非晶合金也称金属玻璃(Metallic glass),是金属合金的熔体从高温冷却到熔点以下没有结晶而直接“冷冻”生成的固体[1]。直径大于1 mm的金属玻璃称为块体金属玻璃(Bulk metallic glasses,BMGs)[2]。Zr-Cu-Al-Ni体系块体金属玻璃具有优异的弹性、强度、韧性、耐磨损和高硬度等力学性能,受到了极大的关注。Zr55Al10Ni5Cu30是最具有代表性的合金之一。20世纪90年代初Inoue等[3]制备的Zr55Cu30Al10Ni5块体金属玻璃,其直径达到30 mm,过冷液相区ΔTx=(Tx-Tg)=84 K,Trg可达0.595[4]。金属合金的Trg值越大则其GFA越大,就越容易形成非晶态。Turnbull[5]根据结晶形核动力学用Trg表征合金玻璃形成能力(Glass forming ability,GFA),Trg=Tg/Tm。Lu等[6]发现,用Tg/Tl (即玻璃转变温度Tg与液相线温度Tl的比值)定义的Trg能更好地表征合金的玻璃形成能力。大量实验结果表明,Tg/Tl比Tg/Tm能更直观反映金属玻璃的GFA。
金属玻璃抵抗晶化的能力等价于其GFA的大小,是表征其晶化过程中激活能的一个重要动力学参数。激活能分为等温晶化激活能和连续升温晶化激活能。热激活能越高其热稳定性越高,抗晶化能力越强,对应的GFA越高。激活能(活化能)是一个原子成为某种激活原子团簇的一部分而必须获得的能量[7]。本文用热分析方法测量连续升温条件下的晶化激活能,从而分析了大块金属玻璃的热稳定性。对于Zr55Cu30Al10Ni5大块金属玻璃,Gao等[8]研究了其在连续加热和等温退火过程中从非晶态到过冷液相区结晶的过程,由Tg、Tx和Tp计算出其激活能分别为335.79±37.52,315.52±0.86和315.58±0.48 kJ/mol,表明其具有较高的热稳定性和抗晶化能力,也预示其具有较高的GFA。
为了从局域结构入手揭示块体金属玻璃的成分根源,本文应用董闯等[16, 17]提出的团簇加连接原子模型。这个模型认为,任何一种近程序结构都可看作由团簇加上位于团簇间隙的连接原子组成,表示成统一的团簇式形式为:[团簇](连接原子) x,其中x为连接原子的个数,通常为1或3。应用团簇加连接原子模型,可设计块体金属玻璃的成分。王增睿等[18]在Ti-Cu二元成分的基础上添加合金化元素Zr和Sn,制备出具有高GFA的Ti-Cu-Zr-Sn四元体系BMG成分Ti40Zr10Cu56.94Sn3.06,其临界尺寸可达5 mm;耿遥祥等[19]应用该模型设计和优化了具有高GFA的Fe-B-Si-Nb块体金属玻璃,制备出临界尺寸为2.5 mm的块体金属玻璃Si8.33B16.66Fe62.5-63.33Nb3.33-4.16。应用团簇加连接原子模型分析块体金属玻璃对应晶化相的局域结构,可得到与金属玻璃形成有关的团簇结构进而得到相应的团簇式以指导金属玻璃的成分设计。
在Zr-Cu-Al-Ni体系中,Zr55Cu30Al10Ni5合金因其较高的GFA可作为进一步优化设计块体金属玻璃成分的基础。本文参照Zr55Cu30Al10Ni5合金成分,基于CuZr2和CuZr两个晶化相,应用团簇加连接原子模型设计一种新的块体金属玻璃成分,测试其GFA、激活能、晶化行为及力学性能并与参照合金Zr55Cu30Al10Ni5对比,全面分析这种合金的综合性能。
1 基于团簇加连接原子模型的成分解析及设计
| Phase name CuZr2 | Structure type MoSi2 | Pearson symbol tI6 | Space group I4/mmm | No.139 | ||
|---|---|---|---|---|---|---|
| a=0.32204(4) nm | c=1.11832(6) nm | |||||
| Cu | 2a | 4/mmm | x=0 | y=0 | z =0 | Occ.=1 |
| Zr | 4e | 4mm | x=0 | y=0 | z =0.34 | Occ.=1 |
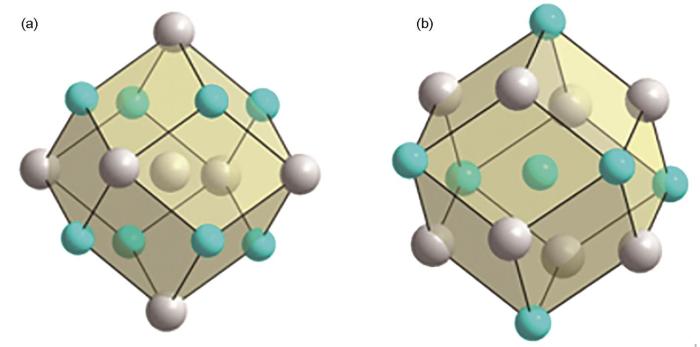
图1
图1
CuZr2相中的两种团簇
Fig.1
Two kinds of clusters in CuZr2 phase (a) [Cu-Zr8Cu4] with Cu atom as the center and (b) [Zr-Cu4Zr9] with Zr atom as the center, with the yellow spheres representing Zr atoms and the red ones representing Cu atoms
| Phase name CuZr | Structure type ClCs | Pearson symbol cP2 | Space group Pm | No.221 | ||
|---|---|---|---|---|---|---|
| a=0.32620(5) nm | ||||||
| Cu | 1a | m | x=0 | y=0 | z=0 | Occ.=1 |
| Zr | 1b | m | x=1/2 | y=1/2 | z=1/2 | Occ.=1 |
图2
图2
CuZr相中的两种团簇
Fig.2
Two kinds of clusters in CuZrphase (a) [Cu-Zr8Cu6] with Cu atom as the center and (b) [Zr-Cu8Zr6] with Zr atom as the center, with the grey spheres representing Zr atoms and the blue ones representing Cu atoms
根据结构遗传性[20],CuZr2相和CuZr相中的所有团簇结构均可作为对应块体金属玻璃的近程序局域结构。在CuZr2相中选择以Cu为心的[Cu-Zr8Cu4],在CuZr相中选择以Cu为中心的[Cu-Zr8Cu6],进而引入双团簇模型[26]并将其按照1∶1混合,连接原子个数可能为2或4或6,双团簇式的总原子个数因此有且仅有三种情况,即30、32、34。作为初步尝试,选择总原子个数为32的双团簇式作为多元块体金属玻璃设计的基础成分,将Zr55Cu30Al10Ni5的成分转换成32原子的成分式,即Zr17.6Cu9.6Al3.2Ni1.6,取整后设计出合金成分Zr17Cu10Al3Ni2,其原子百分比为Zr53.1Cu31.3Al9.4Ni6.3。本文成分式中的原子个数均用下脚标,若数字总和为32则数字表示原子个数,若数字总和为100则数字表示原子百分比。
2 实验验证
实验用原料:纯金属Zr(99.2%)、Cu(99.99%)、Al(99.99%)和Ni(99.9%)。按照上文设计的名义成分配置母合金,用电弧炉熔炼出15 g母合金棒材。为了使各元素混合均匀,每个母合金锭交替翻面重熔4~5次。将合金熔液浇注到铜模具中,铸造出直径为5 mm、长度为46 mm的棒状样品。在熔炼过程中质量损失不超过0.1%。为了减小误差以保证实验对比的科学性,在各合金棒的相同位置截取实验用样品。
用X射线衍射(Cu靶,Kα 辐射,λ=0.15406 nm)和SUPRA 55场发射扫描电镜检测样品是否为完全非晶态。由差示扫描量热仪(Differential scanning calorimetry, DSC, TA Q100)测量非晶合金的玻璃转变温度Tg和晶化温度Tx,用差示热分析仪(Differential thermal analysis, DTA,TA Q600)测量熔点Tm和液相线温度Tl。用连续加热方法测量样品的晶化激活能。
为了了解金属玻璃晶化过程中组织转变,将样品切成直径为5 mm 厚度为1 mm的薄片,在晶化温度附近进行真空热处理。先将热处理炉温升到设置温度。将样品置于石英管中,然后将石英管抽真空至1×10-3 Pa,封管后将其放入设定温度的热处理炉中。因为冷料入炉使炉温降低,重新加热使炉温升到设定的温度,开始计时时间为60 min,这段时间称为保温时间。用X射线衍射、扫描测量处理后样品的密度和硬度。
3 实验结果和讨论
3.1 XRD分析
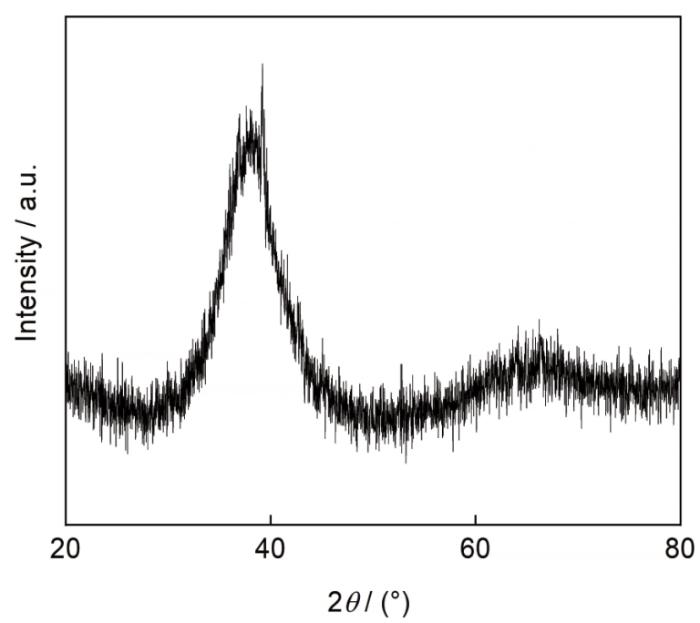
图3
图3
尺寸为5 mm的Zr17Cu10Al3Ni2块体金属玻璃的X射线衍射谱
Fig.3
X-ray diffraction spectrogram of 5 mm Zr17Cu10-Al3Ni2 BMG
3.2 SEM形貌分析
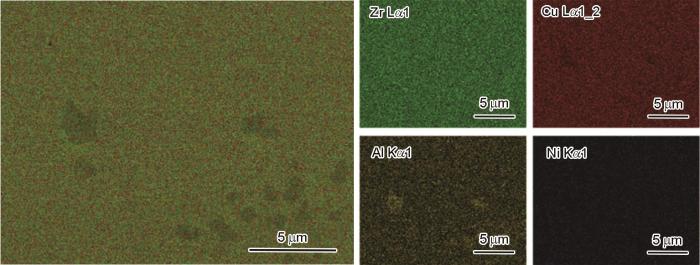
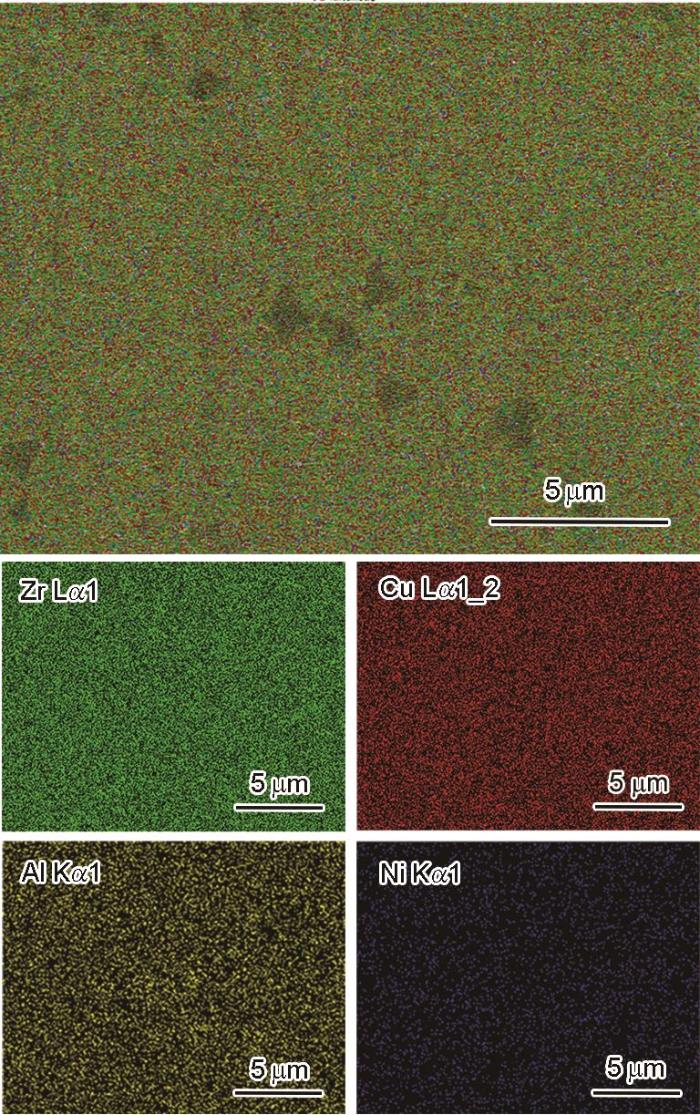
图4给出了SEM面扫描结果,可见金属玻璃中存在少量直径小于0.5 μm级的黑色夹杂物,Zr和Ni元素分布均匀,其中黑色夹杂物Al元素含量较为富集,初步判定析出的晶化相是富Al相。
图4
图4
Zr17Cu10Al3Ni2 BMG能谱面扫分析
Fig.4
EDS area scan analysis of Zr17Cu10Al3Ni2 BMG
从表3可见,金属玻璃样品的成分为Zr51.2Cu33.24-Al8.89Ni6.67,接近于本文设计的成分Zr17Cu10Al3Ni2≈Zr53.1Cu31.3Al9.4Ni6.3。以上的实验结果和分析表明,用吸铸法制备的金属玻璃棒由非晶组成,且元素分布较均匀,是以团簇加连接原子模型为判据设计的合金成分大块金属玻璃。
表3 Zr17Cu10Al3Ni2 面扫描能谱成分
Table 3
| Element | Line type | Concentration / mol·L-1 | Mass fraction/% | Atomic fraction/% |
|---|---|---|---|---|
| Al | K | 1.58 | 3.24 | 8.89 |
| Ni | K | 3.03 | 5.28 | 6.67 |
| Cu | L | 9.02 | 28.48 | 33.24 |
| Zr | L | 27.85 | 63.00 | 51.20 |
| Total | 100.00 | 100.00 |
图5
表4 析出晶化相对应的成分表
Table 4
| Element | Line type | Concentration / mol·L-1 | Mass fraction /% | Atomic fraction /% |
|---|---|---|---|---|
| Al | K | 3.46 | 6.91 | 18.04 |
| Ni | K | 1.86 | 3.31 | 3.98 |
| Cu | L | 8.20 | 25.55 | 28.35 |
| Zr | L | 27.94 | 64.23 | 49.63 |
| Total | 100.00 | 100.00 |
3.3 DSC和DTA分析
可根据玻璃转变温度Tg和晶化温度Tx表征金属玻璃的热稳定性,因为Tg温度越高合金在弛豫期间原子越难以移动;Tx温度越高表明合金在过冷液相区内的组元元素越难扩散和结构重排,而使金属玻璃的热稳定性提高。Inoue等[31]提出用过冷液相区宽度ΔTx(=Tx-Tg)作为表征金属玻璃热稳定性的参数。
图6
图6
Zr17Cu10Al3Ni2块体金属玻璃的DSC、DTA曲线(DSC的升温速率为10 K/min,DTA为恒速度升温)
Fig.6
Curves of DSC (a) and DTA curve (b) of the Zr17Cu10Al3Ni2 bulk metallic glass at the heating rate of 10 K/min and a constant heating rate
文献[32]报道的Zr55Cu30Al10Ni5合金的非晶形成能力强,过冷液相区宽,其玻璃转化温度Tg、晶化温度Tx和过冷液相区ΔTx分别为676、755和79 K。本文在10 K/min条件下得到的实验结果是58 K,产生差异可能与制备方法和原料纯度有关。
3.4 晶化和热激活能分析
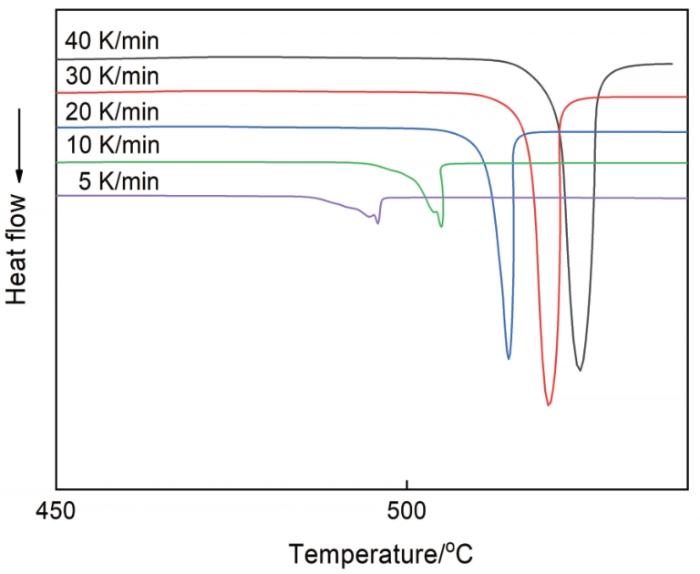
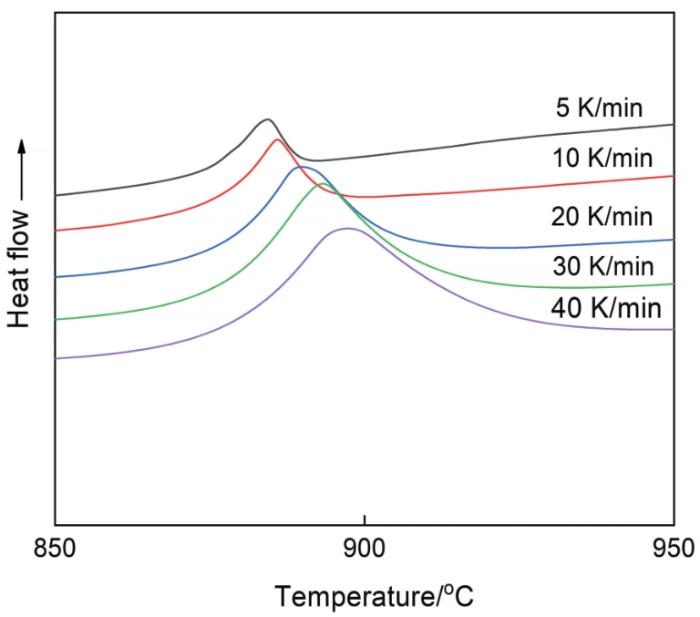
图7
图7
Zr17Cu10Al3Ni2大块金属玻璃在不同升温速率下的DSC曲线
Fig.7
DSC traces of the Zr17Cu10Al3Ni2 bulk metallic glass at different heating rates, where the measured heat flow, in arbitrary unit, points downwards, showing heat-absorption reactions
图8
图8
Zr17Cu10Al3Ni2大块金属玻璃在不同升温速率下的DTA曲线
Fig.8
DTA traces of the Zr17Cu10Al3Ni2 bulk metallic glass at different heating rates, where the measured heat flow, in arbitrary unit, points upwards, showing heat-release reactions
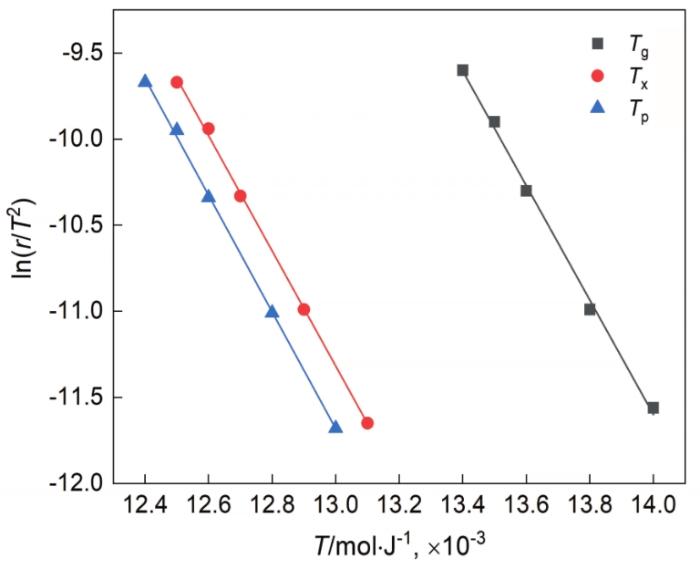
根据Kissinger方程[36],用Tg、Tx和Tp、ln(r/T2)对1/T作图得到如图9所示的直线,其中r为升温速率,T为不同升温速率下的特征点温度值。根据图9中直线的斜率可确定激活能E值,其中Tg温度下的激活能最高为338.793 kJ/mol,Tp温度下的激活能最低为332.328 kJ/mol,Tx温度下的激活能介于Tg与Tp的激活能之间为334.138 kJ/mol。金属玻璃在晶化过程中的激活能,反映晶化过程中所克服的能量势垒。激活能越高,表明需要克服的势垒越大和金属玻璃越稳定。与文献[8]中Zr55Cu30Al10Ni5块体金属玻璃的激活能对比,本文实验中测得的表观激活能都略高,表明Zr17Cu10Al3Ni2大块金属玻璃有较高的热稳定性和较强的抗晶化能力。
图9
图9
Zr17Cu10Al3Ni2大块金属玻璃试样的Kissinger曲线
Fig.9
Kissinger plots of the Zr17Cu10Al3Ni2 bulk metallic glass
表5 不同升温速率下Zr17Cu10Al3Ni2BMG样品的热学参数
Table 5
Heating rates /K·min-1 | Tg /K | Tx /K | ΔTx /K | Tp /K | Tm /K | Tl /K | Trg |
|---|---|---|---|---|---|---|---|
| 5 | 710 | 760 | 50 | 769 | 1138 | 1165 | 0.6 |
| 10 | 715 | 773 | 58 | 778 | 1142 | 1173 | 0.6 |
| 20 | 723 | 784 | 61 | 787 | 1143 | 1193 | 0.6 |
| 30 | 726 | 790 | 64 | 793 | 1147 | 1208 | 0.6 |
| 40 | 727 | 794 | 68 | 798 | 1148 | 1210 | 0.6 |
3.5 晶化退火后的组织
Zr基金属玻璃的过冷液相区为350~500℃。高温下的流变过程伴随着微量的晶化,尤其是在靠近晶化温度Tx晶化现象更严重。热稳定性差的Zr基金属玻璃成分在接近Tx的温度便失去完全非晶的结构,也不再具有金属玻璃的特性。实验结果表明,本文设计的Zr17Cu10Al3Ni2大块金属玻璃具有较高的玻璃形成能力和热稳定性。根据DSC曲线选择在Tx温度进行热处理,因为石英管隔热需使加热温度高于Tx温度(770 K)。在800 K对合金试样进行热处理,鉴定Zr17Cu10Al3Ni2大块金属玻璃的相组成,以检验团簇加连接原子理论的正确性。
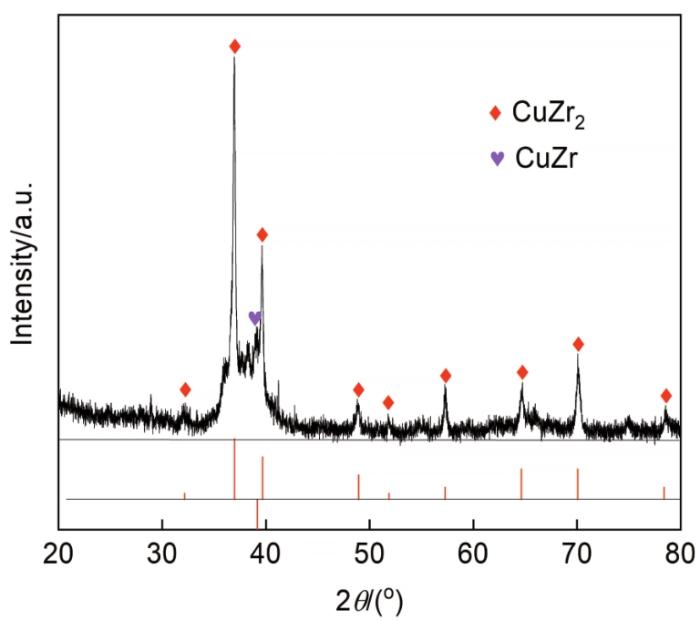
为了确定晶化过程中微观结构的变化,测试了晶化后Zr17Cu10Al3Ni2合金的XRD谱。图10给出了晶化后的Zr17Cu10Al3Ni2大块金属玻璃(在800 K退火60 min,然后随炉冷却)的XRD谱。可以看出,热处理后的样品显示出大部分晶化相。Jade软件分析结果表明,其结晶产物含两个主要相:CuZr2和CuZr, 这个结果与本文在成分设计时参照的晶化相吻合。
图10
图10
在800 K热处理保温1 h后几乎完全晶化的Zr17Cu10Al3Ni2的X射线衍射谱
Fig.10
XRD patterns of Zr17Cu10Al3Ni2 with complete crystallization after heat treatment for 1 hour at 800 K
图11给出了退火态Zr17Cu10Al3Ni2的能谱面扫分析照片。可以看出,晶化后的合金样品中析出了更多尺寸小于0.5 μm的黑色夹杂物,可以确认为析出的晶化相,与铸态扫描结果一致。Zr和Ni元素在析出相中分布均匀,Al元素比较富集,表明是富Al相,Al元素代替Cu元素。还可以看出,析出相有两种,一种是尺寸约为0.5 μm的圆形析出相,另一种的形状不规则。
图11
图11
退火态Zr17Cu10Al3Ni2的能谱面扫分析
Fig.11
EDS area scan analysis of the annealed alloy Zr17Cu10Al3Ni2
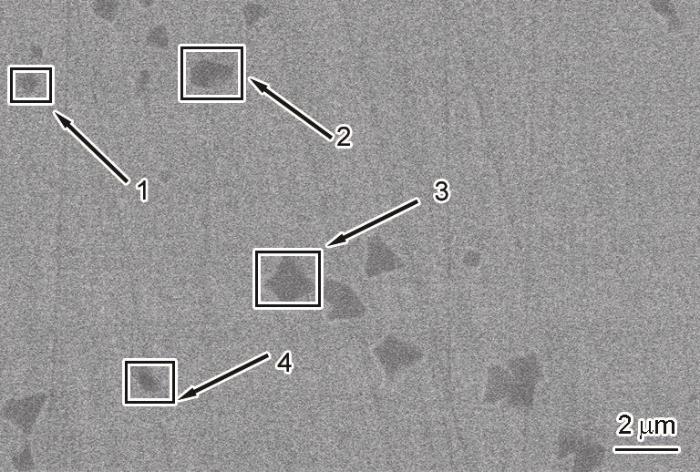
为了确定析出晶相是CuZr2和CuZr并验证团簇加连接原子模型的正确性,对热处理后的Zr17Cu10Al3Ni2大块金属玻璃进行了SEM观察。图12给出了退火态Zr17Cu10Al3Ni2 BMG的微观组织。可以看出,在灰白色金属玻璃试样上存在黑色析出晶化相。EDS能谱分析给出的各检测点Zr17Cu10Al3Ni2的元素构成,列于表6。表6表明,析出相1点的Cu、Zr原子比为Cu(Al、Ni)∶Zr=1∶1.68,析出相2点的Cu、Zr原子比为Cu(Al、Ni)∶Zr=1∶1.57,析出相3点的Cu、Zr原子的比为Cu(Al、Ni)∶Zr=1.02∶1。合金中析出晶化相中Cu与Zr的原子比有两种。在误差范围内,结晶相的Cu、Zr原子比接近1∶1和1∶2,基本符合B2-CuZr和CuZr2。XRD给出的结果也证实,在灰白色金属玻璃试样上的黑色析出结晶相有两种:Zr17Cu10Al3Ni2块体金属玻璃的晶化退火后析出相结构为序列号139、I4/mmm的CuZr2相和221、Pm
图12
图12
退火态Zr17Cu10Al3Ni2 BMG 的微观组织图
Fig.12
Microstructure diagram of the annealed Zr17Cu10Al3Ni2 BMG,in which 1 and 2 representing CuZr2 phases, 3 and 4 representing CuZr phases
表6 试样的定点EDS分析结果
Table 6
| Point | Cu | Zr | Al | Ni |
|---|---|---|---|---|
| 1 | 22.86 | 62.73 | 7.91 | 6.50 |
| 2 | 23.06 | 61.14 | 8.75 | 7.05 |
| 3 | 28.54 | 49.36 | 18.28 | 3.83 |
| 4 | 32.37 | 52.27 | 8.48 | 6.88 |
4 结论
(1) 与合金Zr55Cu30Al10Ni5相比,本文设计的合金成分Zr17Cu10Al3Ni2≈Zr53.1Cu31.3Al9.4Ni6.3具有更高的非晶形成能力。在升温速率为10 K/min的条件下,参照合金Zr55Cu30Al10Ni5的特征参数为Tg=676 K,Tx=755 K,ΔTx=79 K,Trg=0.595;而设计的合金其特征参数为Tg=715 K,Tx=773 K,ΔTx=58 K,Trg=0.6,都略高于参照合金。Zr55Cu30Al10Ni5合金在Tg、Tx和Tp温度下激活能分别为335.79±37.52、315.52±0.86和315.58±0.48 kJ/mol;而设计的合金在Tg、Tx和Tp温度下得到的激活能分别为338.793、334.138和332.328 kJ/mol,均比Zr55Cu30Al10Ni5合金的激活能高。表明本文设计的合金Zr17Cu10Al3Ni2具有更大的GFA。
(2) Zr17Cu10Al3Ni2块体金属玻璃的晶化相为CuZr2相和CuZr相,与应用团簇加连接原子模型设计合金成分的初始假设一致。
参考文献
A brief history of metallic glasses
[J].
金属玻璃研究简史
[J].
Thermodynamic considerations on the formation and stability of metallic glasses
[J].
Fabrication of bulk glassy Zr55Al10Ni5Cu30 alloy of 30 mm in diameter by a suction casting method
[J].
The glass-forming ability and die casting performance of Zr-based bulk metallic glasses
[J].
Zr基块体非晶合金玻璃形成能力与压铸成型性能研究
[J].
Under what conditions can a glass be formed?
[J].
A new glass-forming ability criterion for bulk metallic glasses
[J].
Thermal analysis kinetics and its application in bulk amorphous alloy
[J].
热分析动力学及其在大块非晶合金研究中的应用
[J].
Crystallization behavior of ZrAlNiCu bulk metallic glass with wide supercooled liquid region
[J].
In-situ detection of the onset crystallisation of Zr55Cu30Al10Ni5 from the bulk glass and the liquid states using synchrotron radiation
[J].
Isothermal nanocrystallization of Zr55Al10Ni5-Cu30 bulk amorphous alloy near the glass transition temperature
[J].
Zr55Al10Ni5Cu30块状非晶合金靠近玻璃转变点的等温纳米晶化
[J].
Phases formed during crystallization of Zr55Al10Ni5Cu30 metallic glass containing oxygen
[J]. J.
Crystallization behavior of Zr55Al10Ni5Cu30 amorphous alloys with different morphologies and thermal history conditions
[J].
不同形态和热历史条件下Zr55Al10Ni5Cu30非晶合金的晶化行为
[J].
Effect of differential thermal analysis experimental condition on crystallization behavior of bulk amorphous alloy Zr55Cu30Al10Ni5
[J].
差热分析实验条件对大块非晶合金Zr55Cu30Al10Ni5晶化行为的影响
[J].
Cu-Zr (copper-zirconium)
[J].
Chemical short-range order in liquid and amorphous Cu66Ti34 alloys
[J].
From clusters to phase diagrams: composition rules of quasicrystals and bulk metallic glasses
[J].
Review of structural models for the compositional interpretation of metallic glasses
[J].
Composition design procedures of Ti-based bulk metallic glasses using the cluster-plus-glue-atom model
[J].
Composition design and optimization of Fe-B-Si-Nb bulk amorphous alloys
[J].Fe-based amorphous alloys are well known for their good magnetic properties including ultrahigh saturation magnetization, low coercive force, high magnetic permeability and low core loss. But these alloys were only prepared into ribbon form in early times due to their insufficient glass-forming abilities (GFAs). The present work focuses on the design of Fe-B-Si-Nb bulk metallic glasses with good soft magnetic properties and high glass-forming ability. Glass formation in Fe-B system is first considered with cluster-plus-glue-atom model. A basic composition formula [B-B2Fe8]Fe is proposed as the framework for multi-component alloy design. Considering the structural stability of the model glass, Si and Nb are introduced to the [B-B2Fe8] cluster to replace the center B and shell Fe atoms, from which a series of Fe-B-Si-Nb alloys with composition formulas [Si-B2Fe8-xNbx]Fe (x=0.1~1.2) are derived. Copper mold casting experiments revealed that bulk glass alloys with a critical diameter (dc) exceeding 1.0 mm are readily obtained with the Nb content range of x=0.2~1.2, the largest dc (about 2.5 mm) appears in the vicinity of x=0.4~0.5. Considering the local packing efficiency of Fe-B-Si-Nb glass model structure, another series alloy compositions, namely, [(Si1-yBy)-B2Fe8-xNbx]Fe is reached by increasing Nb and decreasing Si simultaneously in [Si-B2Fe7.6Nb0.4]Fe basal glass alloys. The experimental results show that bulk glass alloys with dc=2.5 mm are available over a wide range of compositions from (x=0.5, y=0.05) to (x=0.9, y=0.25). Excellent magnetic softness with high saturation magnetizations (Bs=1.14~1.46 T) and low coercive forces (Hc=1.6~6.7 A/m) is found in the [Si-B2Fe8-xNbx]Fe (x=0.2~0.6) series glass alloys. A high fracture strength of 4220 MPa with a plasticity of 0.5% is observed in the [(Si0.95B0.05)-B2Fe7.5Nb0.5]Fe bulk glass alloy.
Fe-B-Si-Nb块体非晶合金的成分设计与优化
[J].利用“团簇加连接原子”模型设计和优化具有高形成能力的Fe-B-Si-Nb块体非晶合金. 以源于Fe-B二元共晶相的Fe<sub>2</sub>B局域结构为基础, 结合电子浓度判据, 构建Fe-B二元理想非晶团簇式[B-B<sub>2</sub>Fe<sub>8</sub>]Fe; 考虑到原子间混合焓的大小, 选择Si和Nb原子分别替代[B-B<sub>2</sub>Fe<sub>8</sub>]团簇的中心原子B和壳层原子Fe, 得到[Si-B<sub>2</sub>Fe<sub>8-</sub><sub>x</sub>Nb<sub>x</sub>]Fe系列四元非晶成分. 结果表明, [Si-B<sub>2</sub>Fe<sub>8-</sub><sub>x</sub>Nb<sub>x</sub>]Fe团簇式在x=0.2~1.2成分处均可形成块体非晶合金, 其中在x=0.4~0.5的成分区间内均可形成临界尺寸为2.5 mm的块体非晶合金. 考虑到原子半径的大小, 鉴于增加Nb的同时降低Si的含量可维持[Si-B<sub>2</sub>Fe<sub>7.6</sub>Nb<sub>0.4</sub>]Fe非晶团簇结构的拓扑密堆性, 由此得到另一系列[(Si<sub>1-</sub><sub>y</sub>B<sub>y</sub>)-B<sub>2</sub>Fe<sub>8-</sub><sub>x</sub>Nb<sub>x</sub>]Fe团簇式成分. 结果表明, 在(x=0.5, y=0.05)~(x=0.9, y=0.25)成分区间内均可通过Cu模铸造法获得直径为2.5 mm的块体非晶. 新设计获得的Fe-B-Si-Nb块体非晶合金具有优良的室温软磁性能和力学性能, 其中[Si-B<sub>2</sub>Fe<sub>8-</sub><sub>x</sub>Nb<sub>x</sub>]Fe (x=0.2~0.6)非晶合金的饱和磁化强度为1.14~1.46 T, 矫顽力为1.6~6.7 A/m; [(Si<sub>0.95</sub>B<sub>0.05</sub>)-B<sub>2</sub>Fe<sub>7.5</sub>Nb<sub>0.5</sub>]Fe块体非晶合金的室温压缩断裂强度达4220 MPa, 塑性形变约为0.5%.
Spherical periodicity as structural homology of crystalline and amorphous states
[J].
Characteristics of cluster formulas for binary bulk metallic glasses
[J].
Pearson's handbook desk edition
[J].
Crystallization of metallic glasses
[A]. GüntherodtHJ, BeckH.
ChemInform abstract: the alloy system copper-zirconium. Part 1. phase diagram and structural relations
[J].
High-accuracy X-ray diffraction analysis of phase evolution sequence during devitrification of Cu50Zr50 metallic glass
[J].
Composition formulas of binary eutectics
[J].The present paper addresses the long-standing composition puzzle of eutectic points by introducing a new structural tool for the description of short-range-order structural unit, the cluster-plus-glueatom model. In this model, any structure is dissociated into a 1st-neighbor cluster and a few glue atoms between the clusters, expressed by a cluster formula clusterglue(x). This model is applied here to establish the structural model for eutectic liquids, assuming that a eutectic liquid consist of two subunits issued from the relevant eutectic phases, each being expressed by the cluster formula for ideal metallic glasses, i.e., cluster(glue atom)(1 or 3). A structural unit is then composed of two clusters from the relevant eutectic phases plus 2, 4, or 6 glue atoms. Such a dual cluster formulism is well validated in all boron-containing (except those located by the extreme phase diagram ends) and in some commonly-encountered binary eutectics, within accuracies below 1 at.%. The dual cluster formulas vary extensively and are rarely identical even for eutectics of close compositions. They are generally formed with two distinctly different cluster types, with special cluster matching rules such as cuboctahedron plus capped trigonal prism and rhombidodecahedron plus octahedral antiprism.
Preparation and mechanical properties of Zr-based bulk nanocrystalline alloys containing compound and amorphous phases
[J].
Stabilization of metallic supercooled liquid and bulk amorphous alloys
[J].
Fabrication of bulky Zr-based glassy alloys by suction casting into copper mold
[J].
Workability of the supercooled liquid in the Zr65Al10Ni10Cu15 bulk metallic glass
[J].
Zr-Al-Ni amorphous alloys with high glass transition temperature and significant supercooled liquid region
[J].
Impact fracture energy of bulk amorphous Zr55Al10Cu30Ni5 alloy
[J].
Structural amorphous steels
[J].
Synthesis of high strength bulk amorphous Zr-Al-Ni-Cu-Ag alloys with a nanoscale secondary phase
[J].
Density, thermal stability and mechanical properties of Zr-Ti-Al-Cu-Ni bulk amorphous alloys with high Al plus Ti concentrations
[J].