温度响应性纳米纤维是一种用静电纺丝工艺制备的智能纳米纤维,其平均直径小于1000 nm[1]。温度是容易改变和控制的环境条件,因此这类智能纳米纤维在生物医学、传感与检测、分离与纯化、自动化等领域有广阔的应用前景[2,3]。主要的温度响应性纳米纤维有三种:第一种,是在聚合物纳米纤维表面接枝温度响应性聚合物制备的纳米纤维[4],可响应环境温度变化改变表面的亲疏水性。这种温度响应性纳米纤维在油水分离等方面有潜在的应用价值[5];第二种,是以温度响应性聚合物为成纤聚合物制备的纳米纤维[6],响应环境温度变化时不仅改变表面的亲疏水性,在水中还发生收缩或溶胀,可应用在生物医学领域[7,8];第三种温度响应性纳米纤维,在环境温度变化发生形态改变,在智能微驱动器等方面有潜在的应用前景[9]。对前两种温度响应性纳米纤维研究的较多,对后一种的研究较少[9]。他们采用并列静电纺丝工艺制备了由聚(N-异丙基丙烯酰胺)(PNIPAM)和甲基丙烯酸酯类共聚物(poly(MMA-co-BMA))构成的双面纳米纤维。这种纳米纤维在低温(0℃)下处于卷曲形态,在40℃变为伸直形态,这种温度响应具有可逆性。目前低温下处于伸直形态、升温后变为卷曲形态的温度响应性纳米纤维,还未见报道。这种智能纳米纤维不仅在智能微驱动器等方面有更高的潜在应用价值,还可利用其卷曲形成的纤维之间的缠结作用制备可注射水凝胶以取代传统的可注射化学交联水凝胶[10]。传统的可注射化学交联水凝胶有毒和韧性差,且其稳定性不高和拉伸强度较低[11]。鉴于此,本文采用自由基共聚反应合成分子侧链带可引发光交联反应的二苯基甲酮基团的温度响应性聚合物(poly(NIPAM-co-ABP), PNA)和用甲基丙烯酸缩水甘油酯改性聚乙烯醇(PVA),制备分子侧链带可光交联的甲基丙烯酰氧基团的PVA(MPVA),然后以PNA和MPVA为成纤聚合物分别配制纺丝液采用并列静电纺丝和紫外(UV)光辐照相结合的工艺制备一种具有双面结构的纳米纤维,研究纺丝工艺条件对双面纳米纤维产率及其平均直径的影响以及在水中的稳定性温度响应性。
1 实验方法
1.1 实验用原料
N-异丙基丙烯酰胺(NIPAM)(纯度99%),使用前用体积比为1∶1的正己烷/甲苯混合溶剂进行重结晶纯化。聚乙烯醇(PVA)(聚合度为1700,醇解度为88%)、甲基丙烯酸缩水甘油酯(GMA)(分析纯)、对二甲氨基吡啶(DMAP)(分析纯)、光引发剂2959(分析纯)、4-丙烯酰氧基二苯甲酮(ABP)(分析纯)、偶氮二异丁氰(AIBN)(分析纯)、1,4-二氧六环(分析纯)和二甲基亚砜(DMSO)(分析纯)等化学药品或化学试剂使用前均未做纯化处理。去离子水。
1.2 NIPAM和ABP共聚合成PNA
将5 g纯化过的NIPAM加到装有30 mL 1,4-二氧六环的三口烧瓶中,再依次加入0.073 g AIBN和适量的ABP。搅拌使其溶解后通氮气30 min,以除去烧瓶内的氧气,然后加热到75℃保温反应6 h。反应结束后将冷却至室温的反应溶液倒入无水乙醚中,将反应溶液过滤使沉淀物分离出来。再用1,4-二氧六环和无水乙醚将沉淀物反复“溶解-沉淀”三次,以除去未反应的单体和引发剂。最后将沉淀物放入真空干燥箱中在室温下抽真空12 h,即得到共聚物PNA。
改变合成配方中ABP与NIPAM的摩尔比(1.0%、1.5%、2.0%,摩尔分数),合成出三批PNA样品,其编号分别为PNA-1、PNA-2和PNA-3。
1.3 用GMA改性PVA制备MPVA
将6 g的PVA 加到装有90 mL DMSO的三口烧瓶中并加热搅拌使其溶解,然后冷却至室温。再加入0.132 g的DMAP 和0.28 g的GMA并搅拌使DMAP完全溶解,然后加热至60℃保温反应6 h。反应结束后将聚合物溶液冷却至室温,在搅拌的同时缓慢加入到丙酮中生成絮状沉淀物。倒掉上层清液后再用丙酮两次洗涤沉淀物,最后将沉淀物放在真空干燥箱中在室温下抽真空干燥12 h,即得到MPVA。
1.4 MPVA/PNA双面纳米纤维的制备
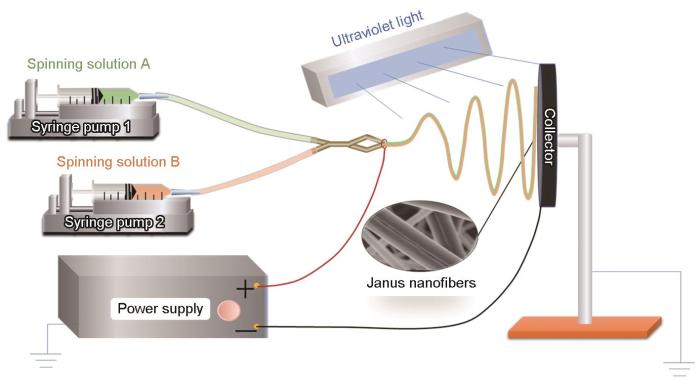
将制得的PNA与MPVA分别溶解在水中,配制成浓度均为18%(质量分数)的纺丝液A和纺丝液B。在纺丝液B中加入质量为MPVA的1%的光引发剂2959,然后用并列静电纺丝装置(图1)在UV光辐照下制备双面纳米纤维(Janus nanofibers)。在静电纺丝过程中使用波长为365 nm、功率为8 W的紫外灯(WFH-203型)辐照收集器,紫外灯管与收集器之间的距离为20 cm。纺丝温度:(24±1)℃,相对湿度:(65±5)%。将收集到的纳米纤维放在温度为105℃真空干燥箱中抽真空干燥12 h,以除去残留的水分。
图1
图1
制备MPVA/PNA双面纳米纤维的装置示意图
Fig.1
Schematic diagram of the apparatus for preparing MPVA/PNA Janus nanofibers
1.5 性能表征
1.5.1 MPVA和PNA的表征
用核磁共振波谱仪(NMR)(Avance600型)分别测定MPVA和PNA的1H NMR谱以及MPVA的13C NMR谱,溶剂均为氘代二甲基亚砜(d6-DMSO)。根据PNA的1H NMR谱中相关谱峰的积分面积计算其分子链中ABP单元与NIPAM单元的摩尔比(ABP/NIPAM(%,摩尔分数)),根据MPVA的1H NMR谱中相关谱峰的积分面积计算其分子侧链上接枝的丙烯酰氧基团占所有重复单元的摩尔比。
用可变温的紫外-可见光谱仪(UV-vis)(Lambda 35型)测试不同温度(T)下、浓度约为1.0 %的PNA水溶液在500 nm处的吸光度(A),测试温度范围为20~40℃,然后做A-T关系曲线的微分曲线(dA/dT~T),其峰顶对应的温度即为PNA的最低临界溶解温度(LCST)。
1.5.2 MPVA/PNA双面纳米纤维的表征
将收集到的纳米纤维膜连同铝箔剪成2 mm×2 mm小方片,用导电胶粘贴到样品台上,喷金处理后用扫描电镜(SEM)(S-4800型i)表征纳米纤维的双面结构。随机观察同一个样品的多张SEM照片中50根纤维是否具有双面结构,统计出其中双面纳米纤维的含量,以此作为该批样品的双面纳米纤维产率。使用ImageJ软件随机测量同一个样品的多张SEM照片中50根双面纳米纤维的直径,计算其平均值和标准偏差。
在静电纺丝过程中将铜网贴在铝箔上收集少量的纳米纤维,用透射电镜(TEM)(JEM-2100)表征纳米纤维的双面结构,以与SEM观察到的双面结构相互印证。
参考文献[2]中的方法,用高速均质乳化机(FJ200-SH型)将浸在叔丁醇中的MPVA/PNA双面纳米纤维切短,冷冻干燥后得到干态的短纤维。将短纤维分散在d6-DMSO中,充分溶胀后测定其1H NMR谱,以表征其中形成的化学交联结构。
1.5.3 MPVA/PNA双面纳米纤维的稳定性
将充分干燥的纳米纤维膜从铝箔上刮下并称重(Wo),然后将其浸泡到去离子水中在振荡摇床上以300 r/min的频率振荡2 h。然后用纱布过滤,将滤渣放入温度为105℃的真空干燥箱中抽真空干燥24 h。称量干燥后滤渣的质量(W'),振荡前后样品的失重率(WLR)为
其值即为样品中未形成交联结构的、可溶于水的聚合物的质量含量,以此评价MPVA/PNA双面纳米纤维在水中的稳定性。每个样品测试三次,取其测试结果的平均值并计算标准偏差。
参考文献[12]中的方法,将收集到的双面纳米纤维膜连同铝箔一起放在水中浸泡6 h,取出后将其充分干燥,采用上述类似方法用SEM观察,比较样品浸泡前后双面纳米纤维的含量和直径的变化,以此判断MPVA/PNA双面纳米纤维中两种组分之间结合的稳定性。
1.5.4 MPVA/PNA双面纳米纤维的温度响应性
将上述冷冻干燥得到的短纤维放在水中,用超声振荡仪将其充分分散后用可控温度的热台偏光显微镜(BX51-P型)观察分散液中短纤维在不同温度下的形态并拍照,以此判定制得的MPVA/PNA双面纳米纤维的升温可弯曲的温度响应性。温度变化范围为25~35℃。
2 结果和讨论
2.1 PVA的改性
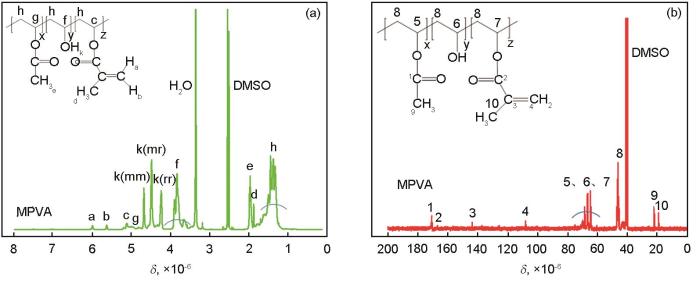
PVA是一种具有良好水溶性和生物相容性的聚合物,制备出的纳米纤维在生物医学领域有良好的应用前景[13]。为了使PVA构成的双面纳米纤维稳定地存在于水中,本文以DMAP为催化剂用GMA对PVA改性,以使PVA分子侧链接枝上可光交联的甲基丙烯酰氧基团。图2a和b给出了改性并纯化的产物MPVA的1H NMR谱和13C NMR谱。本文使用易溶于水的、醇解度为88%的PVA制备MPVA,因此图2a和b中都有未醇解的乙酸乙烯酯单元的氢原子和碳原子特征峰,见图中结构式上的标示。图2a中化学位移分别为4.2×10-6、4.5×10-6、4.7×10-6的三个峰分别对应MPVA分子侧链上未与GMA反应的、处于三种不同立体序列结构(mm,mr,rr)中的羟基上的氢原子[14],相应地由三个峰组成的f峰是与这三种羟基相连的次甲基(CH)上的氢原子,其中碳原子与图2b中的5峰对应。图2a中a、b、c、d四个峰和图2b中2、3、4、10四个峰都是PVA和GMA反应后形成的谱峰,分别归属于与主链上次甲基相连的丙烯酰氧基团上的氢原子和碳原子,见图中结构式上的标示。两个谱中均未出现GMA与PVA侧链羟基发生开环反应形成的-O-CH2-CH(OH)-CH2-O-结构片段上氢原子和碳原子的谱峰,尤其是图2a中c峰的出现,表明用DMAP作催化剂与用N,N,N’N’-四甲基乙二胺作催化剂相同[15],GMA与PVA侧链上羟基之间发生的是酯交换反应,而不是有些文献推测的开环反应[16]。图2中推断出的结构式表明,MPVA是由三种重复单元构成的共聚物,其中甲基丙烯酸乙烯酯单元所占的摩尔分数(%)代表了MPVA分子侧链接枝的可光交联的甲基丙烯酰氧基团的含量。根据图2a中相对独立的a峰、f峰和g峰的积分面积,可计算出其含量约为1.05%。
图2
图2
MPVA的1H NMR谱和MPVA的13C NMR谱
Fig.2
1H NMR spectrum of MPVA (a) and 13C NMR spectrum of MPVA (b)
2.2 PNA的合成
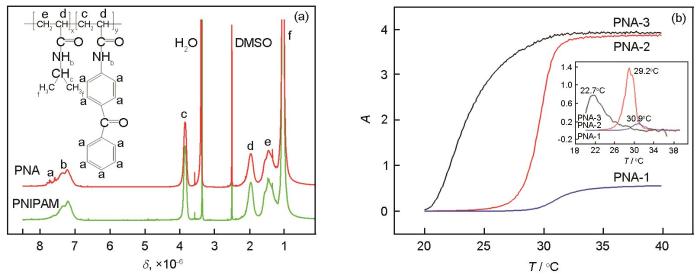
PNIPAM的最低临界溶解温度(LCST)约为32℃,与人的体温接近,因此可用于生物医学领域[17]。本文用含碳碳双键基团和二苯甲酮基团的ABP做为共聚单体合成PNIPAM的衍生物PNA,图3a给出了PNA-2和PNIPAM的1H NMR谱,PNA-1和PNA-3的1H NMR谱图与PNA-2的类似。可以看出,除化学位移和峰形相同的其它谱峰外,PNA-2在化学位移位于7.0×10-6~8.0×10-6之间的宽峰b上出现了一系列尖峰a,应该归属于ABP单元中二苯甲酮基团上的氢原子。根据图3a中a峰b峰的积分面积总和Aa+b和c峰的积分面积Ac,PNA共聚物中ABP单元与NIPAM单元之间的摩尔百分比为
图3
图3
PNA-2和PNIPAM的1H NMR谱、三批PNA的水溶液在500 nm处的吸光度与温度的关系(A-T)及其微分曲线(dA/dT-T)(插图)
Fig.3
1H NMR spectra of PNA-2 and PNIPAM (a); the relationship curves of the absorbance of the aqueous solutions of three batches of PNA at 500 nm vs. temperature (A-T) and their differential curves (dA/dT-T) (b)
合成的三批PNA的R值计算结果列于表1。可以看出,用NMR测得的PNA共聚物中ABP单元与NIPAM单元的之间摩尔百分比都比合成配方中两种单体的摩尔百分比低,表明ABP的反应活性比NIPAM的低。图3b给出了浓度(质量分数)约为1.0%的三批PNA水溶液在500 nm处的吸光度(A)随温度变化的关系曲线,插图为其微分曲线。由此得到的LCST也列于表1。从表1可以看出,随着PNA分子链中ABP单元含量的提高PNA的LCST逐渐减小。其原因是,ABP单元具有较强的疏水性,其含量的提高增强了PNA分子链的疏水性,使其发生相转变的温度也随之降低[18]。因此,改变合成配方中ABP/NIPAM的摩尔百分比可调节PNA的LCST。
表1 三批PNA分子链中ABP/NIPAM摩尔百分比和LCST测试结果
Table 1
| PNA Sample | Feeding percentage of ABP/NIPAM / %, mole fraction | Tested percentage of ABP/NIPAM / %, mole fraction | LCST / ℃ |
|---|---|---|---|
| PNA-1 | 1.0 | 0.8 | 30.9 |
| PNA-2 | 1.5 | 1.1 | 29.2 |
| PNA-3 | 2.0 | 1.7 | 22.7 |
2.3 MPVA/PNA双面纳米纤维的制备与表征
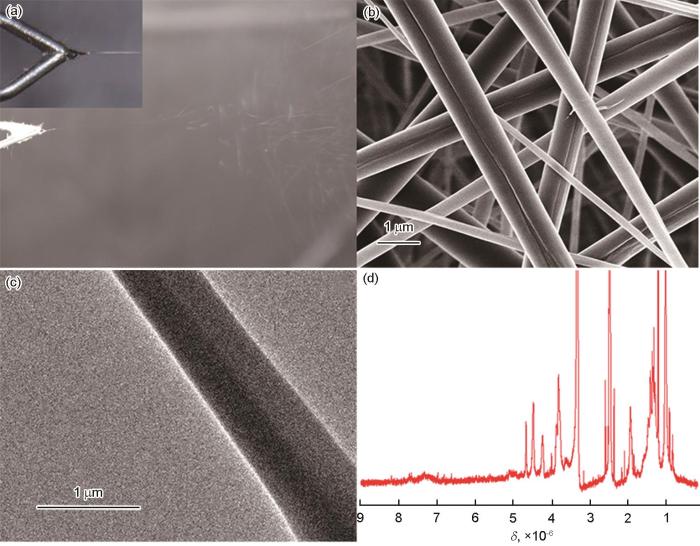
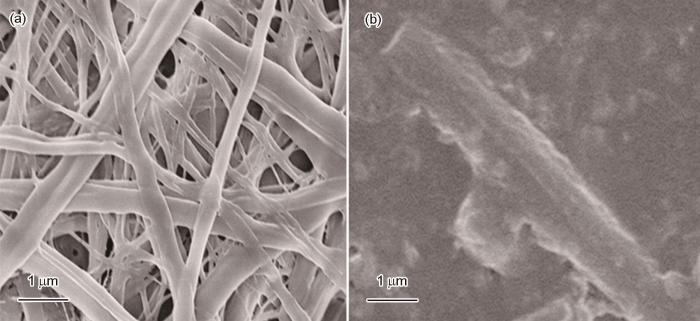
用MPVA和PNA-2为成纤聚合物,用如图1所示的装置制备MPVA/PNA双面纳米纤维。使用的喷丝头中两根输送纺丝液的管子之间有45°夹角,因此两种纺丝液在喷丝头出口处汇合时产生一定的对冲[20],有利于克服其被电场极化后带相同电荷产生的静电排斥力,形成只有一个带电的泰勒锥。泰勒锥在电场应力的作用下喷射出由两种纺丝液平行排列且相互结合的直线射流,如图4a中的插图所示。射流直线行进随后鞭动行进到达收集器,随着水的挥发逐渐固化成双面纳米纤维,如图4a所示。到达收集器的双面纳米纤维在UV光辐照下形成交联结构。图4b给出了纳米纤维的典型SEM照片。可以看出,大多数纤维是由两根纤维结合在一起、平均直径小于1000 nm的双面纳米纤维。只有少数直径较小的非双面纳米纤维。图4c给出了制得的纳米纤维的典型TEM照片,可见纤维轴的两边有明显的衬度,其原因是,双面纳米纤维是由化学组成和直径都不完全相同的两根纤维构成,其间存在所谓的“质厚衬度”,由此进一步证明制得的纳米纤维具有双面结构。图4d给出了双面纳米纤维的1H NMR谱。与图2a对比可见,化学位移为6.1×10-6和5.6×10-6的a峰和b峰消失了,表明到达收集器的双面纳米纤维在波长为365 nm的UV光辐照下其中光引发剂2959产生的自由基可能引发MPVA分子链上的甲基丙烯酰氧基团发生了聚合反应,使MPVA构成的一面形成了交联结构。与图3a对比可见,化学位移位在7.0×10-6~8.0×10-6的一系列尖峰a向低场方向移动。其原因是,PNA分子侧链上的二苯甲酮基团在相同波长的UV光辐照下激发为三线态,夺取其它PNA分子链上的氢原子形成了二苯甲醇基团。苯环与羰基之间的共轭作用消失,使与其相连的氢原子的化学位移增大。夺取氢原子后产生的PNA分子链自由基之间发生偶合使反应终止,使双面纳米纤维中由PNA构成的一面也形成了交联结构。分别用PNA-1和PNA-3取代PNA-2制备的MPVA/PNA双面纳米纤维,SEM和TEM表征结果表明PNA中ABP的含量不影响纤维的形态结构。
图4
图4
用并列静电纺丝制备的MPVA/PNA双面纳米纤维的外观照片、MPVA/PNA双面纳米纤维的SEM照片、MPVA/PNA双面纳米纤维的TEM照片以及MPVA/PNA双面纳米纤维的1H NMR谱
Fig.4
Appearance images of preparing MPVA/PNA Janus nanofibers by side-by-side electrospinning (a); SEM image of the prepared MPVA/PNA Janus nanofibers (b); TEM image of the prepared MPVA/PNA Janus nanofiber (c) and 1H NMR spectrum of MPVA/PNA Janus nanofibers (d)
2.4 纺丝工艺条件对MPVA/PNA双面纳米纤维直径和产率的影响
在并列静电纺丝制得的纳米纤维中,双面纳米纤维的含量即产率不仅与喷丝头结构有关,而且与纺丝工艺条件有关[19]。两种纺丝液的流速相同,有助于形成双面纳米纤维[21]。因此,本文在保持两种纺丝液流速相等的情况下同时改变其流速,或改变纺丝电压与接收距离。以PNA-2和MPVA为成纤聚合物,用上述工艺制备了九批纤维样品,如表2所示。图5给出了这些纤维样品的SEM照片,每一批纤维样品的双面纳米纤维产率和平均直径统计结果列于表2。从表2可见,随着两种纺丝液流速的提高双面纳米纤维的产率相差不大,均高于90%,而纤维的平均直径逐渐增大。其原因是,纺丝液流速的提高使电场中被拉伸的聚合物量增大,从而形成直径更大的双面纳米纤维。但是,如果两种纺丝液流速过高,可能因无法及时被电场应力拉伸而堆积导致相互之间静电排斥力增大,使形成的双面纳米纤维上存在有空洞的串珠,见JNF-3的SEM照片。因此,两种纺丝液的流速不宜超过0.3 mL/h。从表2还可见,在保持其它工艺条件不变的情况下,随着纺丝电压的增大双面纳米纤维的平均直径逐渐减小。其原因是,电压增大导致电场强度增加,两种纺丝液在行进过程中受到电场拉伸的应力也相应增加。纺丝电压为20 kV和22 kV制得的双面纳米纤维的产率高于90%,而电压升高到24 kV双面纳米纤维的产率急剧下降到54%。其原因可能是,喷丝头出口处的两种纺丝液在强电场的极化作用下产生的电荷量大,它们之间的粘性力和表面张力难以克服静电排斥力而形成了两个泰勒锥,然后被拉伸成两个只由一种聚合物构成的纳米纤维。因此,纺丝电压不宜超过22 kV。另外,随着接收距离的增加双面纳米纤维的平均直径逐渐增大,但是产率的变化不大,均高于90%。其原因是,接收距离增加使电场强度降低,两种纺丝液及其形成的双面纤维受到的电场应力降低,使纤维变粗。因此,选择合适的纺丝液流速和纺丝电压以确保双面纳米纤维具有较高的产率后,可在一定范围内改变接收距离以调节双面纳米纤维的粗细。
表2 用不同并列静电纺丝工艺制备的MPVA/PNA双面纳米纤维的产率和平均直径
Table 2
| Sample | Flow rate /mL·h-1 | Spinning voltage/kV | Collection distance/cm | Average width of JNF/nm | Productivity of JNF/% |
|---|---|---|---|---|---|
| JNF-1 | 0.2 | 22 | 15 | 588±83 | 92 |
| JNF-2 | 0.3 | 22 | 15 | 759±53 | 96 |
| JNF-3 | 0.4 | 22 | 15 | / | / |
| JNF-4 | 0.3 | 20 | 15 | 842±69 | 90 |
| JNF-5 | 0.3 | 22 | 15 | 759±53 | 96 |
| JNF-6 | 0.3 | 24 | 15 | 424±346 | 54 |
| JNF-7 | 0.3 | 22 | 10 | 227±62 | 94 |
| JNF-8 | 0.3 | 22 | 15 | 759±53 | 96 |
| JNF-9 | 0.3 | 22 | 20 | 947±72 | 92 |
图5
图5
在不同并列静电纺丝工艺条件下制备的MPVA/PNA双面纳米纤维的SEM照片
Fig.5
SEM images of MPVA/PNA Janus nanofibers prepared by different side-by-side electrospinning process
2.5 MPVA/PNA双面纳米纤维在水中的稳定性
构成MPVA/PNA双面纳米纤维的两种聚合物在室温下具有良好的亲水性,其中的PNA只有在水介质中才会响应温度的改变发生体积相转变,因此有必要评估其在水中的稳定性。分别用ABP单元含量不同的PNA-1、PNA-2或PNA-3为一种成纤聚合物,MPVA为另一种成纤聚合物,在两种纺丝液流速均为0.3 mL/h、纺丝电压为22 kV、接收距离为15 cm的条件下制备双面纳米纤维膜,测定三批样品的WLR值分别为1.63%、1.19%和1.15%。WLR值即其中可溶于水的聚合物含量均小于2.0 %,说明制得的MPVA/PNA双面纳米纤维在水中具有良好的稳定性。其原因是,在UV光辐照下MPVA/PNA双面纳米纤维内形成了交联结构,水分子扩散到纤维内只使其溶胀而不会溶解。另外,随着PNA中ABP单元含量的提高WLR值逐渐降低,表明双面纳米纤维的稳定性提高。其原因是,ABP单元含量越高在UV光辐照下产生的自由基越多,PNA分子链之间形成的交联点也越多。
评价MPVA/PNA双面纳米纤维在水中的稳定性,不仅要考虑其中聚合物可能未形成交联结构而溶于水中,还要了解双面纳米纤维中两种组分在水中是否会分离形成单组分的纳米纤维。参考文献[12]中的方法,将样品JNF-8连同铝箔一起放在水中浸泡6 h,取出充分干燥后用SEM观察,结果如图6a所示。可以看出,绝大部分纤维仍具有双面结构。随机观察多张SEM图中50根纤维的形态,统计出其中双面纳米纤维的含量为90%,与浸泡前相比变化不大。即使浸泡在水中以300 r/min的频率振荡2 h,干燥后用SEM观察到纤维仍具有双面结构,如图6b所示。这些结果表明,MPVA/PNA双面纳米纤维中的两个组分结合牢固,在水中难以分开。当纤维到达收集器受到UV光辐照时,其中MPVA与PNA两种组分都产生聚合物自由基,因此在它们相互接触的界面上也可能发生自由基偶合反应,使两种组分之间产生了化学连接。
图6
图6
样品JNF-8在水中浸泡6 h干燥后的SEM照片和浸在水中的样品JNF-8以300 r/min频率振荡2 h干燥后的SEM照片
Fig. 6
SEM image of the sample JNF-8 after soaking it in water for 6 h and then drying it (a); SEM image of the sample JNF-8 after oscillating in water at 300 r/min for 2 h and then drying it (b)
2.6 MPVA/PNA双面纳米纤维的温度响应性
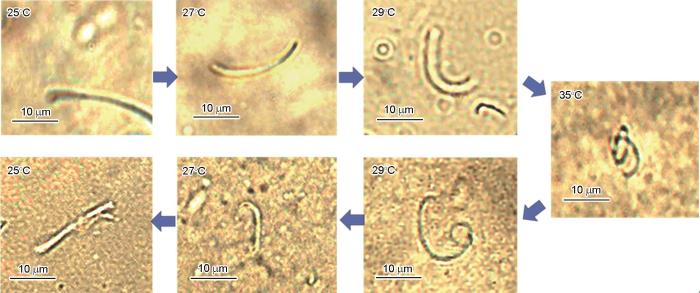
图7给出了用热台偏光显微镜观察到的、不同温度下切短的MPVA/PNA双面纳米纤维(JNF-8)分散在水中的形态。从图7可以看出,在水温以1℃/min的速率从25℃升高到27℃的过程中,纤维处于伸展的形态。水温进一步升高到29℃纤维开始发生卷曲。水温继续上升至35℃则纤维处于完全卷曲的形态,表明MPVA/PNA双面纳米纤维具有升温可卷曲的温度响应性。另外,将分散液的温度逐渐从35℃降到25℃纤维又从卷曲的形态又回复到伸展的形态,表明其升温可弯曲的温度响应性具有可逆性。MPVA/PNA双面纳米纤维升温可卷曲的温度响应性,源自其组成和双面结构。纤维一面的MPVA组分被水溶胀,形成的水凝胶不随温度变化发生体积改变。而纤维另一面的PNA组分具有温度响应性,在室温(25℃)下和MPVA一样被水溶胀,因此整个纤维处于伸展形态。当水介质温度升高超过PNA的LCST(29.2℃)时其分子链由亲水性转变为疏水性,使纤维中PNA面排出所含的水而发生体积收缩。MPVA和PNA两面之间通过化学键连接在一起,因此PNA面单独收缩的结果使双面纳米纤维发生了卷曲。当水介质的温度下降到低于PNA的LCST时PNA面由疏水性又转变为亲水性,重新吸水溶胀后双面纳米纤维恢复到伸展的形态,表明其温度响应性具有可逆性。
图7
图7
在温度不同的水介质中MPVA/PNA双面纳米纤维(JNF-8)的显微照片
Fig.7
Micrographs of MPVA/PNA Janus nanofiber (JNF-8) in aqueous medium at different temperatures (magnification ratio:1000)
3 结论
以分子侧链带可光交联的甲基丙烯酰氧基团的MPVA和分子侧链带可引发光交联反应的二苯基甲酮基团的PNA为成纤聚合物将并列静电纺丝与紫外光辐照相结合,可制备产率超过90%的双面纳米纤维。这种纳米纤维具有双面结构和交联结构,其中可溶于水的聚合物含量小于2.0%,在水介质中的稳定性良好。随着水介质温度从25℃升高到35℃这种双面纳米纤维从伸展的形态转变为卷曲的形态,且具有可逆性。
参考文献
Stimuli-responsive electrospun fibers and their applications
[J].Stimuli-responsive electrospun nanofibers are gaining considerable attention as highly versatile tools which offer great potential in the biomedical field. In this critical review, an overview is given on recent advances made in the development and application of stimuli-responsive fibers. The specific features of these electrospun fibers are highlighted and discussed in view of the properties required for the diverse applications. Furthermore, several novel biomedical applications are discussed and the respective advantages and shortcomings inherent to stimuli-responsive electrospun fibers are addressed (136 references).
Preparation of ultra-fast temperature-responsive nanofiber hydrogels and their use for controlled release of drugs
[J].
超快温度响应性纳米纤维水凝胶的制备及其用于药物的可控释放
[J].
Effects of morphologies of thermo-sensitive electrospun nanofibers on controllable drug release
[J].
Smart nanofibers from combined living radical polymerization, "click chemistry", and electrospinning
[J].
Temperature-responsive nanofibers for controllable oil/water separation
[J].
Silver nanoparticles loaded thermoresponsive hybrid nanofibrous hydrogel as a recyclable dip-catalyst with temperature-tunable catalytic activity
[J].
Electrospunnanofibers for cancer diagnosis and therapy
[J].
Electrospunnanofibers for wound healing
[J].
Tailoring the morphology of responsive bioinspired bicomponent fibers
[J].
Structurally dynamic hydrogels for biomedical applications: pursuing a fine balance between macroscopic stability and microscopic dynamics
[J].Owing to their unique chemical and physical properties, hydrogels are attracting increasing attention in both basic and translational biomedical studies. Although the classical hydrogels with static networks have been widely reported for decades, a growing number of recent studies have shown that structurally dynamic hydrogels can better mimic the dynamics and functions of natural extracellular matrix (ECM) in soft tissues. These synthetic materials with defined compositions can recapitulate key chemical and biophysical properties of living tissues, providing an important means to understanding the mechanisms by which cells sense and remodel their surrounding microenvironments. This review begins with the overall expectation and design principles of dynamic hydrogels. We then highlight recent progress in the fabrication strategies of dynamic hydrogels including both degradation-dependent and degradation-independent approaches, followed by their unique properties and use in biomedical applications such as regenerative medicine, drug delivery, and 3D culture. Finally, challenges and emerging trends in the development and application of dynamic hydrogels are discussed.
Stimuli-responsive polymers and their applications
[J].
Template assisted change in morphology from particles to nanofibers by side‐by‐side electrospinning of block copolymers
[J].
Development of the PVA/CS nanofibers containing silk protein sericin as a wound dressing: In vitro and in vivo assessment
[J].Skin and soft tissue infections are major concerns with respect to wound repair. Recently, anti-bacterial wound dressings have been emerging as promising candidates to reduce infection, thus accelerating the wound healing process. This paper presents our work to develop and characterize poly(vinyl alcohol) (PVA)/chitosan (CS)/silk sericin (SS)/tetracycline (TCN) porous nanofibers, with diameters varying from 305 to 425 nm, both in vitro and in vivo for potential applications as wound dressings. The fabricated nanofibers possess a considerable capacity to take up water through swelling (~325-650%). Sericin addition leads to increased hydrophilicity and elongation at break while decreasing fiber diameter and mechanical strength. Moreover, fibroblasts (L929) cultured on the nanofibers with low sericin content (PVA/CS/1-2SS) displayed greater proliferation compared to those on nanofibers without sericin (PVA/CS). Nanofibers loaded with high sericin and tetracycline content significantly inhibited the growth of Escherichia coli and Staphylococcus aureus. In vivo examination revealed that PVA/CS/2SS-TCN nanofibers enhance wound healing, re-epithelialization, and collagen deposition compared to traditional gauze and nanofibers without sericin. The results of this study demonstrate that the PVA/CS/2SS-TCN nanofiber creates a promising alternative to traditional wound dressing materials.Copyright © 2020 Elsevier B.V. All rights reserved.
Biodegradation of PVAs with various stereoregularities
[J].
Addition of methacryloil groups to poly (vinyl alcohol) in DMSO catalyzed by TEMED: Optimization through response surface methodology
[J].
Reaction of glycidyl methacrylate at the hydroxyl and carboxylic groups of poly (vinyl alcohol) and poly (acrylic acid): is this reaction mechanism still unclear
[J].
Stimulus-responsive electrospun nanofibers
[J].
刺激响应性电纺纳米纤维
[J].
Synthesis and characterization of a novel temperature sensitive microgels based on n-isopropylacrylamide and tert-butyl acrylate
[J], J.
Multifluidelectrospinning for the generation of complex nanostructures
[J].
Structure-tunable Janus fibers fabricated using spinnerets with varying port angles
[J].
Electroconductive 3D polymericnetworkproduction by using polyanilinechitosan-based hydrogel
[J].Herein, polyaniline/chitosan(PANI/CTS)-based electroconductive hydrogel was produced by photocrosslinked network in which CTS was used as a main component. Firstly, glycidyl methacrylate (GMA) was grafted on the CTS backbone to form CTS-g-GMA. Then, a three-dimensional polymeric network consisting of CTS-g-GMA and poly(ethylene glycol)diacrylate (PEGDA) was obtained by using photocrosslinking technique. At last, aniline monomer solution prepared by utilizing three different aniline concentrations (0.08, 0.16 and 0,32 M) was absorbed into (CTS-g-GMA)-PEGDA crosslinked structure to form [(CTS-g-GMA)-PEGDA]-PANI electroconductive semi interpenetrating network. FT-IR, XRD, SEM, TGA analyses and cytotoxicity test were performed for the produced samples. Conductivities of the hydrogels were determined by four-point probe technique. According to the conductivity measurements, among the PANI/CTS-based hydrogels, [(CTS-g-GMA)-PEGDA]-PANI(0.32 M) has the highest conductivity value (7437 × 10 S/cm). The obtained results showed that the fabricated electroconductive hydrogel (ECHs) in this study is a promising candidate owing to its advantages for biomedical applications especially biosensors in the future.Copyright © 2018. Published by Elsevier Ltd.