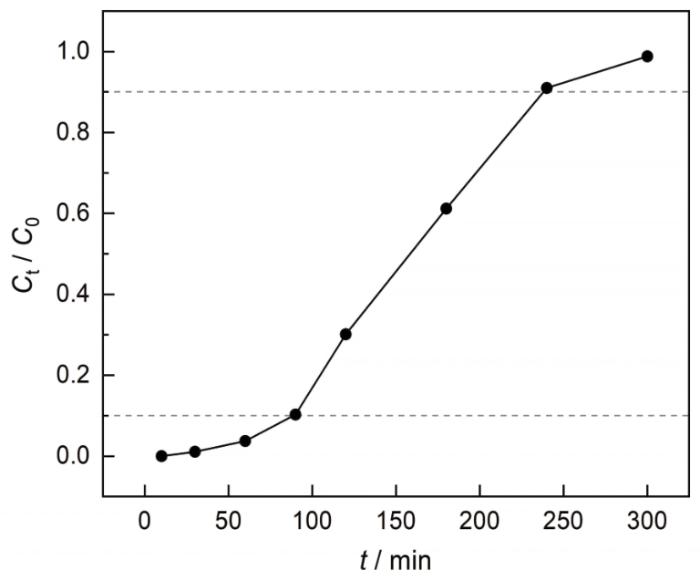
图9给出了NBC800的吸附穿透曲线。可见,在模拟固定床中NBC800作为吸附剂吸附Co2+的穿透时间为89 min,饱和时间为237 min。穿透时间和饱和时间较长,表明NBC800对Co2+有较大的吸附容量,与吸附等温线和吸附动力学的结果相同。
图9
图9
NBC800对Co2+的吸附穿透曲线
Fig.9
Breakthrough curve for Co2+ dynamic adsorption on NBC800
2.9 吸附剂质量对吸附的影响
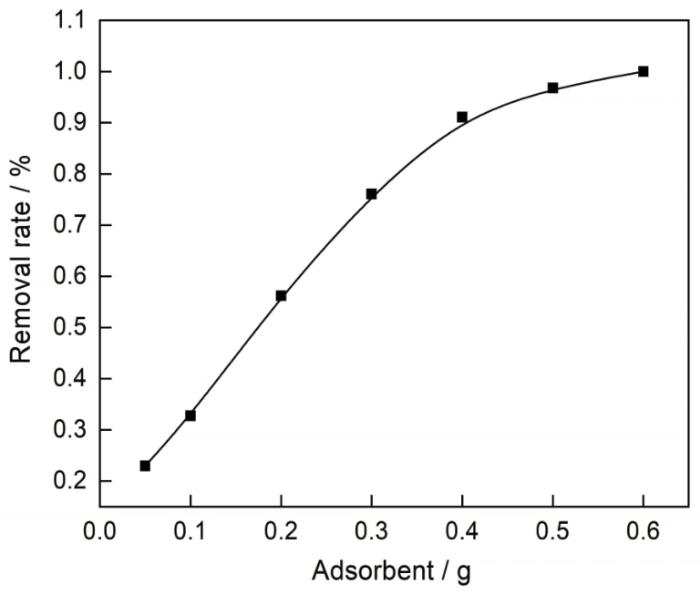
如图10所示,随着NBC800加入量的增加Co2+溶液的平衡浓度越来越低,去除率递增。NBC800的质量为500和600 mg时吸附接近饱和,去除率曲线逐渐平衡,并且接近于1。实验结果表明,NBC800对Co2+有较大的的吸附容量。
图10
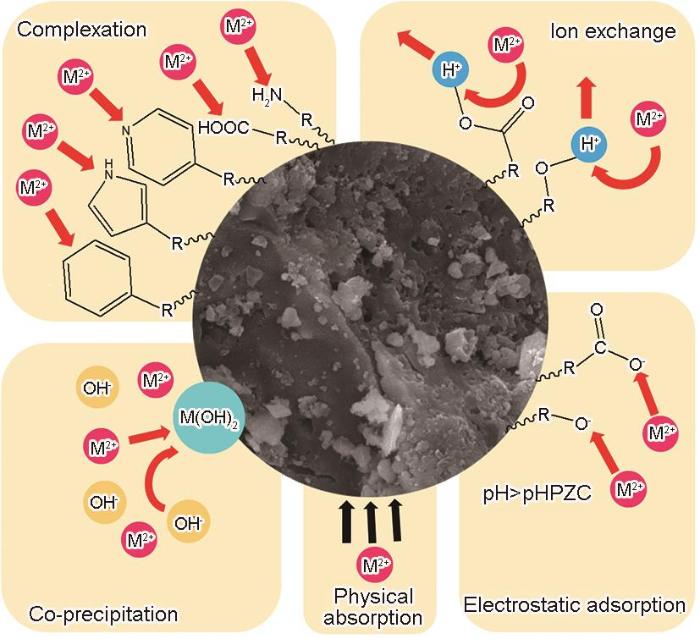
2.10 吸附机理
图11给出了NBCx的吸附机理图,其中M代表Co2+。由表征分析结果和吸附实验数据可知,NBC x 具有高度芳香化和杂环化的结构,表面有丰富的活性官能团。Co2+在NBC x 上的吸附机制可能是离子交换、络合作用、共沉淀和静电吸附等。参与反应的主要活性官能团包括羧基(-COOH)、酯(-COOR)、和氨基(-NH2)基团等。其中羟基和羧酸参与离子交换、静电作用、络合作用等,羧基参与离子交换和络合作用等。在-OH的作用下形成氢氧化物沉淀在NBC x 表面,是可能的吸附方式。FTIR分析结果表明,也可能存在芳香族基团与金属离子的络合;XPS分析结果表明,吡啶和吡咯基团也可能参与了吸附过程。
图11
3 结论
N掺杂生物炭(NBC x )的表面有明显的层块堆积,具有分级多孔结构。在芦荟叶皮与尿素的质量比为2∶1、热解终温为800℃条件下制备的NBC800其比表面积为32 m2·g-1,总孔体积为0.04 cm3·g-1,其中非微孔的比例高达75%。NBC800表面含有丰富的含氧和含氮官能团,N含量和O含量(摩尔分数)高达3.89%和46.35%,可与Co2+发生离子交换、静电吸附、络合作用和共沉淀等反应。NBC800对Co2+的吸附为单分子层吸附,最大理论吸附量高达228.31 mg·g-1,Langmuir等温线模型能很好地描述。拟二级吸附动力学模型表明,这种吸附进行得较快,吸附速率主要由化学吸附控制。
参考文献
Cobalt removal from simulated wastewaters using a novel flow-by fixed bed bio-electrochemical reactor
[J].
Adsorption capabilities of activated carbon derived from detarium microcarpum seeds in removing Co2+ and Pb2+ from wastewater
[J].
Fraction distribution and bioavailability of sediment heavy metals in the environment surrounding MSW landfill: a case study
[J].The aim of this study was to characterize the sediment samples from vicinity of a landfill in Qayen City, Iran. The samples were obtained from four different sampling stations. Sequential extraction was performed via a four-step procedure defined to evaluate the distribution of the element fraction in various samples. In the stations 3 and 4, Cd was found in large quantities during the first extraction F1, accounting for 40.4 and 38.7%, respectively. Pb was primarily presented in F2 of station 1 (approximately 44.80%), station 2 (approximately 41.8%), and station 4 (approximately 37.7%). Moreover, principal component analysis showed that heavy metal fraction in the sediment samples can be explained by two principal components (PCs). PC1 represented Cd, Cr, Ni, and Zn, while PC2 represented Pb and Cu. Pearson correlation coefficient indicated significant correlations in Cu-Pb, Zn-Cu, and Cr-Zn pairings. The present study concluded that the spatial distributions of sediment heavy metals were influenced by MSW landfill.
Potential use of biochar from various waste biomass as biosorbent in Co(II) removal processes
[J].The removal of Co(II) ions from aqueous media was done using three types of biochars obtained from algae waste biomass, mustard waste biomass, and soy waste biomass. The biochar samples were obtained by pyrolysis of waste biomass resulted from biofules production, at relative low temperature (600–650 °C), and this procedure can be considered a suitable alternative to reduce the volume of such waste. FTIR spectra recorded for each type of biochar reveal the presence of several functional groups that can be used as binding sites for Co(II) retention. The batch biosorption experiments were performed as a function of initial Co(II) ions concentration and contact time, at constant solution pH (5.0), sorbent dose (8.0 g/L), and room temperature (25 ± 1 °C). The sorption experiments showed that the Co(II) ions retention reaches the equilibrium in maximum 60 min, and the maximum sorption capacity follows the order: Mustard biochar (MBC—24.21 mg/g) < soy biochar (SBC—19.61 mg/g) < algae biochar (ABC—11.90 mg/g). The modeling of experimental data proves that the retention of Co(II) ions from aqueous solution occurs through electrostatic interactions, and that the sorption process takes place until a monolayer coverage is formed on the outer surface of the biochar. This information is very useful in the design of a suitable desorption system. The desorption results showed that by treating the biochar samples loaded with Co(II) ions with 0.1 mol/L HNO3 solution, over 92% of Co(II) ions are desorbed and can be recovered, and the biochar samples can be used in at least three sorption/desorption cycles. All the experimental observations sustain the potential use of biochar obtained from different types of waste biomass as a promising alternative sorbent for the removal of Co(II) ions from aqueous media.
Mesoporous activated carbon as a green adsorbent for the removal of heavy metals and Congo red: characterization, adsorption kinetics, and isotherm studies
[J].
Purge gas humidity improves microwave-assisted regeneration of polymeric and zeolite adsorbents
[J].
Elucidating the removal of organic micropollutants on biological ion exchange resins
[J].
Recent advances in heavy metal removal by chitosan based adsorbents
[J].
Removal of heavy metals from wastewaters with biochar pyrolyzed from MgAl-layered double hydroxide-coated rice husk: Mechanism and application
[J].
Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption
[J].
A critical review of biochar-based materials for the remediation of heavy metal contaminated environment: applications and practical evaluations
[J].
Synthesis and application of granular activated carbon from biomass waste materials for water treatment: a review
[J].
A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application
[J].
Preparation of high oxygen porous carbon from walnut peel and its adsorption properties for Ni2+
[J].
核桃青皮制备高含氧量多孔炭及其对Ni2+的吸附性能
[J].
Heavy metal ions (lead, cobalt, and nickel) biosorption from aqueous solution onto activated carbon prepared from Citrus limetta leaves
[J].
Synthesis of surfactant modified activated carbon (SMAC) from rice husks as Ni(II) and Cr(VI) adsorbent
[J].
Investigation of heavy metal ions adsorption by magnetically modified aloe vera leaves ash based on equilibrium, kinetic and thermodynamic studies
[J].
Aloe vera waste biomass-based adsorbents for the removal of aquatic pollutants: a review
[J].Aloe vera has been cultivated for many centuries for its beneficial properties, finding application in a wide range of medical and health products. Nowadays, the research has also focused on an alternative use of Aloe vera which is related to environmental applications such as clean water technology/wastewater treatment process. In recent years, biosorption has been shown to be a cost-effective and efficient alternative method for removing various pollutants from wastewater and water. This work provides a comprehensive review on using Aloe vera waste biomass-based sorbents, as well as modified counterparts, for the removal of heavy metals, dyes and other pollutants from aqueous media. The discussed biosorbents have been grouped in five categories based on the treatment of the Aloe vera leaves. Adsorption mechanisms, in addition to the significant factors influencing sorption capability like physical and chemical properties of the adsorbent, initial concentration, initial pH and temperature of the solution, dosage and contact time, have been discussed in detail. Furthermore, the applied equilibrium and kinetic models have been also summarized. The history, taxonomy, botany, and applications of Aloe vera are also presented in brief.Copyright © 2018 Elsevier Ltd. All rights reserved.
Multifunctional binding sites on Nitrogen-doped carboxylated porous carbon for highly efficient adsorption of Pb(II), Hg(II), and Cr(VI) Ions
[J].Heavy metal ions represent one of the most toxic and environmentally harmful pollutants of water sources. This work reports the development of a novel chelating nitrogen-doped carboxylated porous carbon (ND-CPC) adsorbent for the effective removal of the heavy metal ions Pb(II), Hg(II), and Cr(VI) from contaminated and polluted water sources. The ND-CPC adsorbent is designed to combine four different types of nitrogen functional groups (graphitic, pyrrolic, pyridinic, and pyridine oxide) with the carboxylic acid functional groups within a high surface area of 1135 ± 20 m/g of the porous carbon structure. The ND-CPC adsorbent shows exceptionally high adsorption affinity for Pb(II) with a capacity of 721 ± 14 mg/g in addition to high uptake values of 257 ± 5 and 104 ± 2 mg/g for Hg(II) and Cr(VI), respectively. The high adsorption capacity is also coupled with fast kinetics where the equilibrium time required for the 100% removal of Pb(II) from 50 ppb and 10 ppm concentrations is 30 s and 60 min, respectively. Even with the very high concentration of 700 ppm, 74% uptake of Pb(II) is achieved within 90 min. Removal efficiencies of 100% of Pb(II), 96% of Hg(II), 91% of Cu(II), 82% of Zn(II), 25% of Cd(II), and 13% of Ni(II) are achieved from a solution containing 10 ppm concentrations of these ions, thus demonstrating excellent selectivity for Pb(II), Hg(II), and Cu(II) ions. Regeneration of the ND-CPC adsorbent shows excellent desorption efficiencies of 99 and 95% for Pb(II) and Cr(VI) ions, respectively. Because of the fast adsorption kinetics, high removal capacity and excellent regeneration, stability, and reusability, the ND-CPC is proposed as a highly efficient remediation adsorbent for the solid-phase removal of Pb(II), Hg(II), and Cr(VI) from contaminated water.© 2020 American Chemical Society.
Efficiencies and mechanisms of heavy metals adsorption on waste leather-derived high-nitrogen activated carbon
[J].
Effect of surface modification by nitrogen-containing chemicals on morphology and surface characteristics of N-doped pine bark biochars
[J].
Adsorption based recovery of cobalt using chemically modified activated carbon
[J].
The effective removal of heavy metals from water by activated carbon adsorbents of Albizia lebbeck and Melia azedarach seed shells
[J].
Removal of Pb(II) from wastewater using activated carbon prepared from the seeds of Reptonia buxifolia
[J].The potential of activated carbon as a cheap bioadsorbent prepared from Reptonia buxifolia seeds, for the removal of Pb(II) from wastewater was investigated. The morphology and structure of the prepared activated carbon was characterized using different techniques. Adsorption phenomenon was studied by varying the metal ion concentration, contact time, temperature, and pH, in a batch process. The SEM results showed that the thermal treatment significantly altered the topography of synthesized activated carbon due to formation of numerous pores on the surface of the adsorbent. At equilibrium, the Langmuir model gave a better fit to the adsorption isotherm results than the Freundlich model. Kinetics data indicate that equilibrium is established within the first 60 min. The results showed that activated carbon obtained from seeds of R. buxifolia have the potential to be used as alternative economical biosorbent for the removal of heavy metals from wastewater.
Biochar properties and lead (II) adsorption capacity depend on feedstock type, pyrolysis temperature, and steam activation
[J].
Comparative adsorption mechanism of rice straw activated carbon activated with NaOH and KOH
[J].
Adsorption isotherm, kinetic and thermodynamic studies for the removal of Pb(II), Cd(II), Zn(II) and Cu(II) ions from aqueous solutions using Albizia lebbeck pods
[J].
Superefficient removal of heavy metals from wastewater by Mg-Loaded biochars: adsorption characteristics and removal mechanisms
[J].Six types of biochar (BSB, CSB, FSB, CFSB, MSB, and TSB) were prepared from different raw materials by loading magnesium ions (Mg) via an impregnation process. The adsorption kinetics and thermodynamics of heavy metals at high concentrations were analyzed. The adsorption mechanisms were investigated by zeta potential, scanning electron microscopy-energy-dispersive X-ray spectroscopy, Fourier-transform infrared spectroscopy, X-ray diffraction, X-ray photoelectron spectroscopy, and inductively coupled plasma-atomic absorption spectroscopy analyses. The adsorption of heavy metals by BSB, CSB, FSB, CFSB, MSB, and TSB conformed to the Langmuir model and PS-order. The maximum theoretical saturation adsorption capacities for Cd(II), Cu(II), and Pb(II) were 333.33, 238.10, 75.19, 96.15, 66.23, and 185.19 mg·g; 370.37, 294.12, 111.11, 169.49, 84.75, and 217.39 mg·g; and 302.58, 200.00, 61.73, 90.91, 54.47, and 166.67 mg·g, respectively. According to the analysis of the contribution of adsorption, the adsorption process was mainly controlled by cation-π interactions, ion exchange, mineral precipitation, and functional group interactions. Biochars contain ash, functional groups and load a large number of Mg, which can form complexes with metal ions and perform strong ion exchange; therefore, mineral precipitation and cation exchange played dominant roles in the adsorption process. The prepared Mg-loaded biochars presented in this research showed excellent adsorption properties for heavy metals and have great potential for practical application; in particular, BSB had the strongest adsorption capacity for the three heavy metal ions.
Adsorption of heavy metals using activated carbon synthesized from the residues of medicinal herbs
[J].
Unravelling the adsorption disparity mechanism of heavy-metal ions on the biomass-derived hierarchically porous carbon
[J].
A review of recent advances in biomass pyrolysis
[J].
O, N-doped porous biochar by air oxidation for enhancing heavy metal removal: The role of O, N functional groups
[J].
Electrosorption of cadmium and arsenic from wastewaters using nitrogen-doped biochar: mechanism and application
[J].
In situ stabilization of the adsorbed Co2+ and Ni2+ in rice straw biochar based on LDH and its reutilization in the activation of peroxymonosulfate
[J].
Removal of heavy metals from soil with biochar composite: a critical review of the mechanism
[J].
Biomass-derived biochar: from production to application in removing heavy metal-contaminated water
[J].
Hierarchically porous nitrogen-doped carbon materials as efficient adsorbents for removal of heavy metal ions
[J].
Activated carbons produced by pyrolysis of waste potato peels: cobalt ions removal by adsorption
[J].