Advances in nanomaterial based approaches for enhanced fluoride and nitrate removal from contaminated water
1
2016
... 化肥的过度使用、工业废水和生活污水的大量排放,使硝酸盐氮成为水体中的主要污染物[1~3].水体中的硝酸盐氮产生的富营养化加速了水质的恶化[4].因此,世界卫生组织(WHO)和中国的《生活饮用水卫生标准》(GB5749-2022)规定饮用水中硝酸盐氮的浓度不得高于10 mg/L. ...
Ni2P-modified Ta3N5 and TaON for photocatalytic nitrate reduction
0
2020
Fabrication of novel 2D Ag-TiO2/γ-Al2O3/Chitosan nano-composite photocatalyst toward enhanced photocatalytic reduction of nitrate
1
2020
... 化肥的过度使用、工业废水和生活污水的大量排放,使硝酸盐氮成为水体中的主要污染物[1~3].水体中的硝酸盐氮产生的富营养化加速了水质的恶化[4].因此,世界卫生组织(WHO)和中国的《生活饮用水卫生标准》(GB5749-2022)规定饮用水中硝酸盐氮的浓度不得高于10 mg/L. ...
Removal of nitrate nitrogen from water by phosphotungstate-supported TiO2 photocatalytic method
1
2020
... 化肥的过度使用、工业废水和生活污水的大量排放,使硝酸盐氮成为水体中的主要污染物[1~3].水体中的硝酸盐氮产生的富营养化加速了水质的恶化[4].因此,世界卫生组织(WHO)和中国的《生活饮用水卫生标准》(GB5749-2022)规定饮用水中硝酸盐氮的浓度不得高于10 mg/L. ...
Overlooked pathways of denitrification in a sulfur-based denitrification system with organic supplementation
1
2020
... 生物反硝化[5]、化学还原[6]、反渗透[7]、电渗析[8]、离子交换[9]、电催化[10]、光催化[11]等技术,可去除硝酸盐氮.光催化技术的设备简单、环境友好和无二次污染,受到了极大的重视[12,13].但是其电子-空穴对的复合和光能的利用效率较低,限制了光催化转化硝酸盐氮的应用[14].因此,急待开发更高效的光催化剂. ...
Fe(II) enhances simultaneous phosphorus removal and denitrification in heterotrophic denitrification by chemical precipitation and stimulating denitrifiers activity
1
2021
... 生物反硝化[5]、化学还原[6]、反渗透[7]、电渗析[8]、离子交换[9]、电催化[10]、光催化[11]等技术,可去除硝酸盐氮.光催化技术的设备简单、环境友好和无二次污染,受到了极大的重视[12,13].但是其电子-空穴对的复合和光能的利用效率较低,限制了光催化转化硝酸盐氮的应用[14].因此,急待开发更高效的光催化剂. ...
Denitrification performance and microbial community under salinity and MIT stresses for reverse osmosis concentrate treatment
1
2020
... 生物反硝化[5]、化学还原[6]、反渗透[7]、电渗析[8]、离子交换[9]、电催化[10]、光催化[11]等技术,可去除硝酸盐氮.光催化技术的设备简单、环境友好和无二次污染,受到了极大的重视[12,13].但是其电子-空穴对的复合和光能的利用效率较低,限制了光催化转化硝酸盐氮的应用[14].因此,急待开发更高效的光催化剂. ...
Recent discoveries in the reaction mechanism of heterogeneous electrocatalytic nitrate reduction
1
2021
... 生物反硝化[5]、化学还原[6]、反渗透[7]、电渗析[8]、离子交换[9]、电催化[10]、光催化[11]等技术,可去除硝酸盐氮.光催化技术的设备简单、环境友好和无二次污染,受到了极大的重视[12,13].但是其电子-空穴对的复合和光能的利用效率较低,限制了光催化转化硝酸盐氮的应用[14].因此,急待开发更高效的光催化剂. ...
Sulfur-based denitrification treating regeneration water from ion exchange at high performance and low cost
1
2018
... 生物反硝化[5]、化学还原[6]、反渗透[7]、电渗析[8]、离子交换[9]、电催化[10]、光催化[11]等技术,可去除硝酸盐氮.光催化技术的设备简单、环境友好和无二次污染,受到了极大的重视[12,13].但是其电子-空穴对的复合和光能的利用效率较低,限制了光催化转化硝酸盐氮的应用[14].因此,急待开发更高效的光催化剂. ...
Structure sensitivity of Pd facets for enhanced electrochemical nitrate reduction to ammonia
1
2021
... 生物反硝化[5]、化学还原[6]、反渗透[7]、电渗析[8]、离子交换[9]、电催化[10]、光催化[11]等技术,可去除硝酸盐氮.光催化技术的设备简单、环境友好和无二次污染,受到了极大的重视[12,13].但是其电子-空穴对的复合和光能的利用效率较低,限制了光催化转化硝酸盐氮的应用[14].因此,急待开发更高效的光催化剂. ...
Room-temperature catalytic reduction of aqueous nitrate to Ammonia with Ni nanoparticles immobilized on an Fe3O4@n-SiO2@h-SiO2-NH2 support
1
2017
... 生物反硝化[5]、化学还原[6]、反渗透[7]、电渗析[8]、离子交换[9]、电催化[10]、光催化[11]等技术,可去除硝酸盐氮.光催化技术的设备简单、环境友好和无二次污染,受到了极大的重视[12,13].但是其电子-空穴对的复合和光能的利用效率较低,限制了光催化转化硝酸盐氮的应用[14].因此,急待开发更高效的光催化剂. ...
Challenges in photocatalytic reduction of nitrate as a water treatment technology
1
2017
... 生物反硝化[5]、化学还原[6]、反渗透[7]、电渗析[8]、离子交换[9]、电催化[10]、光催化[11]等技术,可去除硝酸盐氮.光催化技术的设备简单、环境友好和无二次污染,受到了极大的重视[12,13].但是其电子-空穴对的复合和光能的利用效率较低,限制了光催化转化硝酸盐氮的应用[14].因此,急待开发更高效的光催化剂. ...
Reaction mechanism and selectivity regulation of photocatalytic nitrate reduction for wastewater purification: progress and challenges
1
2022
... 生物反硝化[5]、化学还原[6]、反渗透[7]、电渗析[8]、离子交换[9]、电催化[10]、光催化[11]等技术,可去除硝酸盐氮.光催化技术的设备简单、环境友好和无二次污染,受到了极大的重视[12,13].但是其电子-空穴对的复合和光能的利用效率较低,限制了光催化转化硝酸盐氮的应用[14].因此,急待开发更高效的光催化剂. ...
Photocatalytic reduction of nitrate pollutants by novel Z-scheme ZnSe/BiVO4 heterostructures with high N2 selectivity
2
2022
... 生物反硝化[5]、化学还原[6]、反渗透[7]、电渗析[8]、离子交换[9]、电催化[10]、光催化[11]等技术,可去除硝酸盐氮.光催化技术的设备简单、环境友好和无二次污染,受到了极大的重视[12,13].但是其电子-空穴对的复合和光能的利用效率较低,限制了光催化转化硝酸盐氮的应用[14].因此,急待开发更高效的光催化剂. ...
... Photocatalytic reduction of nitrate in this work and previous literature studies
Table 1| Photocatalyst | Hole scavenger | Light source | RN/% | S/% | Ref. |
|---|
| g-C3N4/Ag/BiOBr | Formic acid | Halide lamp | 95.2 | 92.4 | This work |
| Ag/TiO2 | Formic acid | Xe lamp | 95 | 90 | [48] |
| Au/TiO2 | Oxalic acid | High-pressure Hg lamp | 44 | 49.9 | [49] |
| 0.5TiO2/Ti3C2/g-C3N4 | Formic acid | High-pressure Hg lamp | 93.3 | 96.62 | [29] |
| Fe-LiNbO3 | Formic acid | High-pressure Hg lamp | 90 | 88 | [50] |
| ZnSe/BiVO4 | Formic acid | High-pressure Hg lamp | 89.84 | 91.03 | [14] |
| NH2-MIL-101(Fe)/BiVO4 | Formic acid | High-pressure Hg lamp | 94.8 | 93.5 | [51] |
| Ag/SiO2@cTiO2 | Formic acid | High-pressure Hg lamp | 95.8 | 93.6 | [22] |
<strong>2.5</strong> 光催化降解机理本文分析对比了g-C3N4及BiOBr复合光催化材料,选择硝酸盐氮(50 mg/L)为研究对象以探索光催化还原效率.经g-C3N4/Ag/BiOBr光催化后硝酸盐氮的浓度可降低到2.4 mg/L(图7),氮气选择性可达92.4%(图8),表明使用Ag强化复合材料光催化作用可有效提高光催化的还原性能. ...
Selective reduction of nitrate to N2 using ilmenite as a low cost photo-catalyst
1
2020
... TiO2是一种常见的催化剂,可用于硝酸盐氮的光催化还原[15].为了提高硝酸盐氮的转化效率和氮气选择性,可掺杂金属(Au[16]、Ag[17]、Cu[18]等)或金属氧化物(Ag2O[19]、Cu2O[20]等);多元材料的复合也可提高TiO2光催化还原硝酸盐氮.Wang等[21]合成多金属氧化物(POM)/TiO2/Cu复合光催化材料,硝酸盐氮的去除效率为76.53%,氮气选择性为82.09%.Hou等[22]合成核壳状Ag/SiO2@cTiO(2)复合光催化材料,硝酸盐氮的去除率为95.8%,氮气选择性为93.6%.但是,由于TiO2的带隙较宽(约3.2 eV),不能直接利用太阳光[23].g-C3N4(带隙约2.7 eV)[24]和BiOBr(带隙2.6~2.9 eV)[25]的带隙较窄,可用于光催化去除污染物 [26].但是,光催化材料单体的比表面积小、载流子重组速度较快,影响其光催化活性[27].多重材料的掺杂复合,是提高光催化材料效能的主要手段[28].Zheng R等[29]采用g-C3N4可实现约50%硝酸盐氮的去除,采用TiO2/Ti3C2/g-C3N4复合材料使硝酸盐氮的去除效率提高到93.03%,氮气选择性达到96.62%.贵金属Ag具有较低的费米能级(EF=4.74 eV),可阻止光催化材料电子-空穴对的复合[30].不同银掺杂改性的复合材料,都能明显提高g-C3N4或BiOBr的光催化性能.杨利伟等[31]研究发现,与pg-C3N4、BiOBr 单体和二元复合材料pg-C3N4/BiOBr相比,pg-C3N4/BiOBr/Ag(5%)光催化降解磺胺甲唑的效果显著提高.Shelton等[32]用Ag/g-C3N4光催化剂对比了紫外光和可见光下还原水中硝酸盐效果,发现Ag强化了还原作用.本文以g-C3N4、BiOBr和银为基质制备一系列的复合光催化材料(Ag/g-C3N4、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr),选择低浓度硝酸盐氮(50 mg/L以氮计)为处理对象,对比分析不同光催化材料对硝酸盐氮的去除性能及其氮气选择性. ...
Photocatalytic nitrate reduction over Au/TiO2
1
2011
... TiO2是一种常见的催化剂,可用于硝酸盐氮的光催化还原[15].为了提高硝酸盐氮的转化效率和氮气选择性,可掺杂金属(Au[16]、Ag[17]、Cu[18]等)或金属氧化物(Ag2O[19]、Cu2O[20]等);多元材料的复合也可提高TiO2光催化还原硝酸盐氮.Wang等[21]合成多金属氧化物(POM)/TiO2/Cu复合光催化材料,硝酸盐氮的去除效率为76.53%,氮气选择性为82.09%.Hou等[22]合成核壳状Ag/SiO2@cTiO(2)复合光催化材料,硝酸盐氮的去除率为95.8%,氮气选择性为93.6%.但是,由于TiO2的带隙较宽(约3.2 eV),不能直接利用太阳光[23].g-C3N4(带隙约2.7 eV)[24]和BiOBr(带隙2.6~2.9 eV)[25]的带隙较窄,可用于光催化去除污染物 [26].但是,光催化材料单体的比表面积小、载流子重组速度较快,影响其光催化活性[27].多重材料的掺杂复合,是提高光催化材料效能的主要手段[28].Zheng R等[29]采用g-C3N4可实现约50%硝酸盐氮的去除,采用TiO2/Ti3C2/g-C3N4复合材料使硝酸盐氮的去除效率提高到93.03%,氮气选择性达到96.62%.贵金属Ag具有较低的费米能级(EF=4.74 eV),可阻止光催化材料电子-空穴对的复合[30].不同银掺杂改性的复合材料,都能明显提高g-C3N4或BiOBr的光催化性能.杨利伟等[31]研究发现,与pg-C3N4、BiOBr 单体和二元复合材料pg-C3N4/BiOBr相比,pg-C3N4/BiOBr/Ag(5%)光催化降解磺胺甲唑的效果显著提高.Shelton等[32]用Ag/g-C3N4光催化剂对比了紫外光和可见光下还原水中硝酸盐效果,发现Ag强化了还原作用.本文以g-C3N4、BiOBr和银为基质制备一系列的复合光催化材料(Ag/g-C3N4、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr),选择低浓度硝酸盐氮(50 mg/L以氮计)为处理对象,对比分析不同光催化材料对硝酸盐氮的去除性能及其氮气选择性. ...
High photocatalytic activity and selectivity for nitrogen in nitrate reduction on Ag/TiO2 catalyst with fine silver clusters
1
2005
... TiO2是一种常见的催化剂,可用于硝酸盐氮的光催化还原[15].为了提高硝酸盐氮的转化效率和氮气选择性,可掺杂金属(Au[16]、Ag[17]、Cu[18]等)或金属氧化物(Ag2O[19]、Cu2O[20]等);多元材料的复合也可提高TiO2光催化还原硝酸盐氮.Wang等[21]合成多金属氧化物(POM)/TiO2/Cu复合光催化材料,硝酸盐氮的去除效率为76.53%,氮气选择性为82.09%.Hou等[22]合成核壳状Ag/SiO2@cTiO(2)复合光催化材料,硝酸盐氮的去除率为95.8%,氮气选择性为93.6%.但是,由于TiO2的带隙较宽(约3.2 eV),不能直接利用太阳光[23].g-C3N4(带隙约2.7 eV)[24]和BiOBr(带隙2.6~2.9 eV)[25]的带隙较窄,可用于光催化去除污染物 [26].但是,光催化材料单体的比表面积小、载流子重组速度较快,影响其光催化活性[27].多重材料的掺杂复合,是提高光催化材料效能的主要手段[28].Zheng R等[29]采用g-C3N4可实现约50%硝酸盐氮的去除,采用TiO2/Ti3C2/g-C3N4复合材料使硝酸盐氮的去除效率提高到93.03%,氮气选择性达到96.62%.贵金属Ag具有较低的费米能级(EF=4.74 eV),可阻止光催化材料电子-空穴对的复合[30].不同银掺杂改性的复合材料,都能明显提高g-C3N4或BiOBr的光催化性能.杨利伟等[31]研究发现,与pg-C3N4、BiOBr 单体和二元复合材料pg-C3N4/BiOBr相比,pg-C3N4/BiOBr/Ag(5%)光催化降解磺胺甲唑的效果显著提高.Shelton等[32]用Ag/g-C3N4光催化剂对比了紫外光和可见光下还原水中硝酸盐效果,发现Ag强化了还原作用.本文以g-C3N4、BiOBr和银为基质制备一系列的复合光催化材料(Ag/g-C3N4、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr),选择低浓度硝酸盐氮(50 mg/L以氮计)为处理对象,对比分析不同光催化材料对硝酸盐氮的去除性能及其氮气选择性. ...
Removal of nitrate and simultaneous hydrogen generation through photocatalytic reforming of glycerol over "in situ" prepared zero-valent nano copper/P25
1
2017
... TiO2是一种常见的催化剂,可用于硝酸盐氮的光催化还原[15].为了提高硝酸盐氮的转化效率和氮气选择性,可掺杂金属(Au[16]、Ag[17]、Cu[18]等)或金属氧化物(Ag2O[19]、Cu2O[20]等);多元材料的复合也可提高TiO2光催化还原硝酸盐氮.Wang等[21]合成多金属氧化物(POM)/TiO2/Cu复合光催化材料,硝酸盐氮的去除效率为76.53%,氮气选择性为82.09%.Hou等[22]合成核壳状Ag/SiO2@cTiO(2)复合光催化材料,硝酸盐氮的去除率为95.8%,氮气选择性为93.6%.但是,由于TiO2的带隙较宽(约3.2 eV),不能直接利用太阳光[23].g-C3N4(带隙约2.7 eV)[24]和BiOBr(带隙2.6~2.9 eV)[25]的带隙较窄,可用于光催化去除污染物 [26].但是,光催化材料单体的比表面积小、载流子重组速度较快,影响其光催化活性[27].多重材料的掺杂复合,是提高光催化材料效能的主要手段[28].Zheng R等[29]采用g-C3N4可实现约50%硝酸盐氮的去除,采用TiO2/Ti3C2/g-C3N4复合材料使硝酸盐氮的去除效率提高到93.03%,氮气选择性达到96.62%.贵金属Ag具有较低的费米能级(EF=4.74 eV),可阻止光催化材料电子-空穴对的复合[30].不同银掺杂改性的复合材料,都能明显提高g-C3N4或BiOBr的光催化性能.杨利伟等[31]研究发现,与pg-C3N4、BiOBr 单体和二元复合材料pg-C3N4/BiOBr相比,pg-C3N4/BiOBr/Ag(5%)光催化降解磺胺甲唑的效果显著提高.Shelton等[32]用Ag/g-C3N4光催化剂对比了紫外光和可见光下还原水中硝酸盐效果,发现Ag强化了还原作用.本文以g-C3N4、BiOBr和银为基质制备一系列的复合光催化材料(Ag/g-C3N4、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr),选择低浓度硝酸盐氮(50 mg/L以氮计)为处理对象,对比分析不同光催化材料对硝酸盐氮的去除性能及其氮气选择性. ...
A facile preparation of Ag2O/P25 photocatalyst for selective reduction of nitrate
1
2015
... TiO2是一种常见的催化剂,可用于硝酸盐氮的光催化还原[15].为了提高硝酸盐氮的转化效率和氮气选择性,可掺杂金属(Au[16]、Ag[17]、Cu[18]等)或金属氧化物(Ag2O[19]、Cu2O[20]等);多元材料的复合也可提高TiO2光催化还原硝酸盐氮.Wang等[21]合成多金属氧化物(POM)/TiO2/Cu复合光催化材料,硝酸盐氮的去除效率为76.53%,氮气选择性为82.09%.Hou等[22]合成核壳状Ag/SiO2@cTiO(2)复合光催化材料,硝酸盐氮的去除率为95.8%,氮气选择性为93.6%.但是,由于TiO2的带隙较宽(约3.2 eV),不能直接利用太阳光[23].g-C3N4(带隙约2.7 eV)[24]和BiOBr(带隙2.6~2.9 eV)[25]的带隙较窄,可用于光催化去除污染物 [26].但是,光催化材料单体的比表面积小、载流子重组速度较快,影响其光催化活性[27].多重材料的掺杂复合,是提高光催化材料效能的主要手段[28].Zheng R等[29]采用g-C3N4可实现约50%硝酸盐氮的去除,采用TiO2/Ti3C2/g-C3N4复合材料使硝酸盐氮的去除效率提高到93.03%,氮气选择性达到96.62%.贵金属Ag具有较低的费米能级(EF=4.74 eV),可阻止光催化材料电子-空穴对的复合[30].不同银掺杂改性的复合材料,都能明显提高g-C3N4或BiOBr的光催化性能.杨利伟等[31]研究发现,与pg-C3N4、BiOBr 单体和二元复合材料pg-C3N4/BiOBr相比,pg-C3N4/BiOBr/Ag(5%)光催化降解磺胺甲唑的效果显著提高.Shelton等[32]用Ag/g-C3N4光催化剂对比了紫外光和可见光下还原水中硝酸盐效果,发现Ag强化了还原作用.本文以g-C3N4、BiOBr和银为基质制备一系列的复合光催化材料(Ag/g-C3N4、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr),选择低浓度硝酸盐氮(50 mg/L以氮计)为处理对象,对比分析不同光催化材料对硝酸盐氮的去除性能及其氮气选择性. ...
Simultaneous photocatalytic removal of nitrate and oxalic acid over Cu2O/TiO2 and Cu2O/TiO2-AC composites
1
2017
... TiO2是一种常见的催化剂,可用于硝酸盐氮的光催化还原[15].为了提高硝酸盐氮的转化效率和氮气选择性,可掺杂金属(Au[16]、Ag[17]、Cu[18]等)或金属氧化物(Ag2O[19]、Cu2O[20]等);多元材料的复合也可提高TiO2光催化还原硝酸盐氮.Wang等[21]合成多金属氧化物(POM)/TiO2/Cu复合光催化材料,硝酸盐氮的去除效率为76.53%,氮气选择性为82.09%.Hou等[22]合成核壳状Ag/SiO2@cTiO(2)复合光催化材料,硝酸盐氮的去除率为95.8%,氮气选择性为93.6%.但是,由于TiO2的带隙较宽(约3.2 eV),不能直接利用太阳光[23].g-C3N4(带隙约2.7 eV)[24]和BiOBr(带隙2.6~2.9 eV)[25]的带隙较窄,可用于光催化去除污染物 [26].但是,光催化材料单体的比表面积小、载流子重组速度较快,影响其光催化活性[27].多重材料的掺杂复合,是提高光催化材料效能的主要手段[28].Zheng R等[29]采用g-C3N4可实现约50%硝酸盐氮的去除,采用TiO2/Ti3C2/g-C3N4复合材料使硝酸盐氮的去除效率提高到93.03%,氮气选择性达到96.62%.贵金属Ag具有较低的费米能级(EF=4.74 eV),可阻止光催化材料电子-空穴对的复合[30].不同银掺杂改性的复合材料,都能明显提高g-C3N4或BiOBr的光催化性能.杨利伟等[31]研究发现,与pg-C3N4、BiOBr 单体和二元复合材料pg-C3N4/BiOBr相比,pg-C3N4/BiOBr/Ag(5%)光催化降解磺胺甲唑的效果显著提高.Shelton等[32]用Ag/g-C3N4光催化剂对比了紫外光和可见光下还原水中硝酸盐效果,发现Ag强化了还原作用.本文以g-C3N4、BiOBr和银为基质制备一系列的复合光催化材料(Ag/g-C3N4、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr),选择低浓度硝酸盐氮(50 mg/L以氮计)为处理对象,对比分析不同光催化材料对硝酸盐氮的去除性能及其氮气选择性. ...
Synthesis of polyoxometalates (POM)/TiO2/Cu and removal of nitrate nitrogen in water by photocatalysis
1
2021
... TiO2是一种常见的催化剂,可用于硝酸盐氮的光催化还原[15].为了提高硝酸盐氮的转化效率和氮气选择性,可掺杂金属(Au[16]、Ag[17]、Cu[18]等)或金属氧化物(Ag2O[19]、Cu2O[20]等);多元材料的复合也可提高TiO2光催化还原硝酸盐氮.Wang等[21]合成多金属氧化物(POM)/TiO2/Cu复合光催化材料,硝酸盐氮的去除效率为76.53%,氮气选择性为82.09%.Hou等[22]合成核壳状Ag/SiO2@cTiO(2)复合光催化材料,硝酸盐氮的去除率为95.8%,氮气选择性为93.6%.但是,由于TiO2的带隙较宽(约3.2 eV),不能直接利用太阳光[23].g-C3N4(带隙约2.7 eV)[24]和BiOBr(带隙2.6~2.9 eV)[25]的带隙较窄,可用于光催化去除污染物 [26].但是,光催化材料单体的比表面积小、载流子重组速度较快,影响其光催化活性[27].多重材料的掺杂复合,是提高光催化材料效能的主要手段[28].Zheng R等[29]采用g-C3N4可实现约50%硝酸盐氮的去除,采用TiO2/Ti3C2/g-C3N4复合材料使硝酸盐氮的去除效率提高到93.03%,氮气选择性达到96.62%.贵金属Ag具有较低的费米能级(EF=4.74 eV),可阻止光催化材料电子-空穴对的复合[30].不同银掺杂改性的复合材料,都能明显提高g-C3N4或BiOBr的光催化性能.杨利伟等[31]研究发现,与pg-C3N4、BiOBr 单体和二元复合材料pg-C3N4/BiOBr相比,pg-C3N4/BiOBr/Ag(5%)光催化降解磺胺甲唑的效果显著提高.Shelton等[32]用Ag/g-C3N4光催化剂对比了紫外光和可见光下还原水中硝酸盐效果,发现Ag强化了还原作用.本文以g-C3N4、BiOBr和银为基质制备一系列的复合光催化材料(Ag/g-C3N4、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr),选择低浓度硝酸盐氮(50 mg/L以氮计)为处理对象,对比分析不同光催化材料对硝酸盐氮的去除性能及其氮气选择性. ...
High efficient photocatalytic reduction of nitrate to N2 by Core-shell Ag/SiO2@cTiO2 with synergistic effect of light scattering and surface plasmon resonance
2
2021
... TiO2是一种常见的催化剂,可用于硝酸盐氮的光催化还原[15].为了提高硝酸盐氮的转化效率和氮气选择性,可掺杂金属(Au[16]、Ag[17]、Cu[18]等)或金属氧化物(Ag2O[19]、Cu2O[20]等);多元材料的复合也可提高TiO2光催化还原硝酸盐氮.Wang等[21]合成多金属氧化物(POM)/TiO2/Cu复合光催化材料,硝酸盐氮的去除效率为76.53%,氮气选择性为82.09%.Hou等[22]合成核壳状Ag/SiO2@cTiO(2)复合光催化材料,硝酸盐氮的去除率为95.8%,氮气选择性为93.6%.但是,由于TiO2的带隙较宽(约3.2 eV),不能直接利用太阳光[23].g-C3N4(带隙约2.7 eV)[24]和BiOBr(带隙2.6~2.9 eV)[25]的带隙较窄,可用于光催化去除污染物 [26].但是,光催化材料单体的比表面积小、载流子重组速度较快,影响其光催化活性[27].多重材料的掺杂复合,是提高光催化材料效能的主要手段[28].Zheng R等[29]采用g-C3N4可实现约50%硝酸盐氮的去除,采用TiO2/Ti3C2/g-C3N4复合材料使硝酸盐氮的去除效率提高到93.03%,氮气选择性达到96.62%.贵金属Ag具有较低的费米能级(EF=4.74 eV),可阻止光催化材料电子-空穴对的复合[30].不同银掺杂改性的复合材料,都能明显提高g-C3N4或BiOBr的光催化性能.杨利伟等[31]研究发现,与pg-C3N4、BiOBr 单体和二元复合材料pg-C3N4/BiOBr相比,pg-C3N4/BiOBr/Ag(5%)光催化降解磺胺甲唑的效果显著提高.Shelton等[32]用Ag/g-C3N4光催化剂对比了紫外光和可见光下还原水中硝酸盐效果,发现Ag强化了还原作用.本文以g-C3N4、BiOBr和银为基质制备一系列的复合光催化材料(Ag/g-C3N4、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr),选择低浓度硝酸盐氮(50 mg/L以氮计)为处理对象,对比分析不同光催化材料对硝酸盐氮的去除性能及其氮气选择性. ...
... Photocatalytic reduction of nitrate in this work and previous literature studies
Table 1| Photocatalyst | Hole scavenger | Light source | RN/% | S/% | Ref. |
|---|
| g-C3N4/Ag/BiOBr | Formic acid | Halide lamp | 95.2 | 92.4 | This work |
| Ag/TiO2 | Formic acid | Xe lamp | 95 | 90 | [48] |
| Au/TiO2 | Oxalic acid | High-pressure Hg lamp | 44 | 49.9 | [49] |
| 0.5TiO2/Ti3C2/g-C3N4 | Formic acid | High-pressure Hg lamp | 93.3 | 96.62 | [29] |
| Fe-LiNbO3 | Formic acid | High-pressure Hg lamp | 90 | 88 | [50] |
| ZnSe/BiVO4 | Formic acid | High-pressure Hg lamp | 89.84 | 91.03 | [14] |
| NH2-MIL-101(Fe)/BiVO4 | Formic acid | High-pressure Hg lamp | 94.8 | 93.5 | [51] |
| Ag/SiO2@cTiO2 | Formic acid | High-pressure Hg lamp | 95.8 | 93.6 | [22] |
<strong>2.5</strong> 光催化降解机理本文分析对比了g-C3N4及BiOBr复合光催化材料,选择硝酸盐氮(50 mg/L)为研究对象以探索光催化还原效率.经g-C3N4/Ag/BiOBr光催化后硝酸盐氮的浓度可降低到2.4 mg/L(图7),氮气选择性可达92.4%(图8),表明使用Ag强化复合材料光催化作用可有效提高光催化的还原性能. ...
The photocatalytic potential of BiOBr for wastewater treatment: A mini-review
1
2021
... TiO2是一种常见的催化剂,可用于硝酸盐氮的光催化还原[15].为了提高硝酸盐氮的转化效率和氮气选择性,可掺杂金属(Au[16]、Ag[17]、Cu[18]等)或金属氧化物(Ag2O[19]、Cu2O[20]等);多元材料的复合也可提高TiO2光催化还原硝酸盐氮.Wang等[21]合成多金属氧化物(POM)/TiO2/Cu复合光催化材料,硝酸盐氮的去除效率为76.53%,氮气选择性为82.09%.Hou等[22]合成核壳状Ag/SiO2@cTiO(2)复合光催化材料,硝酸盐氮的去除率为95.8%,氮气选择性为93.6%.但是,由于TiO2的带隙较宽(约3.2 eV),不能直接利用太阳光[23].g-C3N4(带隙约2.7 eV)[24]和BiOBr(带隙2.6~2.9 eV)[25]的带隙较窄,可用于光催化去除污染物 [26].但是,光催化材料单体的比表面积小、载流子重组速度较快,影响其光催化活性[27].多重材料的掺杂复合,是提高光催化材料效能的主要手段[28].Zheng R等[29]采用g-C3N4可实现约50%硝酸盐氮的去除,采用TiO2/Ti3C2/g-C3N4复合材料使硝酸盐氮的去除效率提高到93.03%,氮气选择性达到96.62%.贵金属Ag具有较低的费米能级(EF=4.74 eV),可阻止光催化材料电子-空穴对的复合[30].不同银掺杂改性的复合材料,都能明显提高g-C3N4或BiOBr的光催化性能.杨利伟等[31]研究发现,与pg-C3N4、BiOBr 单体和二元复合材料pg-C3N4/BiOBr相比,pg-C3N4/BiOBr/Ag(5%)光催化降解磺胺甲唑的效果显著提高.Shelton等[32]用Ag/g-C3N4光催化剂对比了紫外光和可见光下还原水中硝酸盐效果,发现Ag强化了还原作用.本文以g-C3N4、BiOBr和银为基质制备一系列的复合光催化材料(Ag/g-C3N4、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr),选择低浓度硝酸盐氮(50 mg/L以氮计)为处理对象,对比分析不同光催化材料对硝酸盐氮的去除性能及其氮气选择性. ...
g-C3N4-based heterostructured photocatalysts
1
2018
... TiO2是一种常见的催化剂,可用于硝酸盐氮的光催化还原[15].为了提高硝酸盐氮的转化效率和氮气选择性,可掺杂金属(Au[16]、Ag[17]、Cu[18]等)或金属氧化物(Ag2O[19]、Cu2O[20]等);多元材料的复合也可提高TiO2光催化还原硝酸盐氮.Wang等[21]合成多金属氧化物(POM)/TiO2/Cu复合光催化材料,硝酸盐氮的去除效率为76.53%,氮气选择性为82.09%.Hou等[22]合成核壳状Ag/SiO2@cTiO(2)复合光催化材料,硝酸盐氮的去除率为95.8%,氮气选择性为93.6%.但是,由于TiO2的带隙较宽(约3.2 eV),不能直接利用太阳光[23].g-C3N4(带隙约2.7 eV)[24]和BiOBr(带隙2.6~2.9 eV)[25]的带隙较窄,可用于光催化去除污染物 [26].但是,光催化材料单体的比表面积小、载流子重组速度较快,影响其光催化活性[27].多重材料的掺杂复合,是提高光催化材料效能的主要手段[28].Zheng R等[29]采用g-C3N4可实现约50%硝酸盐氮的去除,采用TiO2/Ti3C2/g-C3N4复合材料使硝酸盐氮的去除效率提高到93.03%,氮气选择性达到96.62%.贵金属Ag具有较低的费米能级(EF=4.74 eV),可阻止光催化材料电子-空穴对的复合[30].不同银掺杂改性的复合材料,都能明显提高g-C3N4或BiOBr的光催化性能.杨利伟等[31]研究发现,与pg-C3N4、BiOBr 单体和二元复合材料pg-C3N4/BiOBr相比,pg-C3N4/BiOBr/Ag(5%)光催化降解磺胺甲唑的效果显著提高.Shelton等[32]用Ag/g-C3N4光催化剂对比了紫外光和可见光下还原水中硝酸盐效果,发现Ag强化了还原作用.本文以g-C3N4、BiOBr和银为基质制备一系列的复合光催化材料(Ag/g-C3N4、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr),选择低浓度硝酸盐氮(50 mg/L以氮计)为处理对象,对比分析不同光催化材料对硝酸盐氮的去除性能及其氮气选择性. ...
Bi-based photocatalysts for light-driven environmental and energy applications: Structural tuning, reaction mechanisms, and challenges
2
2020
... TiO2是一种常见的催化剂,可用于硝酸盐氮的光催化还原[15].为了提高硝酸盐氮的转化效率和氮气选择性,可掺杂金属(Au[16]、Ag[17]、Cu[18]等)或金属氧化物(Ag2O[19]、Cu2O[20]等);多元材料的复合也可提高TiO2光催化还原硝酸盐氮.Wang等[21]合成多金属氧化物(POM)/TiO2/Cu复合光催化材料,硝酸盐氮的去除效率为76.53%,氮气选择性为82.09%.Hou等[22]合成核壳状Ag/SiO2@cTiO(2)复合光催化材料,硝酸盐氮的去除率为95.8%,氮气选择性为93.6%.但是,由于TiO2的带隙较宽(约3.2 eV),不能直接利用太阳光[23].g-C3N4(带隙约2.7 eV)[24]和BiOBr(带隙2.6~2.9 eV)[25]的带隙较窄,可用于光催化去除污染物 [26].但是,光催化材料单体的比表面积小、载流子重组速度较快,影响其光催化活性[27].多重材料的掺杂复合,是提高光催化材料效能的主要手段[28].Zheng R等[29]采用g-C3N4可实现约50%硝酸盐氮的去除,采用TiO2/Ti3C2/g-C3N4复合材料使硝酸盐氮的去除效率提高到93.03%,氮气选择性达到96.62%.贵金属Ag具有较低的费米能级(EF=4.74 eV),可阻止光催化材料电子-空穴对的复合[30].不同银掺杂改性的复合材料,都能明显提高g-C3N4或BiOBr的光催化性能.杨利伟等[31]研究发现,与pg-C3N4、BiOBr 单体和二元复合材料pg-C3N4/BiOBr相比,pg-C3N4/BiOBr/Ag(5%)光催化降解磺胺甲唑的效果显著提高.Shelton等[32]用Ag/g-C3N4光催化剂对比了紫外光和可见光下还原水中硝酸盐效果,发现Ag强化了还原作用.本文以g-C3N4、BiOBr和银为基质制备一系列的复合光催化材料(Ag/g-C3N4、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr),选择低浓度硝酸盐氮(50 mg/L以氮计)为处理对象,对比分析不同光催化材料对硝酸盐氮的去除性能及其氮气选择性. ...
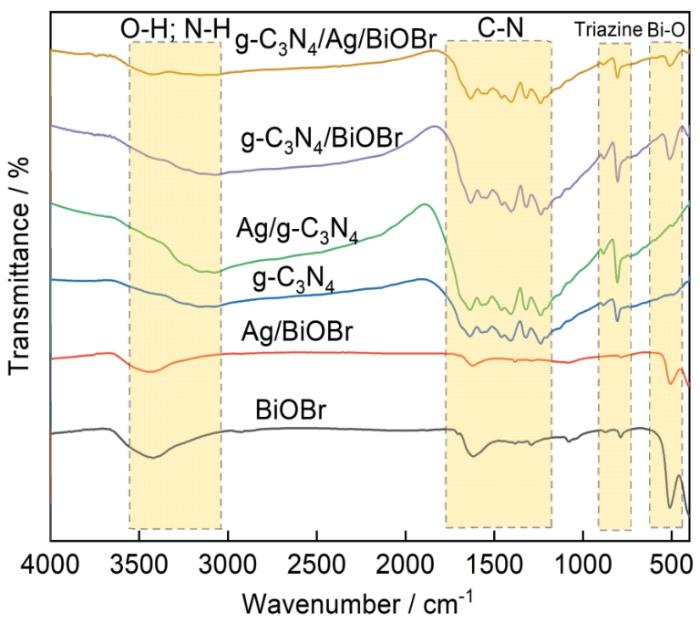
... 为了揭示光催化剂的化学组成及化学键情况,用傅里叶变换红外光谱(FT-IR)分析了不同催化剂(g-C3N4、Ag/g-C3N4、BiOBr、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr)(图3).由图3可见,在1200~1700 cm-1之间出现了明显的吸收峰,对应C-N结构[39~41],是g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂中的典型结构.与g-C3N4、Ag/g-C3N4对比表明,Ag的沉积明显强化了吸收峰的强度,而g-C3N4/Ag/BiOBr催化剂的吸收峰反而减弱,其原因可能是g-C3N4作为基质被Ag、BiOBr包裹(图1、2).在3100~3600 cm-1的峰分别对应-NH基团和-OH基团[42,43],在g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的FT-IR光谱中较为明显.在700~800 cm-1处的峰主要涉及三嗪结构[39~41],为g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的典型结构,Ag的沉积同样提高了吸收峰的强度.在450~600 cm-1的吸收峰对应Bi-O键的伸缩振动[25,44,45],是BiOBr、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的典型结构. ...
Recent advances in BiOBr-based photocatalysts for environmental remediation
1
2021
... TiO2是一种常见的催化剂,可用于硝酸盐氮的光催化还原[15].为了提高硝酸盐氮的转化效率和氮气选择性,可掺杂金属(Au[16]、Ag[17]、Cu[18]等)或金属氧化物(Ag2O[19]、Cu2O[20]等);多元材料的复合也可提高TiO2光催化还原硝酸盐氮.Wang等[21]合成多金属氧化物(POM)/TiO2/Cu复合光催化材料,硝酸盐氮的去除效率为76.53%,氮气选择性为82.09%.Hou等[22]合成核壳状Ag/SiO2@cTiO(2)复合光催化材料,硝酸盐氮的去除率为95.8%,氮气选择性为93.6%.但是,由于TiO2的带隙较宽(约3.2 eV),不能直接利用太阳光[23].g-C3N4(带隙约2.7 eV)[24]和BiOBr(带隙2.6~2.9 eV)[25]的带隙较窄,可用于光催化去除污染物 [26].但是,光催化材料单体的比表面积小、载流子重组速度较快,影响其光催化活性[27].多重材料的掺杂复合,是提高光催化材料效能的主要手段[28].Zheng R等[29]采用g-C3N4可实现约50%硝酸盐氮的去除,采用TiO2/Ti3C2/g-C3N4复合材料使硝酸盐氮的去除效率提高到93.03%,氮气选择性达到96.62%.贵金属Ag具有较低的费米能级(EF=4.74 eV),可阻止光催化材料电子-空穴对的复合[30].不同银掺杂改性的复合材料,都能明显提高g-C3N4或BiOBr的光催化性能.杨利伟等[31]研究发现,与pg-C3N4、BiOBr 单体和二元复合材料pg-C3N4/BiOBr相比,pg-C3N4/BiOBr/Ag(5%)光催化降解磺胺甲唑的效果显著提高.Shelton等[32]用Ag/g-C3N4光催化剂对比了紫外光和可见光下还原水中硝酸盐效果,发现Ag强化了还原作用.本文以g-C3N4、BiOBr和银为基质制备一系列的复合光催化材料(Ag/g-C3N4、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr),选择低浓度硝酸盐氮(50 mg/L以氮计)为处理对象,对比分析不同光催化材料对硝酸盐氮的去除性能及其氮气选择性. ...
Chemically bonded Ni cocatalyst onto the S doped g-C3N4 nanosheets and their synergistic enhancement in H2 production under sunlight irradiation
1
2018
... TiO2是一种常见的催化剂,可用于硝酸盐氮的光催化还原[15].为了提高硝酸盐氮的转化效率和氮气选择性,可掺杂金属(Au[16]、Ag[17]、Cu[18]等)或金属氧化物(Ag2O[19]、Cu2O[20]等);多元材料的复合也可提高TiO2光催化还原硝酸盐氮.Wang等[21]合成多金属氧化物(POM)/TiO2/Cu复合光催化材料,硝酸盐氮的去除效率为76.53%,氮气选择性为82.09%.Hou等[22]合成核壳状Ag/SiO2@cTiO(2)复合光催化材料,硝酸盐氮的去除率为95.8%,氮气选择性为93.6%.但是,由于TiO2的带隙较宽(约3.2 eV),不能直接利用太阳光[23].g-C3N4(带隙约2.7 eV)[24]和BiOBr(带隙2.6~2.9 eV)[25]的带隙较窄,可用于光催化去除污染物 [26].但是,光催化材料单体的比表面积小、载流子重组速度较快,影响其光催化活性[27].多重材料的掺杂复合,是提高光催化材料效能的主要手段[28].Zheng R等[29]采用g-C3N4可实现约50%硝酸盐氮的去除,采用TiO2/Ti3C2/g-C3N4复合材料使硝酸盐氮的去除效率提高到93.03%,氮气选择性达到96.62%.贵金属Ag具有较低的费米能级(EF=4.74 eV),可阻止光催化材料电子-空穴对的复合[30].不同银掺杂改性的复合材料,都能明显提高g-C3N4或BiOBr的光催化性能.杨利伟等[31]研究发现,与pg-C3N4、BiOBr 单体和二元复合材料pg-C3N4/BiOBr相比,pg-C3N4/BiOBr/Ag(5%)光催化降解磺胺甲唑的效果显著提高.Shelton等[32]用Ag/g-C3N4光催化剂对比了紫外光和可见光下还原水中硝酸盐效果,发现Ag强化了还原作用.本文以g-C3N4、BiOBr和银为基质制备一系列的复合光催化材料(Ag/g-C3N4、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr),选择低浓度硝酸盐氮(50 mg/L以氮计)为处理对象,对比分析不同光催化材料对硝酸盐氮的去除性能及其氮气选择性. ...
Preparation, structure and application of g-C3N4/BiOX composite photocatalyst
1
2021
... TiO2是一种常见的催化剂,可用于硝酸盐氮的光催化还原[15].为了提高硝酸盐氮的转化效率和氮气选择性,可掺杂金属(Au[16]、Ag[17]、Cu[18]等)或金属氧化物(Ag2O[19]、Cu2O[20]等);多元材料的复合也可提高TiO2光催化还原硝酸盐氮.Wang等[21]合成多金属氧化物(POM)/TiO2/Cu复合光催化材料,硝酸盐氮的去除效率为76.53%,氮气选择性为82.09%.Hou等[22]合成核壳状Ag/SiO2@cTiO(2)复合光催化材料,硝酸盐氮的去除率为95.8%,氮气选择性为93.6%.但是,由于TiO2的带隙较宽(约3.2 eV),不能直接利用太阳光[23].g-C3N4(带隙约2.7 eV)[24]和BiOBr(带隙2.6~2.9 eV)[25]的带隙较窄,可用于光催化去除污染物 [26].但是,光催化材料单体的比表面积小、载流子重组速度较快,影响其光催化活性[27].多重材料的掺杂复合,是提高光催化材料效能的主要手段[28].Zheng R等[29]采用g-C3N4可实现约50%硝酸盐氮的去除,采用TiO2/Ti3C2/g-C3N4复合材料使硝酸盐氮的去除效率提高到93.03%,氮气选择性达到96.62%.贵金属Ag具有较低的费米能级(EF=4.74 eV),可阻止光催化材料电子-空穴对的复合[30].不同银掺杂改性的复合材料,都能明显提高g-C3N4或BiOBr的光催化性能.杨利伟等[31]研究发现,与pg-C3N4、BiOBr 单体和二元复合材料pg-C3N4/BiOBr相比,pg-C3N4/BiOBr/Ag(5%)光催化降解磺胺甲唑的效果显著提高.Shelton等[32]用Ag/g-C3N4光催化剂对比了紫外光和可见光下还原水中硝酸盐效果,发现Ag强化了还原作用.本文以g-C3N4、BiOBr和银为基质制备一系列的复合光催化材料(Ag/g-C3N4、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr),选择低浓度硝酸盐氮(50 mg/L以氮计)为处理对象,对比分析不同光催化材料对硝酸盐氮的去除性能及其氮气选择性. ...
TiO2/Ti3C2 intercalated with g-C3N4 nanosheets as 3D/2D ternary heterojunctions photocatalyst for the enhanced photocatalytic reduction of nitrate with high N2 selectivity in aqueous solution
3
2021
... TiO2是一种常见的催化剂,可用于硝酸盐氮的光催化还原[15].为了提高硝酸盐氮的转化效率和氮气选择性,可掺杂金属(Au[16]、Ag[17]、Cu[18]等)或金属氧化物(Ag2O[19]、Cu2O[20]等);多元材料的复合也可提高TiO2光催化还原硝酸盐氮.Wang等[21]合成多金属氧化物(POM)/TiO2/Cu复合光催化材料,硝酸盐氮的去除效率为76.53%,氮气选择性为82.09%.Hou等[22]合成核壳状Ag/SiO2@cTiO(2)复合光催化材料,硝酸盐氮的去除率为95.8%,氮气选择性为93.6%.但是,由于TiO2的带隙较宽(约3.2 eV),不能直接利用太阳光[23].g-C3N4(带隙约2.7 eV)[24]和BiOBr(带隙2.6~2.9 eV)[25]的带隙较窄,可用于光催化去除污染物 [26].但是,光催化材料单体的比表面积小、载流子重组速度较快,影响其光催化活性[27].多重材料的掺杂复合,是提高光催化材料效能的主要手段[28].Zheng R等[29]采用g-C3N4可实现约50%硝酸盐氮的去除,采用TiO2/Ti3C2/g-C3N4复合材料使硝酸盐氮的去除效率提高到93.03%,氮气选择性达到96.62%.贵金属Ag具有较低的费米能级(EF=4.74 eV),可阻止光催化材料电子-空穴对的复合[30].不同银掺杂改性的复合材料,都能明显提高g-C3N4或BiOBr的光催化性能.杨利伟等[31]研究发现,与pg-C3N4、BiOBr 单体和二元复合材料pg-C3N4/BiOBr相比,pg-C3N4/BiOBr/Ag(5%)光催化降解磺胺甲唑的效果显著提高.Shelton等[32]用Ag/g-C3N4光催化剂对比了紫外光和可见光下还原水中硝酸盐效果,发现Ag强化了还原作用.本文以g-C3N4、BiOBr和银为基质制备一系列的复合光催化材料(Ag/g-C3N4、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr),选择低浓度硝酸盐氮(50 mg/L以氮计)为处理对象,对比分析不同光催化材料对硝酸盐氮的去除性能及其氮气选择性. ...
... 将预先配置好的50 mg/L(以N计)硝酸钾(KNO3)溶液倒入200 mL反应器中,加入0.2 mg上述光催化剂,通氩气30 min后开启磁力搅拌,暗反应30 min后取样测试;加入0.1 mol/L的甲酸作为空穴清除剂,调整pH值为4,在功率为300 W的金卤灯下开始计时反应.用注射器每30 min取一次水样,用0.22 μm滤头过滤,分别测定水样中硝酸盐氮(NO-N)、亚硝酸盐氮(NO-N)、氨氮(NH-N)的浓度,重复3次实验.用以下公式计算NO3—N的去除率和氮气选择性[29] ...
... Photocatalytic reduction of nitrate in this work and previous literature studies
Table 1| Photocatalyst | Hole scavenger | Light source | RN/% | S/% | Ref. |
|---|
| g-C3N4/Ag/BiOBr | Formic acid | Halide lamp | 95.2 | 92.4 | This work |
| Ag/TiO2 | Formic acid | Xe lamp | 95 | 90 | [48] |
| Au/TiO2 | Oxalic acid | High-pressure Hg lamp | 44 | 49.9 | [49] |
| 0.5TiO2/Ti3C2/g-C3N4 | Formic acid | High-pressure Hg lamp | 93.3 | 96.62 | [29] |
| Fe-LiNbO3 | Formic acid | High-pressure Hg lamp | 90 | 88 | [50] |
| ZnSe/BiVO4 | Formic acid | High-pressure Hg lamp | 89.84 | 91.03 | [14] |
| NH2-MIL-101(Fe)/BiVO4 | Formic acid | High-pressure Hg lamp | 94.8 | 93.5 | [51] |
| Ag/SiO2@cTiO2 | Formic acid | High-pressure Hg lamp | 95.8 | 93.6 | [22] |
<strong>2.5</strong> 光催化降解机理本文分析对比了g-C3N4及BiOBr复合光催化材料,选择硝酸盐氮(50 mg/L)为研究对象以探索光催化还原效率.经g-C3N4/Ag/BiOBr光催化后硝酸盐氮的浓度可降低到2.4 mg/L(图7),氮气选择性可达92.4%(图8),表明使用Ag强化复合材料光催化作用可有效提高光催化的还原性能. ...
Magnetically recoverable Pd-loaded BiFeO3 microcomposite with enhanced visible light photocatalytic performance for pollutant, bacterial and fungal elimination
1
2020
... TiO2是一种常见的催化剂,可用于硝酸盐氮的光催化还原[15].为了提高硝酸盐氮的转化效率和氮气选择性,可掺杂金属(Au[16]、Ag[17]、Cu[18]等)或金属氧化物(Ag2O[19]、Cu2O[20]等);多元材料的复合也可提高TiO2光催化还原硝酸盐氮.Wang等[21]合成多金属氧化物(POM)/TiO2/Cu复合光催化材料,硝酸盐氮的去除效率为76.53%,氮气选择性为82.09%.Hou等[22]合成核壳状Ag/SiO2@cTiO(2)复合光催化材料,硝酸盐氮的去除率为95.8%,氮气选择性为93.6%.但是,由于TiO2的带隙较宽(约3.2 eV),不能直接利用太阳光[23].g-C3N4(带隙约2.7 eV)[24]和BiOBr(带隙2.6~2.9 eV)[25]的带隙较窄,可用于光催化去除污染物 [26].但是,光催化材料单体的比表面积小、载流子重组速度较快,影响其光催化活性[27].多重材料的掺杂复合,是提高光催化材料效能的主要手段[28].Zheng R等[29]采用g-C3N4可实现约50%硝酸盐氮的去除,采用TiO2/Ti3C2/g-C3N4复合材料使硝酸盐氮的去除效率提高到93.03%,氮气选择性达到96.62%.贵金属Ag具有较低的费米能级(EF=4.74 eV),可阻止光催化材料电子-空穴对的复合[30].不同银掺杂改性的复合材料,都能明显提高g-C3N4或BiOBr的光催化性能.杨利伟等[31]研究发现,与pg-C3N4、BiOBr 单体和二元复合材料pg-C3N4/BiOBr相比,pg-C3N4/BiOBr/Ag(5%)光催化降解磺胺甲唑的效果显著提高.Shelton等[32]用Ag/g-C3N4光催化剂对比了紫外光和可见光下还原水中硝酸盐效果,发现Ag强化了还原作用.本文以g-C3N4、BiOBr和银为基质制备一系列的复合光催化材料(Ag/g-C3N4、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr),选择低浓度硝酸盐氮(50 mg/L以氮计)为处理对象,对比分析不同光催化材料对硝酸盐氮的去除性能及其氮气选择性. ...
Preparation of pg-C3N4/BiOBr/Ag composite and photocatalytic degradation of sulfamethoxazole
1
2021
... TiO2是一种常见的催化剂,可用于硝酸盐氮的光催化还原[15].为了提高硝酸盐氮的转化效率和氮气选择性,可掺杂金属(Au[16]、Ag[17]、Cu[18]等)或金属氧化物(Ag2O[19]、Cu2O[20]等);多元材料的复合也可提高TiO2光催化还原硝酸盐氮.Wang等[21]合成多金属氧化物(POM)/TiO2/Cu复合光催化材料,硝酸盐氮的去除效率为76.53%,氮气选择性为82.09%.Hou等[22]合成核壳状Ag/SiO2@cTiO(2)复合光催化材料,硝酸盐氮的去除率为95.8%,氮气选择性为93.6%.但是,由于TiO2的带隙较宽(约3.2 eV),不能直接利用太阳光[23].g-C3N4(带隙约2.7 eV)[24]和BiOBr(带隙2.6~2.9 eV)[25]的带隙较窄,可用于光催化去除污染物 [26].但是,光催化材料单体的比表面积小、载流子重组速度较快,影响其光催化活性[27].多重材料的掺杂复合,是提高光催化材料效能的主要手段[28].Zheng R等[29]采用g-C3N4可实现约50%硝酸盐氮的去除,采用TiO2/Ti3C2/g-C3N4复合材料使硝酸盐氮的去除效率提高到93.03%,氮气选择性达到96.62%.贵金属Ag具有较低的费米能级(EF=4.74 eV),可阻止光催化材料电子-空穴对的复合[30].不同银掺杂改性的复合材料,都能明显提高g-C3N4或BiOBr的光催化性能.杨利伟等[31]研究发现,与pg-C3N4、BiOBr 单体和二元复合材料pg-C3N4/BiOBr相比,pg-C3N4/BiOBr/Ag(5%)光催化降解磺胺甲唑的效果显著提高.Shelton等[32]用Ag/g-C3N4光催化剂对比了紫外光和可见光下还原水中硝酸盐效果,发现Ag强化了还原作用.本文以g-C3N4、BiOBr和银为基质制备一系列的复合光催化材料(Ag/g-C3N4、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr),选择低浓度硝酸盐氮(50 mg/L以氮计)为处理对象,对比分析不同光催化材料对硝酸盐氮的去除性能及其氮气选择性. ...
pg-C3N4/BiOBr/Ag复合材料的制备及其光催化降解磺胺甲噁唑
1
2021
... TiO2是一种常见的催化剂,可用于硝酸盐氮的光催化还原[15].为了提高硝酸盐氮的转化效率和氮气选择性,可掺杂金属(Au[16]、Ag[17]、Cu[18]等)或金属氧化物(Ag2O[19]、Cu2O[20]等);多元材料的复合也可提高TiO2光催化还原硝酸盐氮.Wang等[21]合成多金属氧化物(POM)/TiO2/Cu复合光催化材料,硝酸盐氮的去除效率为76.53%,氮气选择性为82.09%.Hou等[22]合成核壳状Ag/SiO2@cTiO(2)复合光催化材料,硝酸盐氮的去除率为95.8%,氮气选择性为93.6%.但是,由于TiO2的带隙较宽(约3.2 eV),不能直接利用太阳光[23].g-C3N4(带隙约2.7 eV)[24]和BiOBr(带隙2.6~2.9 eV)[25]的带隙较窄,可用于光催化去除污染物 [26].但是,光催化材料单体的比表面积小、载流子重组速度较快,影响其光催化活性[27].多重材料的掺杂复合,是提高光催化材料效能的主要手段[28].Zheng R等[29]采用g-C3N4可实现约50%硝酸盐氮的去除,采用TiO2/Ti3C2/g-C3N4复合材料使硝酸盐氮的去除效率提高到93.03%,氮气选择性达到96.62%.贵金属Ag具有较低的费米能级(EF=4.74 eV),可阻止光催化材料电子-空穴对的复合[30].不同银掺杂改性的复合材料,都能明显提高g-C3N4或BiOBr的光催化性能.杨利伟等[31]研究发现,与pg-C3N4、BiOBr 单体和二元复合材料pg-C3N4/BiOBr相比,pg-C3N4/BiOBr/Ag(5%)光催化降解磺胺甲唑的效果显著提高.Shelton等[32]用Ag/g-C3N4光催化剂对比了紫外光和可见光下还原水中硝酸盐效果,发现Ag强化了还原作用.本文以g-C3N4、BiOBr和银为基质制备一系列的复合光催化材料(Ag/g-C3N4、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr),选择低浓度硝酸盐氮(50 mg/L以氮计)为处理对象,对比分析不同光催化材料对硝酸盐氮的去除性能及其氮气选择性. ...
Photocatalytic reduction of aqueous nitrate with hybrid Ag/g-C3N4 under ultraviolet and visible light
1
2021
... TiO2是一种常见的催化剂,可用于硝酸盐氮的光催化还原[15].为了提高硝酸盐氮的转化效率和氮气选择性,可掺杂金属(Au[16]、Ag[17]、Cu[18]等)或金属氧化物(Ag2O[19]、Cu2O[20]等);多元材料的复合也可提高TiO2光催化还原硝酸盐氮.Wang等[21]合成多金属氧化物(POM)/TiO2/Cu复合光催化材料,硝酸盐氮的去除效率为76.53%,氮气选择性为82.09%.Hou等[22]合成核壳状Ag/SiO2@cTiO(2)复合光催化材料,硝酸盐氮的去除率为95.8%,氮气选择性为93.6%.但是,由于TiO2的带隙较宽(约3.2 eV),不能直接利用太阳光[23].g-C3N4(带隙约2.7 eV)[24]和BiOBr(带隙2.6~2.9 eV)[25]的带隙较窄,可用于光催化去除污染物 [26].但是,光催化材料单体的比表面积小、载流子重组速度较快,影响其光催化活性[27].多重材料的掺杂复合,是提高光催化材料效能的主要手段[28].Zheng R等[29]采用g-C3N4可实现约50%硝酸盐氮的去除,采用TiO2/Ti3C2/g-C3N4复合材料使硝酸盐氮的去除效率提高到93.03%,氮气选择性达到96.62%.贵金属Ag具有较低的费米能级(EF=4.74 eV),可阻止光催化材料电子-空穴对的复合[30].不同银掺杂改性的复合材料,都能明显提高g-C3N4或BiOBr的光催化性能.杨利伟等[31]研究发现,与pg-C3N4、BiOBr 单体和二元复合材料pg-C3N4/BiOBr相比,pg-C3N4/BiOBr/Ag(5%)光催化降解磺胺甲唑的效果显著提高.Shelton等[32]用Ag/g-C3N4光催化剂对比了紫外光和可见光下还原水中硝酸盐效果,发现Ag强化了还原作用.本文以g-C3N4、BiOBr和银为基质制备一系列的复合光催化材料(Ag/g-C3N4、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr),选择低浓度硝酸盐氮(50 mg/L以氮计)为处理对象,对比分析不同光催化材料对硝酸盐氮的去除性能及其氮气选择性. ...
A g-C3N4/BiOBr visible-light-driven composite: Synthesis via a reactable ionic liquid and improved photocatalytic activity
2
2013
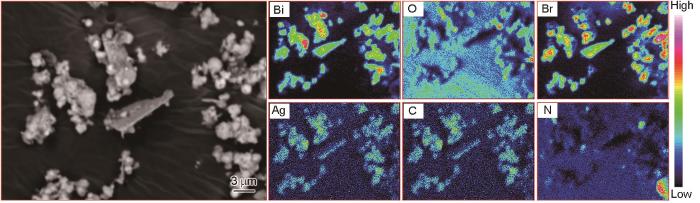
... 为了了解光催化材料的表面化学组成及状态,采用X射线光电子能谱(XPS)对BiOBr、g-C3N4、g-C3N4/Ag/BiOBr光催化剂进行了分析(图2).图2a中为BiOBr、g-C3N4、g-C3N4/Ag/BiOBr全谱分析图,其中元素C和元素N能在g-C3N4、g-C3N4/Ag/BiOBr光催化剂中发现,Bi、O、Br等元素能在BiOBr、g-C3N4/Ag/BiOBr光催化剂中发现,但g-C3N4/Ag/BiOBr中的Ag峰相对较弱,这可能是由于催化剂中银含量较低所致.图2b BiOBr、g-C3N4/Ag/BiOBr中Bi的结合能均为159.2和164.5 eV,峰值均相差5.3 eV,说明两者均有Bi3+的存在[33].图2c中BiOBr中Br的结合能为68.3和69.4 eV,分别对应于Br3d5/2和Br3d3/2[34].图2d中g-C3N4和g-C3N4/Ag/BiOBr均存在典型C-C键(284.8 eV)[35]以及 N=C-N键(288.2 eV),而g-C3N4中存在C-NH2(285.1 eV),这可能是g-C3N4/Ag/BiOBr中其他元素的遮蔽作用导致.图2e中g-C3N4存在C-N=C键(398.8 eV)和C-NH2键(400.7 eV),g-C3N4/Ag/BiOBr结合能为299.2 eV(C-N=C键)和400.4 eV(C-NH2键),N的结合能产生了轻微的偏移,这可能是材料杂化导致.图2f中BiOBr 中结合能为530.0 eV(Bi-O键)和531.2 eV(O-H键)[36],g-C3N4/Ag/BiOBr中结合能为530.1 eV(Bi-O键)和531.2 eV(O-H键),O的结合能也出现轻微的偏移.图2g中Ag结合能为267.9 eV和373.9 eV,两个峰间距为6.0 eV,说明有单质银的存在[37]. ...
... Ag作为电子捕捉剂,能减缓光催化材料的电子-空穴对的复合[52],使光催化剂的催化性能提高(图6~8).图6中的紫外-可见漫反射光谱分析表明,Ag强化了材料对可见光的响应,Ag/g-C3N4、Ag/BiOBr、g-C3N4/Ag/BiOBr在可见光区域光吸收能力明显加强,进一步促进复合光催化材料的光还原性能.g-C3N4/Ag/BiOBr光催化材料硝酸盐的光还原机制,如图9 所示.g-C3N4价带电势为1.52 eV,导带电势为-1.2 eV[33];BiOBr价带电势为3.18 eV,导带电势为0.30 eV[39].这表明,g-C3N4和BiOBr导带电势接近,在银的作用下BiOBr的光生电子经银单质转移到g-C3N4的价带上形成了Z型复合光催化结构.部分硝酸盐氮可被复合光催化剂直接氧化.亚硝酸盐氮的氧化电势较低,可被迅速还原,系统中亚硝酸盐氮的浓度较低(图8).同时,实验中使用甲酸作为空穴清除剂,甲酸在复合材料空穴作用下转化成强氧化性物质(COO.-)[46,47],其较高的电势可进一步还原硝酸盐氮,从而促进了硝酸盐氮的去除. ...
Novel direct dual Z-scheme AgBr(Ag)/MIL-101(Cr)/CuFe2O4 for efficient conversion of nitrate to nitrogen
1
2020
... 为了了解光催化材料的表面化学组成及状态,采用X射线光电子能谱(XPS)对BiOBr、g-C3N4、g-C3N4/Ag/BiOBr光催化剂进行了分析(图2).图2a中为BiOBr、g-C3N4、g-C3N4/Ag/BiOBr全谱分析图,其中元素C和元素N能在g-C3N4、g-C3N4/Ag/BiOBr光催化剂中发现,Bi、O、Br等元素能在BiOBr、g-C3N4/Ag/BiOBr光催化剂中发现,但g-C3N4/Ag/BiOBr中的Ag峰相对较弱,这可能是由于催化剂中银含量较低所致.图2b BiOBr、g-C3N4/Ag/BiOBr中Bi的结合能均为159.2和164.5 eV,峰值均相差5.3 eV,说明两者均有Bi3+的存在[33].图2c中BiOBr中Br的结合能为68.3和69.4 eV,分别对应于Br3d5/2和Br3d3/2[34].图2d中g-C3N4和g-C3N4/Ag/BiOBr均存在典型C-C键(284.8 eV)[35]以及 N=C-N键(288.2 eV),而g-C3N4中存在C-NH2(285.1 eV),这可能是g-C3N4/Ag/BiOBr中其他元素的遮蔽作用导致.图2e中g-C3N4存在C-N=C键(398.8 eV)和C-NH2键(400.7 eV),g-C3N4/Ag/BiOBr结合能为299.2 eV(C-N=C键)和400.4 eV(C-NH2键),N的结合能产生了轻微的偏移,这可能是材料杂化导致.图2f中BiOBr 中结合能为530.0 eV(Bi-O键)和531.2 eV(O-H键)[36],g-C3N4/Ag/BiOBr中结合能为530.1 eV(Bi-O键)和531.2 eV(O-H键),O的结合能也出现轻微的偏移.图2g中Ag结合能为267.9 eV和373.9 eV,两个峰间距为6.0 eV,说明有单质银的存在[37]. ...
IR and Raman spectra properties of Bi2O3-ZnO-B2O3-BaO quaternary glass system
1
2014
... 为了了解光催化材料的表面化学组成及状态,采用X射线光电子能谱(XPS)对BiOBr、g-C3N4、g-C3N4/Ag/BiOBr光催化剂进行了分析(图2).图2a中为BiOBr、g-C3N4、g-C3N4/Ag/BiOBr全谱分析图,其中元素C和元素N能在g-C3N4、g-C3N4/Ag/BiOBr光催化剂中发现,Bi、O、Br等元素能在BiOBr、g-C3N4/Ag/BiOBr光催化剂中发现,但g-C3N4/Ag/BiOBr中的Ag峰相对较弱,这可能是由于催化剂中银含量较低所致.图2b BiOBr、g-C3N4/Ag/BiOBr中Bi的结合能均为159.2和164.5 eV,峰值均相差5.3 eV,说明两者均有Bi3+的存在[33].图2c中BiOBr中Br的结合能为68.3和69.4 eV,分别对应于Br3d5/2和Br3d3/2[34].图2d中g-C3N4和g-C3N4/Ag/BiOBr均存在典型C-C键(284.8 eV)[35]以及 N=C-N键(288.2 eV),而g-C3N4中存在C-NH2(285.1 eV),这可能是g-C3N4/Ag/BiOBr中其他元素的遮蔽作用导致.图2e中g-C3N4存在C-N=C键(398.8 eV)和C-NH2键(400.7 eV),g-C3N4/Ag/BiOBr结合能为299.2 eV(C-N=C键)和400.4 eV(C-NH2键),N的结合能产生了轻微的偏移,这可能是材料杂化导致.图2f中BiOBr 中结合能为530.0 eV(Bi-O键)和531.2 eV(O-H键)[36],g-C3N4/Ag/BiOBr中结合能为530.1 eV(Bi-O键)和531.2 eV(O-H键),O的结合能也出现轻微的偏移.图2g中Ag结合能为267.9 eV和373.9 eV,两个峰间距为6.0 eV,说明有单质银的存在[37]. ...
Highly porous carbon nitride by supramolecular preassembly of monomers for photocatalytic removal of sulfamethazine under visible light driven
1
2018
... 为了了解光催化材料的表面化学组成及状态,采用X射线光电子能谱(XPS)对BiOBr、g-C3N4、g-C3N4/Ag/BiOBr光催化剂进行了分析(图2).图2a中为BiOBr、g-C3N4、g-C3N4/Ag/BiOBr全谱分析图,其中元素C和元素N能在g-C3N4、g-C3N4/Ag/BiOBr光催化剂中发现,Bi、O、Br等元素能在BiOBr、g-C3N4/Ag/BiOBr光催化剂中发现,但g-C3N4/Ag/BiOBr中的Ag峰相对较弱,这可能是由于催化剂中银含量较低所致.图2b BiOBr、g-C3N4/Ag/BiOBr中Bi的结合能均为159.2和164.5 eV,峰值均相差5.3 eV,说明两者均有Bi3+的存在[33].图2c中BiOBr中Br的结合能为68.3和69.4 eV,分别对应于Br3d5/2和Br3d3/2[34].图2d中g-C3N4和g-C3N4/Ag/BiOBr均存在典型C-C键(284.8 eV)[35]以及 N=C-N键(288.2 eV),而g-C3N4中存在C-NH2(285.1 eV),这可能是g-C3N4/Ag/BiOBr中其他元素的遮蔽作用导致.图2e中g-C3N4存在C-N=C键(398.8 eV)和C-NH2键(400.7 eV),g-C3N4/Ag/BiOBr结合能为299.2 eV(C-N=C键)和400.4 eV(C-NH2键),N的结合能产生了轻微的偏移,这可能是材料杂化导致.图2f中BiOBr 中结合能为530.0 eV(Bi-O键)和531.2 eV(O-H键)[36],g-C3N4/Ag/BiOBr中结合能为530.1 eV(Bi-O键)和531.2 eV(O-H键),O的结合能也出现轻微的偏移.图2g中Ag结合能为267.9 eV和373.9 eV,两个峰间距为6.0 eV,说明有单质银的存在[37]. ...
Synthesis and photocatalytic property of BiOBr/palygorskite composites
1
2014
... 为了了解光催化材料的表面化学组成及状态,采用X射线光电子能谱(XPS)对BiOBr、g-C3N4、g-C3N4/Ag/BiOBr光催化剂进行了分析(图2).图2a中为BiOBr、g-C3N4、g-C3N4/Ag/BiOBr全谱分析图,其中元素C和元素N能在g-C3N4、g-C3N4/Ag/BiOBr光催化剂中发现,Bi、O、Br等元素能在BiOBr、g-C3N4/Ag/BiOBr光催化剂中发现,但g-C3N4/Ag/BiOBr中的Ag峰相对较弱,这可能是由于催化剂中银含量较低所致.图2b BiOBr、g-C3N4/Ag/BiOBr中Bi的结合能均为159.2和164.5 eV,峰值均相差5.3 eV,说明两者均有Bi3+的存在[33].图2c中BiOBr中Br的结合能为68.3和69.4 eV,分别对应于Br3d5/2和Br3d3/2[34].图2d中g-C3N4和g-C3N4/Ag/BiOBr均存在典型C-C键(284.8 eV)[35]以及 N=C-N键(288.2 eV),而g-C3N4中存在C-NH2(285.1 eV),这可能是g-C3N4/Ag/BiOBr中其他元素的遮蔽作用导致.图2e中g-C3N4存在C-N=C键(398.8 eV)和C-NH2键(400.7 eV),g-C3N4/Ag/BiOBr结合能为299.2 eV(C-N=C键)和400.4 eV(C-NH2键),N的结合能产生了轻微的偏移,这可能是材料杂化导致.图2f中BiOBr 中结合能为530.0 eV(Bi-O键)和531.2 eV(O-H键)[36],g-C3N4/Ag/BiOBr中结合能为530.1 eV(Bi-O键)和531.2 eV(O-H键),O的结合能也出现轻微的偏移.图2g中Ag结合能为267.9 eV和373.9 eV,两个峰间距为6.0 eV,说明有单质银的存在[37]. ...
MoS2 quantum dots decorated g-C3N4/Ag heterostructures for enhanced visible light photocatalytic activity
1
2018
... 从图3可以看出,Ag/g-C3N4、Ag/BiOBr和g-C3N4/Ag/BiOBr的FT-IR谱中中未出现明显的Ag衍射峰,其原因是Ag沉积量(5%)较少[38].同时g-C3N4/BiOBr、g-C3N4/Ag/BiOBr中g-C3N4衍射峰也不明显(在27.46°出现了较弱的g-C3N4(110)的晶面特征衍射峰),其主要原因是在催化剂制作过程中g-C3N4为主要基质,表层为BiOBr覆盖. ...
Pyrolysis synthesized g-C3N4 for photocatalytic degradation of methylene blue
3
2013
... 为了揭示光催化剂的化学组成及化学键情况,用傅里叶变换红外光谱(FT-IR)分析了不同催化剂(g-C3N4、Ag/g-C3N4、BiOBr、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr)(图3).由图3可见,在1200~1700 cm-1之间出现了明显的吸收峰,对应C-N结构[39~41],是g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂中的典型结构.与g-C3N4、Ag/g-C3N4对比表明,Ag的沉积明显强化了吸收峰的强度,而g-C3N4/Ag/BiOBr催化剂的吸收峰反而减弱,其原因可能是g-C3N4作为基质被Ag、BiOBr包裹(图1、2).在3100~3600 cm-1的峰分别对应-NH基团和-OH基团[42,43],在g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的FT-IR光谱中较为明显.在700~800 cm-1处的峰主要涉及三嗪结构[39~41],为g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的典型结构,Ag的沉积同样提高了吸收峰的强度.在450~600 cm-1的吸收峰对应Bi-O键的伸缩振动[25,44,45],是BiOBr、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的典型结构. ...
... [39~41],为g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的典型结构,Ag的沉积同样提高了吸收峰的强度.在450~600 cm-1的吸收峰对应Bi-O键的伸缩振动[25,44,45],是BiOBr、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的典型结构. ...
... Ag作为电子捕捉剂,能减缓光催化材料的电子-空穴对的复合[52],使光催化剂的催化性能提高(图6~8).图6中的紫外-可见漫反射光谱分析表明,Ag强化了材料对可见光的响应,Ag/g-C3N4、Ag/BiOBr、g-C3N4/Ag/BiOBr在可见光区域光吸收能力明显加强,进一步促进复合光催化材料的光还原性能.g-C3N4/Ag/BiOBr光催化材料硝酸盐的光还原机制,如图9 所示.g-C3N4价带电势为1.52 eV,导带电势为-1.2 eV[33];BiOBr价带电势为3.18 eV,导带电势为0.30 eV[39].这表明,g-C3N4和BiOBr导带电势接近,在银的作用下BiOBr的光生电子经银单质转移到g-C3N4的价带上形成了Z型复合光催化结构.部分硝酸盐氮可被复合光催化剂直接氧化.亚硝酸盐氮的氧化电势较低,可被迅速还原,系统中亚硝酸盐氮的浓度较低(图8).同时,实验中使用甲酸作为空穴清除剂,甲酸在复合材料空穴作用下转化成强氧化性物质(COO.-)[46,47],其较高的电势可进一步还原硝酸盐氮,从而促进了硝酸盐氮的去除. ...
Features of the synthesis of carbon nitride oxide (g-C3N4)O at urea pyrolysis
0
2016
Synthesis and characterization of graphitic carbon nitride sub-microspheres using microwave method under mild condition
2
2013
... 为了揭示光催化剂的化学组成及化学键情况,用傅里叶变换红外光谱(FT-IR)分析了不同催化剂(g-C3N4、Ag/g-C3N4、BiOBr、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr)(图3).由图3可见,在1200~1700 cm-1之间出现了明显的吸收峰,对应C-N结构[39~41],是g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂中的典型结构.与g-C3N4、Ag/g-C3N4对比表明,Ag的沉积明显强化了吸收峰的强度,而g-C3N4/Ag/BiOBr催化剂的吸收峰反而减弱,其原因可能是g-C3N4作为基质被Ag、BiOBr包裹(图1、2).在3100~3600 cm-1的峰分别对应-NH基团和-OH基团[42,43],在g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的FT-IR光谱中较为明显.在700~800 cm-1处的峰主要涉及三嗪结构[39~41],为g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的典型结构,Ag的沉积同样提高了吸收峰的强度.在450~600 cm-1的吸收峰对应Bi-O键的伸缩振动[25,44,45],是BiOBr、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的典型结构. ...
... ~41],为g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的典型结构,Ag的沉积同样提高了吸收峰的强度.在450~600 cm-1的吸收峰对应Bi-O键的伸缩振动[25,44,45],是BiOBr、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的典型结构. ...
Ag/g-C3N4 catalyst with superior catalytic performance for the degradation of dyes: a borohydride-generated superoxide radical approach
1
2015
... 为了揭示光催化剂的化学组成及化学键情况,用傅里叶变换红外光谱(FT-IR)分析了不同催化剂(g-C3N4、Ag/g-C3N4、BiOBr、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr)(图3).由图3可见,在1200~1700 cm-1之间出现了明显的吸收峰,对应C-N结构[39~41],是g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂中的典型结构.与g-C3N4、Ag/g-C3N4对比表明,Ag的沉积明显强化了吸收峰的强度,而g-C3N4/Ag/BiOBr催化剂的吸收峰反而减弱,其原因可能是g-C3N4作为基质被Ag、BiOBr包裹(图1、2).在3100~3600 cm-1的峰分别对应-NH基团和-OH基团[42,43],在g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的FT-IR光谱中较为明显.在700~800 cm-1处的峰主要涉及三嗪结构[39~41],为g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的典型结构,Ag的沉积同样提高了吸收峰的强度.在450~600 cm-1的吸收峰对应Bi-O键的伸缩振动[25,44,45],是BiOBr、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的典型结构. ...
Photocatalytic degradation of levofloxacin in aqueous phase using Ag/AgBr/BiOBr microplates under visible light
1
2017
... 为了揭示光催化剂的化学组成及化学键情况,用傅里叶变换红外光谱(FT-IR)分析了不同催化剂(g-C3N4、Ag/g-C3N4、BiOBr、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr)(图3).由图3可见,在1200~1700 cm-1之间出现了明显的吸收峰,对应C-N结构[39~41],是g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂中的典型结构.与g-C3N4、Ag/g-C3N4对比表明,Ag的沉积明显强化了吸收峰的强度,而g-C3N4/Ag/BiOBr催化剂的吸收峰反而减弱,其原因可能是g-C3N4作为基质被Ag、BiOBr包裹(图1、2).在3100~3600 cm-1的峰分别对应-NH基团和-OH基团[42,43],在g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的FT-IR光谱中较为明显.在700~800 cm-1处的峰主要涉及三嗪结构[39~41],为g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的典型结构,Ag的沉积同样提高了吸收峰的强度.在450~600 cm-1的吸收峰对应Bi-O键的伸缩振动[25,44,45],是BiOBr、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的典型结构. ...
Visible-light-driven photodegradation of rhodamine B on Ag-modified BiOBr
1
2012
... 为了揭示光催化剂的化学组成及化学键情况,用傅里叶变换红外光谱(FT-IR)分析了不同催化剂(g-C3N4、Ag/g-C3N4、BiOBr、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr)(图3).由图3可见,在1200~1700 cm-1之间出现了明显的吸收峰,对应C-N结构[39~41],是g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂中的典型结构.与g-C3N4、Ag/g-C3N4对比表明,Ag的沉积明显强化了吸收峰的强度,而g-C3N4/Ag/BiOBr催化剂的吸收峰反而减弱,其原因可能是g-C3N4作为基质被Ag、BiOBr包裹(图1、2).在3100~3600 cm-1的峰分别对应-NH基团和-OH基团[42,43],在g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的FT-IR光谱中较为明显.在700~800 cm-1处的峰主要涉及三嗪结构[39~41],为g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的典型结构,Ag的沉积同样提高了吸收峰的强度.在450~600 cm-1的吸收峰对应Bi-O键的伸缩振动[25,44,45],是BiOBr、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的典型结构. ...
Ag/AgBr/BiOBr hollow hierarchical microspheres with enhanced activity and stability for RhB degradation under visible light irradiation
1
2013
... 为了揭示光催化剂的化学组成及化学键情况,用傅里叶变换红外光谱(FT-IR)分析了不同催化剂(g-C3N4、Ag/g-C3N4、BiOBr、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr)(图3).由图3可见,在1200~1700 cm-1之间出现了明显的吸收峰,对应C-N结构[39~41],是g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂中的典型结构.与g-C3N4、Ag/g-C3N4对比表明,Ag的沉积明显强化了吸收峰的强度,而g-C3N4/Ag/BiOBr催化剂的吸收峰反而减弱,其原因可能是g-C3N4作为基质被Ag、BiOBr包裹(图1、2).在3100~3600 cm-1的峰分别对应-NH基团和-OH基团[42,43],在g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的FT-IR光谱中较为明显.在700~800 cm-1处的峰主要涉及三嗪结构[39~41],为g-C3N4、Ag/g-C3N4、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的典型结构,Ag的沉积同样提高了吸收峰的强度.在450~600 cm-1的吸收峰对应Bi-O键的伸缩振动[25,44,45],是BiOBr、Ag/BiOBr、g-C3N4/BiOBr、g-C3N4/Ag/BiOBr催化剂的典型结构. ...
In situ synthesis of N-doped TiO2 on Ti3C2 MXene with enhanced photocatalytic activity in the selective reduction of nitrate to N2
2
2022
... 为了评估复合光催化剂还原效果,在表1给出了相关复合光催化还原硝酸盐效果的对比.研究光催化还原硝酸盐氮,多使用甲酸作为光催化剂剂的空穴清除剂,而用草酸作为空穴清除剂时硝酸盐氮的转化率相对较低(表1).其原因是,甲酸在捕捉空穴清除剂生成强氧化性物质(COO.-)[46,47],可进一步促进硝酸盐氮的还原.由于硝酸盐氮相对较为稳定,在光催化过程中多采用高压汞灯提供紫外光源.近年来,在硝酸盐氮的转化率和氮气选择性方面均取得了较好的效果.本文采用对可见光有较强响应的g-C3N4、BiOBr催化材料进行合成,并使用贵金属银进行掺杂以提高g-C3N4/Ag/BiOBr复合材料的性能.实验中用金卤灯光源,硝酸盐氮的去除效率仅次于Ag/SiO2@cTiO2复合材料,氮气选择性也较高,表明本文制作的复合光催化材料具有较高的性能. ...
... Ag作为电子捕捉剂,能减缓光催化材料的电子-空穴对的复合[52],使光催化剂的催化性能提高(图6~8).图6中的紫外-可见漫反射光谱分析表明,Ag强化了材料对可见光的响应,Ag/g-C3N4、Ag/BiOBr、g-C3N4/Ag/BiOBr在可见光区域光吸收能力明显加强,进一步促进复合光催化材料的光还原性能.g-C3N4/Ag/BiOBr光催化材料硝酸盐的光还原机制,如图9 所示.g-C3N4价带电势为1.52 eV,导带电势为-1.2 eV[33];BiOBr价带电势为3.18 eV,导带电势为0.30 eV[39].这表明,g-C3N4和BiOBr导带电势接近,在银的作用下BiOBr的光生电子经银单质转移到g-C3N4的价带上形成了Z型复合光催化结构.部分硝酸盐氮可被复合光催化剂直接氧化.亚硝酸盐氮的氧化电势较低,可被迅速还原,系统中亚硝酸盐氮的浓度较低(图8).同时,实验中使用甲酸作为空穴清除剂,甲酸在复合材料空穴作用下转化成强氧化性物质(COO.-)[46,47],其较高的电势可进一步还原硝酸盐氮,从而促进了硝酸盐氮的去除. ...
Selective reduction of nitrate to nitrogen gas by novel Cu2O-Cu0@Fe0 composite combined with HCOOH under UV radiation
2
2019
... 为了评估复合光催化剂还原效果,在表1给出了相关复合光催化还原硝酸盐效果的对比.研究光催化还原硝酸盐氮,多使用甲酸作为光催化剂剂的空穴清除剂,而用草酸作为空穴清除剂时硝酸盐氮的转化率相对较低(表1).其原因是,甲酸在捕捉空穴清除剂生成强氧化性物质(COO.-)[46,47],可进一步促进硝酸盐氮的还原.由于硝酸盐氮相对较为稳定,在光催化过程中多采用高压汞灯提供紫外光源.近年来,在硝酸盐氮的转化率和氮气选择性方面均取得了较好的效果.本文采用对可见光有较强响应的g-C3N4、BiOBr催化材料进行合成,并使用贵金属银进行掺杂以提高g-C3N4/Ag/BiOBr复合材料的性能.实验中用金卤灯光源,硝酸盐氮的去除效率仅次于Ag/SiO2@cTiO2复合材料,氮气选择性也较高,表明本文制作的复合光催化材料具有较高的性能. ...
... Ag作为电子捕捉剂,能减缓光催化材料的电子-空穴对的复合[52],使光催化剂的催化性能提高(图6~8).图6中的紫外-可见漫反射光谱分析表明,Ag强化了材料对可见光的响应,Ag/g-C3N4、Ag/BiOBr、g-C3N4/Ag/BiOBr在可见光区域光吸收能力明显加强,进一步促进复合光催化材料的光还原性能.g-C3N4/Ag/BiOBr光催化材料硝酸盐的光还原机制,如图9 所示.g-C3N4价带电势为1.52 eV,导带电势为-1.2 eV[33];BiOBr价带电势为3.18 eV,导带电势为0.30 eV[39].这表明,g-C3N4和BiOBr导带电势接近,在银的作用下BiOBr的光生电子经银单质转移到g-C3N4的价带上形成了Z型复合光催化结构.部分硝酸盐氮可被复合光催化剂直接氧化.亚硝酸盐氮的氧化电势较低,可被迅速还原,系统中亚硝酸盐氮的浓度较低(图8).同时,实验中使用甲酸作为空穴清除剂,甲酸在复合材料空穴作用下转化成强氧化性物质(COO.-)[46,47],其较高的电势可进一步还原硝酸盐氮,从而促进了硝酸盐氮的去除. ...
The selective deposition of silver nanoparticles onto {1 0 1} facets of TiO2 nanocrystals with co-exposed {0 0 1}/{1 0 1} facets, and their enhanced photocatalytic reduction of aqueous nitrate under simulated solar illumination
1
2016
... Photocatalytic reduction of nitrate in this work and previous literature studies
Table 1| Photocatalyst | Hole scavenger | Light source | RN/% | S/% | Ref. |
|---|
| g-C3N4/Ag/BiOBr | Formic acid | Halide lamp | 95.2 | 92.4 | This work |
| Ag/TiO2 | Formic acid | Xe lamp | 95 | 90 | [48] |
| Au/TiO2 | Oxalic acid | High-pressure Hg lamp | 44 | 49.9 | [49] |
| 0.5TiO2/Ti3C2/g-C3N4 | Formic acid | High-pressure Hg lamp | 93.3 | 96.62 | [29] |
| Fe-LiNbO3 | Formic acid | High-pressure Hg lamp | 90 | 88 | [50] |
| ZnSe/BiVO4 | Formic acid | High-pressure Hg lamp | 89.84 | 91.03 | [14] |
| NH2-MIL-101(Fe)/BiVO4 | Formic acid | High-pressure Hg lamp | 94.8 | 93.5 | [51] |
| Ag/SiO2@cTiO2 | Formic acid | High-pressure Hg lamp | 95.8 | 93.6 | [22] |
<strong>2.5</strong> 光催化降解机理本文分析对比了g-C3N4及BiOBr复合光催化材料,选择硝酸盐氮(50 mg/L)为研究对象以探索光催化还原效率.经g-C3N4/Ag/BiOBr光催化后硝酸盐氮的浓度可降低到2.4 mg/L(图7),氮气选择性可达92.4%(图8),表明使用Ag强化复合材料光催化作用可有效提高光催化的还原性能. ...
Effective photocatalytic reduction of nitrate to ammonia in an aqueous suspension of metal-loaded titanium (IV) oxide particles in the presence of oxalic acid
1
2001
... Photocatalytic reduction of nitrate in this work and previous literature studies
Table 1| Photocatalyst | Hole scavenger | Light source | RN/% | S/% | Ref. |
|---|
| g-C3N4/Ag/BiOBr | Formic acid | Halide lamp | 95.2 | 92.4 | This work |
| Ag/TiO2 | Formic acid | Xe lamp | 95 | 90 | [48] |
| Au/TiO2 | Oxalic acid | High-pressure Hg lamp | 44 | 49.9 | [49] |
| 0.5TiO2/Ti3C2/g-C3N4 | Formic acid | High-pressure Hg lamp | 93.3 | 96.62 | [29] |
| Fe-LiNbO3 | Formic acid | High-pressure Hg lamp | 90 | 88 | [50] |
| ZnSe/BiVO4 | Formic acid | High-pressure Hg lamp | 89.84 | 91.03 | [14] |
| NH2-MIL-101(Fe)/BiVO4 | Formic acid | High-pressure Hg lamp | 94.8 | 93.5 | [51] |
| Ag/SiO2@cTiO2 | Formic acid | High-pressure Hg lamp | 95.8 | 93.6 | [22] |
<strong>2.5</strong> 光催化降解机理本文分析对比了g-C3N4及BiOBr复合光催化材料,选择硝酸盐氮(50 mg/L)为研究对象以探索光催化还原效率.经g-C3N4/Ag/BiOBr光催化后硝酸盐氮的浓度可降低到2.4 mg/L(图7),氮气选择性可达92.4%(图8),表明使用Ag强化复合材料光催化作用可有效提高光催化的还原性能. ...
Enhanced photocatalytic reduction of nitrate enabled by Fe-doped LiNbO3 materials in water: Performance and mechanism
1
2021
... Photocatalytic reduction of nitrate in this work and previous literature studies
Table 1| Photocatalyst | Hole scavenger | Light source | RN/% | S/% | Ref. |
|---|
| g-C3N4/Ag/BiOBr | Formic acid | Halide lamp | 95.2 | 92.4 | This work |
| Ag/TiO2 | Formic acid | Xe lamp | 95 | 90 | [48] |
| Au/TiO2 | Oxalic acid | High-pressure Hg lamp | 44 | 49.9 | [49] |
| 0.5TiO2/Ti3C2/g-C3N4 | Formic acid | High-pressure Hg lamp | 93.3 | 96.62 | [29] |
| Fe-LiNbO3 | Formic acid | High-pressure Hg lamp | 90 | 88 | [50] |
| ZnSe/BiVO4 | Formic acid | High-pressure Hg lamp | 89.84 | 91.03 | [14] |
| NH2-MIL-101(Fe)/BiVO4 | Formic acid | High-pressure Hg lamp | 94.8 | 93.5 | [51] |
| Ag/SiO2@cTiO2 | Formic acid | High-pressure Hg lamp | 95.8 | 93.6 | [22] |
<strong>2.5</strong> 光催化降解机理本文分析对比了g-C3N4及BiOBr复合光催化材料,选择硝酸盐氮(50 mg/L)为研究对象以探索光催化还原效率.经g-C3N4/Ag/BiOBr光催化后硝酸盐氮的浓度可降低到2.4 mg/L(图7),氮气选择性可达92.4%(图8),表明使用Ag强化复合材料光催化作用可有效提高光催化的还原性能. ...
Selective reduction of nitrate into N2 by novel Z-scheme NH2-MIL-101(Fe)/BiVO4 heterojunction with enhanced photocatalytic activity
1
2022
... Photocatalytic reduction of nitrate in this work and previous literature studies
Table 1| Photocatalyst | Hole scavenger | Light source | RN/% | S/% | Ref. |
|---|
| g-C3N4/Ag/BiOBr | Formic acid | Halide lamp | 95.2 | 92.4 | This work |
| Ag/TiO2 | Formic acid | Xe lamp | 95 | 90 | [48] |
| Au/TiO2 | Oxalic acid | High-pressure Hg lamp | 44 | 49.9 | [49] |
| 0.5TiO2/Ti3C2/g-C3N4 | Formic acid | High-pressure Hg lamp | 93.3 | 96.62 | [29] |
| Fe-LiNbO3 | Formic acid | High-pressure Hg lamp | 90 | 88 | [50] |
| ZnSe/BiVO4 | Formic acid | High-pressure Hg lamp | 89.84 | 91.03 | [14] |
| NH2-MIL-101(Fe)/BiVO4 | Formic acid | High-pressure Hg lamp | 94.8 | 93.5 | [51] |
| Ag/SiO2@cTiO2 | Formic acid | High-pressure Hg lamp | 95.8 | 93.6 | [22] |
<strong>2.5</strong> 光催化降解机理本文分析对比了g-C3N4及BiOBr复合光催化材料,选择硝酸盐氮(50 mg/L)为研究对象以探索光催化还原效率.经g-C3N4/Ag/BiOBr光催化后硝酸盐氮的浓度可降低到2.4 mg/L(图7),氮气选择性可达92.4%(图8),表明使用Ag强化复合材料光催化作用可有效提高光催化的还原性能. ...
Enhanced visible light photocatalytic activity of novel polymeric g-C3N4 loaded with Ag nanoparticles
1
2011
... Ag作为电子捕捉剂,能减缓光催化材料的电子-空穴对的复合[52],使光催化剂的催化性能提高(图6~8).图6中的紫外-可见漫反射光谱分析表明,Ag强化了材料对可见光的响应,Ag/g-C3N4、Ag/BiOBr、g-C3N4/Ag/BiOBr在可见光区域光吸收能力明显加强,进一步促进复合光催化材料的光还原性能.g-C3N4/Ag/BiOBr光催化材料硝酸盐的光还原机制,如图9 所示.g-C3N4价带电势为1.52 eV,导带电势为-1.2 eV[33];BiOBr价带电势为3.18 eV,导带电势为0.30 eV[39].这表明,g-C3N4和BiOBr导带电势接近,在银的作用下BiOBr的光生电子经银单质转移到g-C3N4的价带上形成了Z型复合光催化结构.部分硝酸盐氮可被复合光催化剂直接氧化.亚硝酸盐氮的氧化电势较低,可被迅速还原,系统中亚硝酸盐氮的浓度较低(图8).同时,实验中使用甲酸作为空穴清除剂,甲酸在复合材料空穴作用下转化成强氧化性物质(COO.-)[46,47],其较高的电势可进一步还原硝酸盐氮,从而促进了硝酸盐氮的去除. ...