1
2006
... 微晶玻璃是一类重要的新型无机材料,由性能显著不同的玻璃相和微晶相组成.根据复合材料的协同效应,特定成分的微晶玻璃其综合性能优于其各组成相的性能 [1,2].微晶玻璃的一个重要应用,是制作储能无机电介质[3,4].根据高介电性能的微晶相和高击穿强度的玻璃相的协同效应,可设计出兼具高介电与耐击穿性能的微晶玻璃电介质材料.目前研发的介电微晶玻璃,大都基于硅酸盐玻璃体系.尽管这种硅酸盐玻璃体系的制备工艺成熟和化学性质稳定,但是过高的制备温度(>1400℃)产生高能耗和安全问题[5~8].与硅酸盐玻璃不同,磷酸盐玻璃的熔制温度低(<1300℃)且工艺简便易行[9~11]. ...
1
2006
... 微晶玻璃是一类重要的新型无机材料,由性能显著不同的玻璃相和微晶相组成.根据复合材料的协同效应,特定成分的微晶玻璃其综合性能优于其各组成相的性能 [1,2].微晶玻璃的一个重要应用,是制作储能无机电介质[3,4].根据高介电性能的微晶相和高击穿强度的玻璃相的协同效应,可设计出兼具高介电与耐击穿性能的微晶玻璃电介质材料.目前研发的介电微晶玻璃,大都基于硅酸盐玻璃体系.尽管这种硅酸盐玻璃体系的制备工艺成熟和化学性质稳定,但是过高的制备温度(>1400℃)产生高能耗和安全问题[5~8].与硅酸盐玻璃不同,磷酸盐玻璃的熔制温度低(<1300℃)且工艺简便易行[9~11]. ...
1
1964
... 微晶玻璃是一类重要的新型无机材料,由性能显著不同的玻璃相和微晶相组成.根据复合材料的协同效应,特定成分的微晶玻璃其综合性能优于其各组成相的性能 [1,2].微晶玻璃的一个重要应用,是制作储能无机电介质[3,4].根据高介电性能的微晶相和高击穿强度的玻璃相的协同效应,可设计出兼具高介电与耐击穿性能的微晶玻璃电介质材料.目前研发的介电微晶玻璃,大都基于硅酸盐玻璃体系.尽管这种硅酸盐玻璃体系的制备工艺成熟和化学性质稳定,但是过高的制备温度(>1400℃)产生高能耗和安全问题[5~8].与硅酸盐玻璃不同,磷酸盐玻璃的熔制温度低(<1300℃)且工艺简便易行[9~11]. ...
Application of glass-ceramics for electronic components and circuits
1
1973
... 微晶玻璃是一类重要的新型无机材料,由性能显著不同的玻璃相和微晶相组成.根据复合材料的协同效应,特定成分的微晶玻璃其综合性能优于其各组成相的性能 [1,2].微晶玻璃的一个重要应用,是制作储能无机电介质[3,4].根据高介电性能的微晶相和高击穿强度的玻璃相的协同效应,可设计出兼具高介电与耐击穿性能的微晶玻璃电介质材料.目前研发的介电微晶玻璃,大都基于硅酸盐玻璃体系.尽管这种硅酸盐玻璃体系的制备工艺成熟和化学性质稳定,但是过高的制备温度(>1400℃)产生高能耗和安全问题[5~8].与硅酸盐玻璃不同,磷酸盐玻璃的熔制温度低(<1300℃)且工艺简便易行[9~11]. ...
Effect of additives on the crystallization kinetics of barium strontium titanate glass-ceramics
1
2008
... 微晶玻璃是一类重要的新型无机材料,由性能显著不同的玻璃相和微晶相组成.根据复合材料的协同效应,特定成分的微晶玻璃其综合性能优于其各组成相的性能 [1,2].微晶玻璃的一个重要应用,是制作储能无机电介质[3,4].根据高介电性能的微晶相和高击穿强度的玻璃相的协同效应,可设计出兼具高介电与耐击穿性能的微晶玻璃电介质材料.目前研发的介电微晶玻璃,大都基于硅酸盐玻璃体系.尽管这种硅酸盐玻璃体系的制备工艺成熟和化学性质稳定,但是过高的制备温度(>1400℃)产生高能耗和安全问题[5~8].与硅酸盐玻璃不同,磷酸盐玻璃的熔制温度低(<1300℃)且工艺简便易行[9~11]. ...
A novel high-strength lithium disilicate glass-ceramic featuring a highly intertwined microstructure
1
2017
... 微晶玻璃是一类重要的新型无机材料,由性能显著不同的玻璃相和微晶相组成.根据复合材料的协同效应,特定成分的微晶玻璃其综合性能优于其各组成相的性能 [1,2].微晶玻璃的一个重要应用,是制作储能无机电介质[3,4].根据高介电性能的微晶相和高击穿强度的玻璃相的协同效应,可设计出兼具高介电与耐击穿性能的微晶玻璃电介质材料.目前研发的介电微晶玻璃,大都基于硅酸盐玻璃体系.尽管这种硅酸盐玻璃体系的制备工艺成熟和化学性质稳定,但是过高的制备温度(>1400℃)产生高能耗和安全问题[5~8].与硅酸盐玻璃不同,磷酸盐玻璃的熔制温度低(<1300℃)且工艺简便易行[9~11]. ...
Investigation of structure, dielectric and energy-storage properties of lead-free niobate glass and glass-ceramics
0
2018
Improvement in structural, dielectric and energy-storage properties of lead-free niobate glass-ceramic with Sm2O3
1
2017
... Electric property of glass-ceramics
Table 1| No. | Glass-ceramic system | εr | tan δ | E /kV·mm-1 | Ud /mJ·cm-3 | η | Ref. |
|---|
| 1 | PbO-SrO-Na2O-Nb2O5-SiO2 | 427 | 0.022 | 44.2 | 850 | - | [31] |
| 2 | BaO-Na2O-Nb2O5-SiO2 | 312 | 0.016 | 16.3 | 370 | 72 | [7] |
| 3 | BaO-SrO-TiO2-Al2O3-SiO2 | 282 | 0.018 | 26.5 | 178 | 42.3 | [32] |
| 4 | BaTiO3-glass (BaO-Bi2O3-P2O5) | 1750 | 0.088 | 1.5 | 2.3 | 42.3 | [25] |
| 5 | BaO-Na2O-P2O5-Nb2O5-WO3 | 38 | 0.021 | 9 | 25.6 | 73.7 | [26] |
| 6 | NaNbO3-glass (Na2O-Nb2O5- P2O5) | 406 | 0.01 | 6 | 68.4 | 84.8 | [33] |
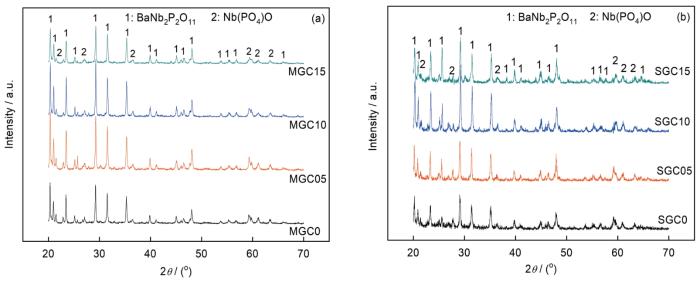
<strong>3</strong> 结论(1) 用熔融法和烧结法制备的P2O5-Nb2O5-BaO-Na2O-CeO2系磷酸盐微晶玻璃,都由BaNb2P2O11和Nb(PO4)O两个结晶相组成.随着CeO2的添加量从0提高到1%微晶玻璃的结晶度逐渐提高,且其内部结构逐渐致密化. ...
Preparation and dielectric characterization of lead-free niobate glass-ceramic composites added with Lu2O3
1
2014
... 微晶玻璃是一类重要的新型无机材料,由性能显著不同的玻璃相和微晶相组成.根据复合材料的协同效应,特定成分的微晶玻璃其综合性能优于其各组成相的性能 [1,2].微晶玻璃的一个重要应用,是制作储能无机电介质[3,4].根据高介电性能的微晶相和高击穿强度的玻璃相的协同效应,可设计出兼具高介电与耐击穿性能的微晶玻璃电介质材料.目前研发的介电微晶玻璃,大都基于硅酸盐玻璃体系.尽管这种硅酸盐玻璃体系的制备工艺成熟和化学性质稳定,但是过高的制备温度(>1400℃)产生高能耗和安全问题[5~8].与硅酸盐玻璃不同,磷酸盐玻璃的熔制温度低(<1300℃)且工艺简便易行[9~11]. ...
Optimized structural and mechanical properties of borophosphate glass
1
2020
... 微晶玻璃是一类重要的新型无机材料,由性能显著不同的玻璃相和微晶相组成.根据复合材料的协同效应,特定成分的微晶玻璃其综合性能优于其各组成相的性能 [1,2].微晶玻璃的一个重要应用,是制作储能无机电介质[3,4].根据高介电性能的微晶相和高击穿强度的玻璃相的协同效应,可设计出兼具高介电与耐击穿性能的微晶玻璃电介质材料.目前研发的介电微晶玻璃,大都基于硅酸盐玻璃体系.尽管这种硅酸盐玻璃体系的制备工艺成熟和化学性质稳定,但是过高的制备温度(>1400℃)产生高能耗和安全问题[5~8].与硅酸盐玻璃不同,磷酸盐玻璃的熔制温度低(<1300℃)且工艺简便易行[9~11]. ...
Structural and dielectric properties of K2O-TiO2-P2O5 glass and its associated glass-ceramic
0
2020
Raman spectroscopic investigations on UV irradiated phosphate glasses with high content of silver or sodium
1
2020
... 微晶玻璃是一类重要的新型无机材料,由性能显著不同的玻璃相和微晶相组成.根据复合材料的协同效应,特定成分的微晶玻璃其综合性能优于其各组成相的性能 [1,2].微晶玻璃的一个重要应用,是制作储能无机电介质[3,4].根据高介电性能的微晶相和高击穿强度的玻璃相的协同效应,可设计出兼具高介电与耐击穿性能的微晶玻璃电介质材料.目前研发的介电微晶玻璃,大都基于硅酸盐玻璃体系.尽管这种硅酸盐玻璃体系的制备工艺成熟和化学性质稳定,但是过高的制备温度(>1400℃)产生高能耗和安全问题[5~8].与硅酸盐玻璃不同,磷酸盐玻璃的熔制温度低(<1300℃)且工艺简便易行[9~11]. ...
Sintering and mechanical properties of lithium disilicate glass-ceramics prepared by sol-gel method
1
2021
... 制备微晶玻璃的方法,有熔融快冷法(简称熔融法),烧结法和溶胶凝胶法.溶胶凝胶法使用的原料是金属有机物,原料成本较高、工艺复杂流程较长且产率低;熔融法和烧结法没有溶胶凝胶法的这些不足[12~14].用熔融法制备微晶玻璃材料,先将原料混合物熔融并均匀化,然后将其浇注到金属模具或金属轧辊中快冷生成玻璃原片,再将其去应力退火,在特定温度下控制析晶的生成使晶粒均匀分布在玻璃基体中.烧结法是将配制的原料熔融并均匀化,然后将熔液倒入水中形成玻璃熔块,再将其研磨或球磨制成玻璃粉.用类似制备陶瓷的工艺将玻璃粉配以粘结剂在金属模具中干压成型,然后在特定温度烧结而制备出微晶玻璃.本文用熔融法和烧结法制备磷酸盐微晶玻璃,并研究其对结构、力学与介电性能的影响. ...
Preparation and structural transformation of PbF2·SiO2 based glass ceramics prepared by sol-gel method
0
2004
PbF2·SiO2基玻璃陶瓷的溶胶-凝胶法制备及结构转变研究
0
2004
Development of preparing bioglass ceramic coatings by sol-gel
1
2004
... 制备微晶玻璃的方法,有熔融快冷法(简称熔融法),烧结法和溶胶凝胶法.溶胶凝胶法使用的原料是金属有机物,原料成本较高、工艺复杂流程较长且产率低;熔融法和烧结法没有溶胶凝胶法的这些不足[12~14].用熔融法制备微晶玻璃材料,先将原料混合物熔融并均匀化,然后将其浇注到金属模具或金属轧辊中快冷生成玻璃原片,再将其去应力退火,在特定温度下控制析晶的生成使晶粒均匀分布在玻璃基体中.烧结法是将配制的原料熔融并均匀化,然后将熔液倒入水中形成玻璃熔块,再将其研磨或球磨制成玻璃粉.用类似制备陶瓷的工艺将玻璃粉配以粘结剂在金属模具中干压成型,然后在特定温度烧结而制备出微晶玻璃.本文用熔融法和烧结法制备磷酸盐微晶玻璃,并研究其对结构、力学与介电性能的影响. ...
溶胶-凝胶法制备生物玻璃陶瓷涂层的发展
1
2004
... 制备微晶玻璃的方法,有熔融快冷法(简称熔融法),烧结法和溶胶凝胶法.溶胶凝胶法使用的原料是金属有机物,原料成本较高、工艺复杂流程较长且产率低;熔融法和烧结法没有溶胶凝胶法的这些不足[12~14].用熔融法制备微晶玻璃材料,先将原料混合物熔融并均匀化,然后将其浇注到金属模具或金属轧辊中快冷生成玻璃原片,再将其去应力退火,在特定温度下控制析晶的生成使晶粒均匀分布在玻璃基体中.烧结法是将配制的原料熔融并均匀化,然后将熔液倒入水中形成玻璃熔块,再将其研磨或球磨制成玻璃粉.用类似制备陶瓷的工艺将玻璃粉配以粘结剂在金属模具中干压成型,然后在特定温度烧结而制备出微晶玻璃.本文用熔融法和烧结法制备磷酸盐微晶玻璃,并研究其对结构、力学与介电性能的影响. ...
1
2000
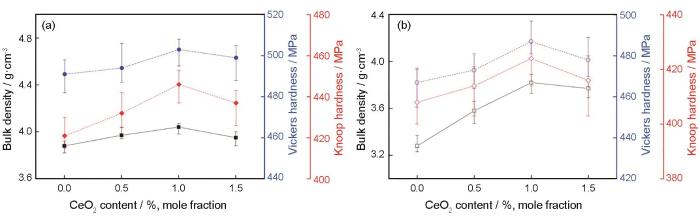
... 图4给出了用熔融法和烧结法制备的微晶玻璃的介电常数与介电损耗随频率的变化.可以看出,介电常数和介电损耗均随着频率的升高而降低,尤其是在低频区.根据电介质理论,电介质的介电常数是电子位移极化、离子位移极化、偶极子转向极化和空间电荷极化四种极化方式的贡献[15].在低频段介电常数主要由空间电荷极化和偶极子转向极化贡献,而在高频段因空间电荷与偶极子转向跟不上频率变化介电常数只是电子位移极化和离子位移极化的贡献.本文用两种方法制备的样品其介电常数频率特征符合Maxwell-Wagner型极化,即当频率升高时极化降低.根据Koop唯象理论,在低频段微晶玻璃电介质中的空间电荷极化起主导作用[16].随着CeO2添加量从0增加到1%,用两种方法制备的微晶玻璃电介质其介电常数都逐渐升高,然后随着CeO2含量的继续提高而降低.介电常数的升高,可能是CeO2的添加促进了样品结晶引起的.随着CeO2含量的继续提高介电常数降低,可能是CeO2含量超过1%时结晶程度降低所致,这由图1可以看出. ...
1
2000
... 图4给出了用熔融法和烧结法制备的微晶玻璃的介电常数与介电损耗随频率的变化.可以看出,介电常数和介电损耗均随着频率的升高而降低,尤其是在低频区.根据电介质理论,电介质的介电常数是电子位移极化、离子位移极化、偶极子转向极化和空间电荷极化四种极化方式的贡献[15].在低频段介电常数主要由空间电荷极化和偶极子转向极化贡献,而在高频段因空间电荷与偶极子转向跟不上频率变化介电常数只是电子位移极化和离子位移极化的贡献.本文用两种方法制备的样品其介电常数频率特征符合Maxwell-Wagner型极化,即当频率升高时极化降低.根据Koop唯象理论,在低频段微晶玻璃电介质中的空间电荷极化起主导作用[16].随着CeO2添加量从0增加到1%,用两种方法制备的微晶玻璃电介质其介电常数都逐渐升高,然后随着CeO2含量的继续提高而降低.介电常数的升高,可能是CeO2的添加促进了样品结晶引起的.随着CeO2含量的继续提高介电常数降低,可能是CeO2含量超过1%时结晶程度降低所致,这由图1可以看出. ...
Low-loss spinel nanoferrite with matching permeability and permittivity in the ultrahigh frequency range
1
2010
... 图4给出了用熔融法和烧结法制备的微晶玻璃的介电常数与介电损耗随频率的变化.可以看出,介电常数和介电损耗均随着频率的升高而降低,尤其是在低频区.根据电介质理论,电介质的介电常数是电子位移极化、离子位移极化、偶极子转向极化和空间电荷极化四种极化方式的贡献[15].在低频段介电常数主要由空间电荷极化和偶极子转向极化贡献,而在高频段因空间电荷与偶极子转向跟不上频率变化介电常数只是电子位移极化和离子位移极化的贡献.本文用两种方法制备的样品其介电常数频率特征符合Maxwell-Wagner型极化,即当频率升高时极化降低.根据Koop唯象理论,在低频段微晶玻璃电介质中的空间电荷极化起主导作用[16].随着CeO2添加量从0增加到1%,用两种方法制备的微晶玻璃电介质其介电常数都逐渐升高,然后随着CeO2含量的继续提高而降低.介电常数的升高,可能是CeO2的添加促进了样品结晶引起的.随着CeO2含量的继续提高介电常数降低,可能是CeO2含量超过1%时结晶程度降低所致,这由图1可以看出. ...
Comparative study of dielectric behavior of Mn0.4Zn0.6Fe2O4 nanoferrite by citrate precursor method
1
2008
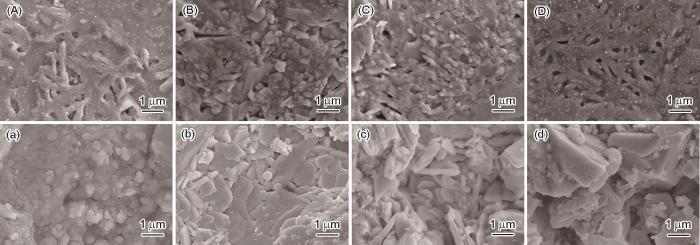
... 介电损耗,也呈现出随着频率的升高而降低的趋势.随着频率的升高只有电子和离子的位移能跟上外电场的变化,因此损耗快速降低.这也反映出,在低频段空间电荷和偶极子转向是产生电介质损耗的主要因素.类似地,介电损耗都随着CeO2含量的升高而下降.材料内部缺陷,如晶体缺陷、孔隙等因素对损耗的促进[17],以及图2给出的微观形貌变化表明,添加CeO2促进了微晶玻璃的结构致密化,从而使介电损耗降低. ...
Electrical tree characteristics of XLPE under repetitive pulse voltage in low temperature
1
2015
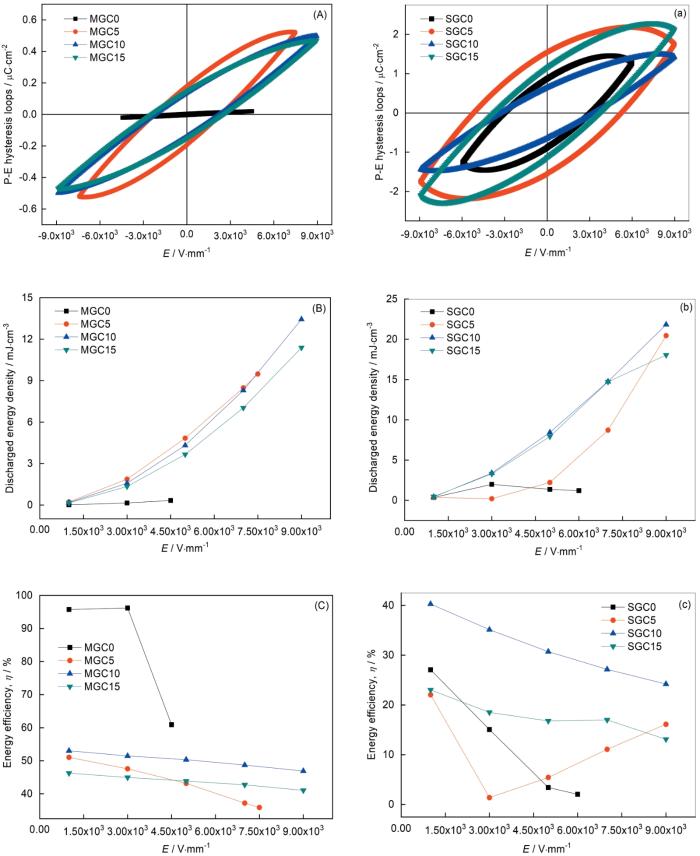
... 图5给出了用熔融法和烧结法制备的微晶玻璃的极化曲线和储能行为.可以看出,用熔融法制备的未添加CeO2样品在场强加到4.5 kV/mm时便击穿.其原因是,内部树枝状且边缘粗糙的颗粒使局部电荷集中[18].随着CeO2添加量的提高微晶玻璃能承受的场强也显著提高,尤其是CeO2添加量超过1%时击穿场强均在9 kV/mm以上.电介质的击穿场强(即最高工作场强)受内部晶粒尺寸、排布、孔隙、样品外形、尺寸、粗糙度、电极等多种因素的影响,而內部结构的影响尤其重要[19~21].电介质致密度的提高将整体改善其电学性能,包括提高击穿场强[22,23].图2给出了用熔融法制备的添加CeO2的微晶玻璃,可见其晶粒细化并排列致密化.正是结构的致密化使添加CeO2的微晶玻璃工作场强显著提高.从图5a还可以看出,CeO2添加量为1%的微晶玻璃其剩余极化强度为0.12 μC/cm2,饱和极化强度为0.51 μC/cm2.添加量为1.5%时剩余极化强度升高到0.15 μC/cm2,而饱和极化强度却降低到0.47 μC/cm2.图5b给出了用熔融法制备的样品的能量释放密度曲线.可以看出,能量释放密度与应用场强之间有非线性关系.CeO2添加量为1%的微晶玻璃样品,在场强为9 kV/mm时能量释放密度最高(13.5 mJ/cm3),是较高饱和极化强度、较低剩余极化强度和较高的应用场强的共同贡献.图5c给出了用熔融法制备的样品的储能效率随场强的变化.可以看出,在最高场强为9 kV/mm、CeO2添加量为1%的微晶玻璃其储能效率最高(50.1%).用烧结法制备的微晶玻璃样品,具有高损耗型电滞回线[24].图5a也表明,CeO2的加入使工作场强的提高.从图5b可以看出,CeO2添加量为1%用烧结法制备的微晶玻璃样品具有最高的能量释放密度(22.5 mJ/cm3).虽然此值高于其他体系磷酸盐微晶玻璃,但是与硅酸盐体系微晶玻璃材料相比还有较大的差距[25,26].图5c给出了用烧结法制备的样品的储能效率随场强的变化.可以看出,尽管CeO2添加量为1%的样品表现出极高的储能效率水平,但是最高场强为9 kV/mm时其储能效率只有27.3%. ...
Structural and dielectric characterization of Gd2O3-added BaO-Na2O-Nb2O5-SiO2 glass-ceramic composites
1
2011
... 图5给出了用熔融法和烧结法制备的微晶玻璃的极化曲线和储能行为.可以看出,用熔融法制备的未添加CeO2样品在场强加到4.5 kV/mm时便击穿.其原因是,内部树枝状且边缘粗糙的颗粒使局部电荷集中[18].随着CeO2添加量的提高微晶玻璃能承受的场强也显著提高,尤其是CeO2添加量超过1%时击穿场强均在9 kV/mm以上.电介质的击穿场强(即最高工作场强)受内部晶粒尺寸、排布、孔隙、样品外形、尺寸、粗糙度、电极等多种因素的影响,而內部结构的影响尤其重要[19~21].电介质致密度的提高将整体改善其电学性能,包括提高击穿场强[22,23].图2给出了用熔融法制备的添加CeO2的微晶玻璃,可见其晶粒细化并排列致密化.正是结构的致密化使添加CeO2的微晶玻璃工作场强显著提高.从图5a还可以看出,CeO2添加量为1%的微晶玻璃其剩余极化强度为0.12 μC/cm2,饱和极化强度为0.51 μC/cm2.添加量为1.5%时剩余极化强度升高到0.15 μC/cm2,而饱和极化强度却降低到0.47 μC/cm2.图5b给出了用熔融法制备的样品的能量释放密度曲线.可以看出,能量释放密度与应用场强之间有非线性关系.CeO2添加量为1%的微晶玻璃样品,在场强为9 kV/mm时能量释放密度最高(13.5 mJ/cm3),是较高饱和极化强度、较低剩余极化强度和较高的应用场强的共同贡献.图5c给出了用熔融法制备的样品的储能效率随场强的变化.可以看出,在最高场强为9 kV/mm、CeO2添加量为1%的微晶玻璃其储能效率最高(50.1%).用烧结法制备的微晶玻璃样品,具有高损耗型电滞回线[24].图5a也表明,CeO2的加入使工作场强的提高.从图5b可以看出,CeO2添加量为1%用烧结法制备的微晶玻璃样品具有最高的能量释放密度(22.5 mJ/cm3).虽然此值高于其他体系磷酸盐微晶玻璃,但是与硅酸盐体系微晶玻璃材料相比还有较大的差距[25,26].图5c给出了用烧结法制备的样品的储能效率随场强的变化.可以看出,尽管CeO2添加量为1%的样品表现出极高的储能效率水平,但是最高场强为9 kV/mm时其储能效率只有27.3%. ...
Improved energy storage density in barium strontium titanate by addition of BaO-SiO2-B2O3 glass
0
2009
Dielectric strength of fine grained barium titanate ceramics
1
1996
... 图5给出了用熔融法和烧结法制备的微晶玻璃的极化曲线和储能行为.可以看出,用熔融法制备的未添加CeO2样品在场强加到4.5 kV/mm时便击穿.其原因是,内部树枝状且边缘粗糙的颗粒使局部电荷集中[18].随着CeO2添加量的提高微晶玻璃能承受的场强也显著提高,尤其是CeO2添加量超过1%时击穿场强均在9 kV/mm以上.电介质的击穿场强(即最高工作场强)受内部晶粒尺寸、排布、孔隙、样品外形、尺寸、粗糙度、电极等多种因素的影响,而內部结构的影响尤其重要[19~21].电介质致密度的提高将整体改善其电学性能,包括提高击穿场强[22,23].图2给出了用熔融法制备的添加CeO2的微晶玻璃,可见其晶粒细化并排列致密化.正是结构的致密化使添加CeO2的微晶玻璃工作场强显著提高.从图5a还可以看出,CeO2添加量为1%的微晶玻璃其剩余极化强度为0.12 μC/cm2,饱和极化强度为0.51 μC/cm2.添加量为1.5%时剩余极化强度升高到0.15 μC/cm2,而饱和极化强度却降低到0.47 μC/cm2.图5b给出了用熔融法制备的样品的能量释放密度曲线.可以看出,能量释放密度与应用场强之间有非线性关系.CeO2添加量为1%的微晶玻璃样品,在场强为9 kV/mm时能量释放密度最高(13.5 mJ/cm3),是较高饱和极化强度、较低剩余极化强度和较高的应用场强的共同贡献.图5c给出了用熔融法制备的样品的储能效率随场强的变化.可以看出,在最高场强为9 kV/mm、CeO2添加量为1%的微晶玻璃其储能效率最高(50.1%).用烧结法制备的微晶玻璃样品,具有高损耗型电滞回线[24].图5a也表明,CeO2的加入使工作场强的提高.从图5b可以看出,CeO2添加量为1%用烧结法制备的微晶玻璃样品具有最高的能量释放密度(22.5 mJ/cm3).虽然此值高于其他体系磷酸盐微晶玻璃,但是与硅酸盐体系微晶玻璃材料相比还有较大的差距[25,26].图5c给出了用烧结法制备的样品的储能效率随场强的变化.可以看出,尽管CeO2添加量为1%的样品表现出极高的储能效率水平,但是最高场强为9 kV/mm时其储能效率只有27.3%. ...
Properties of ZnO varistor ceramics Co-doped with B2O3 and Al2O3
1
2021
... 图5给出了用熔融法和烧结法制备的微晶玻璃的极化曲线和储能行为.可以看出,用熔融法制备的未添加CeO2样品在场强加到4.5 kV/mm时便击穿.其原因是,内部树枝状且边缘粗糙的颗粒使局部电荷集中[18].随着CeO2添加量的提高微晶玻璃能承受的场强也显著提高,尤其是CeO2添加量超过1%时击穿场强均在9 kV/mm以上.电介质的击穿场强(即最高工作场强)受内部晶粒尺寸、排布、孔隙、样品外形、尺寸、粗糙度、电极等多种因素的影响,而內部结构的影响尤其重要[19~21].电介质致密度的提高将整体改善其电学性能,包括提高击穿场强[22,23].图2给出了用熔融法制备的添加CeO2的微晶玻璃,可见其晶粒细化并排列致密化.正是结构的致密化使添加CeO2的微晶玻璃工作场强显著提高.从图5a还可以看出,CeO2添加量为1%的微晶玻璃其剩余极化强度为0.12 μC/cm2,饱和极化强度为0.51 μC/cm2.添加量为1.5%时剩余极化强度升高到0.15 μC/cm2,而饱和极化强度却降低到0.47 μC/cm2.图5b给出了用熔融法制备的样品的能量释放密度曲线.可以看出,能量释放密度与应用场强之间有非线性关系.CeO2添加量为1%的微晶玻璃样品,在场强为9 kV/mm时能量释放密度最高(13.5 mJ/cm3),是较高饱和极化强度、较低剩余极化强度和较高的应用场强的共同贡献.图5c给出了用熔融法制备的样品的储能效率随场强的变化.可以看出,在最高场强为9 kV/mm、CeO2添加量为1%的微晶玻璃其储能效率最高(50.1%).用烧结法制备的微晶玻璃样品,具有高损耗型电滞回线[24].图5a也表明,CeO2的加入使工作场强的提高.从图5b可以看出,CeO2添加量为1%用烧结法制备的微晶玻璃样品具有最高的能量释放密度(22.5 mJ/cm3).虽然此值高于其他体系磷酸盐微晶玻璃,但是与硅酸盐体系微晶玻璃材料相比还有较大的差距[25,26].图5c给出了用烧结法制备的样品的储能效率随场强的变化.可以看出,尽管CeO2添加量为1%的样品表现出极高的储能效率水平,但是最高场强为9 kV/mm时其储能效率只有27.3%. ...
B2O3和Al2O3共同掺杂ZnO压敏陶瓷的性能
1
2021
... 图5给出了用熔融法和烧结法制备的微晶玻璃的极化曲线和储能行为.可以看出,用熔融法制备的未添加CeO2样品在场强加到4.5 kV/mm时便击穿.其原因是,内部树枝状且边缘粗糙的颗粒使局部电荷集中[18].随着CeO2添加量的提高微晶玻璃能承受的场强也显著提高,尤其是CeO2添加量超过1%时击穿场强均在9 kV/mm以上.电介质的击穿场强(即最高工作场强)受内部晶粒尺寸、排布、孔隙、样品外形、尺寸、粗糙度、电极等多种因素的影响,而內部结构的影响尤其重要[19~21].电介质致密度的提高将整体改善其电学性能,包括提高击穿场强[22,23].图2给出了用熔融法制备的添加CeO2的微晶玻璃,可见其晶粒细化并排列致密化.正是结构的致密化使添加CeO2的微晶玻璃工作场强显著提高.从图5a还可以看出,CeO2添加量为1%的微晶玻璃其剩余极化强度为0.12 μC/cm2,饱和极化强度为0.51 μC/cm2.添加量为1.5%时剩余极化强度升高到0.15 μC/cm2,而饱和极化强度却降低到0.47 μC/cm2.图5b给出了用熔融法制备的样品的能量释放密度曲线.可以看出,能量释放密度与应用场强之间有非线性关系.CeO2添加量为1%的微晶玻璃样品,在场强为9 kV/mm时能量释放密度最高(13.5 mJ/cm3),是较高饱和极化强度、较低剩余极化强度和较高的应用场强的共同贡献.图5c给出了用熔融法制备的样品的储能效率随场强的变化.可以看出,在最高场强为9 kV/mm、CeO2添加量为1%的微晶玻璃其储能效率最高(50.1%).用烧结法制备的微晶玻璃样品,具有高损耗型电滞回线[24].图5a也表明,CeO2的加入使工作场强的提高.从图5b可以看出,CeO2添加量为1%用烧结法制备的微晶玻璃样品具有最高的能量释放密度(22.5 mJ/cm3).虽然此值高于其他体系磷酸盐微晶玻璃,但是与硅酸盐体系微晶玻璃材料相比还有较大的差距[25,26].图5c给出了用烧结法制备的样品的储能效率随场强的变化.可以看出,尽管CeO2添加量为1%的样品表现出极高的储能效率水平,但是最高场强为9 kV/mm时其储能效率只有27.3%. ...
Effect of Cr2O3 addition on the microstructure and electrical properties of SnO2-based varistor
1
2013
... 图5给出了用熔融法和烧结法制备的微晶玻璃的极化曲线和储能行为.可以看出,用熔融法制备的未添加CeO2样品在场强加到4.5 kV/mm时便击穿.其原因是,内部树枝状且边缘粗糙的颗粒使局部电荷集中[18].随着CeO2添加量的提高微晶玻璃能承受的场强也显著提高,尤其是CeO2添加量超过1%时击穿场强均在9 kV/mm以上.电介质的击穿场强(即最高工作场强)受内部晶粒尺寸、排布、孔隙、样品外形、尺寸、粗糙度、电极等多种因素的影响,而內部结构的影响尤其重要[19~21].电介质致密度的提高将整体改善其电学性能,包括提高击穿场强[22,23].图2给出了用熔融法制备的添加CeO2的微晶玻璃,可见其晶粒细化并排列致密化.正是结构的致密化使添加CeO2的微晶玻璃工作场强显著提高.从图5a还可以看出,CeO2添加量为1%的微晶玻璃其剩余极化强度为0.12 μC/cm2,饱和极化强度为0.51 μC/cm2.添加量为1.5%时剩余极化强度升高到0.15 μC/cm2,而饱和极化强度却降低到0.47 μC/cm2.图5b给出了用熔融法制备的样品的能量释放密度曲线.可以看出,能量释放密度与应用场强之间有非线性关系.CeO2添加量为1%的微晶玻璃样品,在场强为9 kV/mm时能量释放密度最高(13.5 mJ/cm3),是较高饱和极化强度、较低剩余极化强度和较高的应用场强的共同贡献.图5c给出了用熔融法制备的样品的储能效率随场强的变化.可以看出,在最高场强为9 kV/mm、CeO2添加量为1%的微晶玻璃其储能效率最高(50.1%).用烧结法制备的微晶玻璃样品,具有高损耗型电滞回线[24].图5a也表明,CeO2的加入使工作场强的提高.从图5b可以看出,CeO2添加量为1%用烧结法制备的微晶玻璃样品具有最高的能量释放密度(22.5 mJ/cm3).虽然此值高于其他体系磷酸盐微晶玻璃,但是与硅酸盐体系微晶玻璃材料相比还有较大的差距[25,26].图5c给出了用烧结法制备的样品的储能效率随场强的变化.可以看出,尽管CeO2添加量为1%的样品表现出极高的储能效率水平,但是最高场强为9 kV/mm时其储能效率只有27.3%. ...
Dielectric hysteresis measurement in lossy ferroelectrics
1
1999
... 图5给出了用熔融法和烧结法制备的微晶玻璃的极化曲线和储能行为.可以看出,用熔融法制备的未添加CeO2样品在场强加到4.5 kV/mm时便击穿.其原因是,内部树枝状且边缘粗糙的颗粒使局部电荷集中[18].随着CeO2添加量的提高微晶玻璃能承受的场强也显著提高,尤其是CeO2添加量超过1%时击穿场强均在9 kV/mm以上.电介质的击穿场强(即最高工作场强)受内部晶粒尺寸、排布、孔隙、样品外形、尺寸、粗糙度、电极等多种因素的影响,而內部结构的影响尤其重要[19~21].电介质致密度的提高将整体改善其电学性能,包括提高击穿场强[22,23].图2给出了用熔融法制备的添加CeO2的微晶玻璃,可见其晶粒细化并排列致密化.正是结构的致密化使添加CeO2的微晶玻璃工作场强显著提高.从图5a还可以看出,CeO2添加量为1%的微晶玻璃其剩余极化强度为0.12 μC/cm2,饱和极化强度为0.51 μC/cm2.添加量为1.5%时剩余极化强度升高到0.15 μC/cm2,而饱和极化强度却降低到0.47 μC/cm2.图5b给出了用熔融法制备的样品的能量释放密度曲线.可以看出,能量释放密度与应用场强之间有非线性关系.CeO2添加量为1%的微晶玻璃样品,在场强为9 kV/mm时能量释放密度最高(13.5 mJ/cm3),是较高饱和极化强度、较低剩余极化强度和较高的应用场强的共同贡献.图5c给出了用熔融法制备的样品的储能效率随场强的变化.可以看出,在最高场强为9 kV/mm、CeO2添加量为1%的微晶玻璃其储能效率最高(50.1%).用烧结法制备的微晶玻璃样品,具有高损耗型电滞回线[24].图5a也表明,CeO2的加入使工作场强的提高.从图5b可以看出,CeO2添加量为1%用烧结法制备的微晶玻璃样品具有最高的能量释放密度(22.5 mJ/cm3).虽然此值高于其他体系磷酸盐微晶玻璃,但是与硅酸盐体系微晶玻璃材料相比还有较大的差距[25,26].图5c给出了用烧结法制备的样品的储能效率随场强的变化.可以看出,尽管CeO2添加量为1%的样品表现出极高的储能效率水平,但是最高场强为9 kV/mm时其储能效率只有27.3%. ...
Effect of BaO-Bi2O3-P2O5 glass additive on structural, dielectric and energy storage properties of BaTiO3 ceramics
3
2020
... 图5给出了用熔融法和烧结法制备的微晶玻璃的极化曲线和储能行为.可以看出,用熔融法制备的未添加CeO2样品在场强加到4.5 kV/mm时便击穿.其原因是,内部树枝状且边缘粗糙的颗粒使局部电荷集中[18].随着CeO2添加量的提高微晶玻璃能承受的场强也显著提高,尤其是CeO2添加量超过1%时击穿场强均在9 kV/mm以上.电介质的击穿场强(即最高工作场强)受内部晶粒尺寸、排布、孔隙、样品外形、尺寸、粗糙度、电极等多种因素的影响,而內部结构的影响尤其重要[19~21].电介质致密度的提高将整体改善其电学性能,包括提高击穿场强[22,23].图2给出了用熔融法制备的添加CeO2的微晶玻璃,可见其晶粒细化并排列致密化.正是结构的致密化使添加CeO2的微晶玻璃工作场强显著提高.从图5a还可以看出,CeO2添加量为1%的微晶玻璃其剩余极化强度为0.12 μC/cm2,饱和极化强度为0.51 μC/cm2.添加量为1.5%时剩余极化强度升高到0.15 μC/cm2,而饱和极化强度却降低到0.47 μC/cm2.图5b给出了用熔融法制备的样品的能量释放密度曲线.可以看出,能量释放密度与应用场强之间有非线性关系.CeO2添加量为1%的微晶玻璃样品,在场强为9 kV/mm时能量释放密度最高(13.5 mJ/cm3),是较高饱和极化强度、较低剩余极化强度和较高的应用场强的共同贡献.图5c给出了用熔融法制备的样品的储能效率随场强的变化.可以看出,在最高场强为9 kV/mm、CeO2添加量为1%的微晶玻璃其储能效率最高(50.1%).用烧结法制备的微晶玻璃样品,具有高损耗型电滞回线[24].图5a也表明,CeO2的加入使工作场强的提高.从图5b可以看出,CeO2添加量为1%用烧结法制备的微晶玻璃样品具有最高的能量释放密度(22.5 mJ/cm3).虽然此值高于其他体系磷酸盐微晶玻璃,但是与硅酸盐体系微晶玻璃材料相比还有较大的差距[25,26].图5c给出了用烧结法制备的样品的储能效率随场强的变化.可以看出,尽管CeO2添加量为1%的样品表现出极高的储能效率水平,但是最高场强为9 kV/mm时其储能效率只有27.3%. ...
... 用于储能电容器的微晶玻璃电介质,目前主要是硅酸盐玻璃基,磷酸盐玻璃基微晶玻璃报道较少,目前只有Bih L.组作了相关报导[25,26,33].表1列出了目前基于两种玻璃体系的介电微晶玻璃的电学性能.可以看出,硅酸盐玻璃基和磷酸盐玻璃基的微晶玻璃的介电常数普遍较高且范围可调,介电损耗可控制在0.022以内.本文制备的磷酸盐微晶玻璃的介电常数为37~43,介电损耗低于0.02,与表1列出的水平相当.从表1还可以看出,基于硅酸盐微晶玻璃的工作场强普遍高于基于磷酸盐的微晶玻璃,是其能量释放密度也普遍偏高.本文制备的磷酸盐微晶玻璃的最高工作场强和能量释放密度与表1给出的磷酸盐的微晶玻璃的相当,也反映出磷酸盐微晶玻璃的最高工作场强和能量释放密度还有待提高.由于储能效率属于多变量参数,与应用场强和电介质类型有关,场强越大弛豫型(或反铁电型)电介质的储能效率随之提高.磷酸盐体系较低的工作场强使本文制备的微晶玻璃的储能效率受到限制.本文制备的添加CeO2为1%的微晶玻璃储能效率最佳,为50.1%. ...
... Electric property of glass-ceramics
Table 1| No. | Glass-ceramic system | εr | tan δ | E /kV·mm-1 | Ud /mJ·cm-3 | η | Ref. |
|---|
| 1 | PbO-SrO-Na2O-Nb2O5-SiO2 | 427 | 0.022 | 44.2 | 850 | - | [31] |
| 2 | BaO-Na2O-Nb2O5-SiO2 | 312 | 0.016 | 16.3 | 370 | 72 | [7] |
| 3 | BaO-SrO-TiO2-Al2O3-SiO2 | 282 | 0.018 | 26.5 | 178 | 42.3 | [32] |
| 4 | BaTiO3-glass (BaO-Bi2O3-P2O5) | 1750 | 0.088 | 1.5 | 2.3 | 42.3 | [25] |
| 5 | BaO-Na2O-P2O5-Nb2O5-WO3 | 38 | 0.021 | 9 | 25.6 | 73.7 | [26] |
| 6 | NaNbO3-glass (Na2O-Nb2O5- P2O5) | 406 | 0.01 | 6 | 68.4 | 84.8 | [33] |
<strong>3</strong> 结论(1) 用熔融法和烧结法制备的P2O5-Nb2O5-BaO-Na2O-CeO2系磷酸盐微晶玻璃,都由BaNb2P2O11和Nb(PO4)O两个结晶相组成.随着CeO2的添加量从0提高到1%微晶玻璃的结晶度逐渐提高,且其内部结构逐渐致密化. ...
Structural, elelectrical and energy storage properties of BaO-Na2O-Nb2O5-WO3-P2O5 glass-ceramics system
3
2019
... 图5给出了用熔融法和烧结法制备的微晶玻璃的极化曲线和储能行为.可以看出,用熔融法制备的未添加CeO2样品在场强加到4.5 kV/mm时便击穿.其原因是,内部树枝状且边缘粗糙的颗粒使局部电荷集中[18].随着CeO2添加量的提高微晶玻璃能承受的场强也显著提高,尤其是CeO2添加量超过1%时击穿场强均在9 kV/mm以上.电介质的击穿场强(即最高工作场强)受内部晶粒尺寸、排布、孔隙、样品外形、尺寸、粗糙度、电极等多种因素的影响,而內部结构的影响尤其重要[19~21].电介质致密度的提高将整体改善其电学性能,包括提高击穿场强[22,23].图2给出了用熔融法制备的添加CeO2的微晶玻璃,可见其晶粒细化并排列致密化.正是结构的致密化使添加CeO2的微晶玻璃工作场强显著提高.从图5a还可以看出,CeO2添加量为1%的微晶玻璃其剩余极化强度为0.12 μC/cm2,饱和极化强度为0.51 μC/cm2.添加量为1.5%时剩余极化强度升高到0.15 μC/cm2,而饱和极化强度却降低到0.47 μC/cm2.图5b给出了用熔融法制备的样品的能量释放密度曲线.可以看出,能量释放密度与应用场强之间有非线性关系.CeO2添加量为1%的微晶玻璃样品,在场强为9 kV/mm时能量释放密度最高(13.5 mJ/cm3),是较高饱和极化强度、较低剩余极化强度和较高的应用场强的共同贡献.图5c给出了用熔融法制备的样品的储能效率随场强的变化.可以看出,在最高场强为9 kV/mm、CeO2添加量为1%的微晶玻璃其储能效率最高(50.1%).用烧结法制备的微晶玻璃样品,具有高损耗型电滞回线[24].图5a也表明,CeO2的加入使工作场强的提高.从图5b可以看出,CeO2添加量为1%用烧结法制备的微晶玻璃样品具有最高的能量释放密度(22.5 mJ/cm3).虽然此值高于其他体系磷酸盐微晶玻璃,但是与硅酸盐体系微晶玻璃材料相比还有较大的差距[25,26].图5c给出了用烧结法制备的样品的储能效率随场强的变化.可以看出,尽管CeO2添加量为1%的样品表现出极高的储能效率水平,但是最高场强为9 kV/mm时其储能效率只有27.3%. ...
... 用于储能电容器的微晶玻璃电介质,目前主要是硅酸盐玻璃基,磷酸盐玻璃基微晶玻璃报道较少,目前只有Bih L.组作了相关报导[25,26,33].表1列出了目前基于两种玻璃体系的介电微晶玻璃的电学性能.可以看出,硅酸盐玻璃基和磷酸盐玻璃基的微晶玻璃的介电常数普遍较高且范围可调,介电损耗可控制在0.022以内.本文制备的磷酸盐微晶玻璃的介电常数为37~43,介电损耗低于0.02,与表1列出的水平相当.从表1还可以看出,基于硅酸盐微晶玻璃的工作场强普遍高于基于磷酸盐的微晶玻璃,是其能量释放密度也普遍偏高.本文制备的磷酸盐微晶玻璃的最高工作场强和能量释放密度与表1给出的磷酸盐的微晶玻璃的相当,也反映出磷酸盐微晶玻璃的最高工作场强和能量释放密度还有待提高.由于储能效率属于多变量参数,与应用场强和电介质类型有关,场强越大弛豫型(或反铁电型)电介质的储能效率随之提高.磷酸盐体系较低的工作场强使本文制备的微晶玻璃的储能效率受到限制.本文制备的添加CeO2为1%的微晶玻璃储能效率最佳,为50.1%. ...
... Electric property of glass-ceramics
Table 1| No. | Glass-ceramic system | εr | tan δ | E /kV·mm-1 | Ud /mJ·cm-3 | η | Ref. |
|---|
| 1 | PbO-SrO-Na2O-Nb2O5-SiO2 | 427 | 0.022 | 44.2 | 850 | - | [31] |
| 2 | BaO-Na2O-Nb2O5-SiO2 | 312 | 0.016 | 16.3 | 370 | 72 | [7] |
| 3 | BaO-SrO-TiO2-Al2O3-SiO2 | 282 | 0.018 | 26.5 | 178 | 42.3 | [32] |
| 4 | BaTiO3-glass (BaO-Bi2O3-P2O5) | 1750 | 0.088 | 1.5 | 2.3 | 42.3 | [25] |
| 5 | BaO-Na2O-P2O5-Nb2O5-WO3 | 38 | 0.021 | 9 | 25.6 | 73.7 | [26] |
| 6 | NaNbO3-glass (Na2O-Nb2O5- P2O5) | 406 | 0.01 | 6 | 68.4 | 84.8 | [33] |
<strong>3</strong> 结论(1) 用熔融法和烧结法制备的P2O5-Nb2O5-BaO-Na2O-CeO2系磷酸盐微晶玻璃,都由BaNb2P2O11和Nb(PO4)O两个结晶相组成.随着CeO2的添加量从0提高到1%微晶玻璃的结晶度逐渐提高,且其内部结构逐渐致密化. ...
Ultrahigh discharge efficiency in multilayered polymer nanocomposites of high energy density
1
2019
... 有机电介质即聚合物基电介质具有较高的击穿场强(>400 kV/mm)、较低的介电损耗(<0.01)、较高的储能效率(>60%),但是其介电常数水平很低(<5),且易老化变质[27,28].无机电介质材料的介电常数较高(>30)且范围可调,但是其击穿场强(<15 kV/mm)较低和介电损耗(>0.05)较高[29,30].微晶玻璃是一种新型复合材料,其内部的无孔隙玻璃相击穿场强较高,内部的高极化铁电晶体相的介电常数较高.这表明,介电微晶玻璃兼具高介电常数和高击穿场强的潜质.但是界面效应和制备过程中引起微观结构不均匀性,其内部大量玻璃相和晶体相界面引起的空间电荷使局部电荷集中而降低了整体的击穿场强,从而限制了微晶玻璃电介质整体的储能行为. ...
Reduction of dielectric hysteresis in multilayered films via nanoconfinement
1
2012
... 有机电介质即聚合物基电介质具有较高的击穿场强(>400 kV/mm)、较低的介电损耗(<0.01)、较高的储能效率(>60%),但是其介电常数水平很低(<5),且易老化变质[27,28].无机电介质材料的介电常数较高(>30)且范围可调,但是其击穿场强(<15 kV/mm)较低和介电损耗(>0.05)较高[29,30].微晶玻璃是一种新型复合材料,其内部的无孔隙玻璃相击穿场强较高,内部的高极化铁电晶体相的介电常数较高.这表明,介电微晶玻璃兼具高介电常数和高击穿场强的潜质.但是界面效应和制备过程中引起微观结构不均匀性,其内部大量玻璃相和晶体相界面引起的空间电荷使局部电荷集中而降低了整体的击穿场强,从而限制了微晶玻璃电介质整体的储能行为. ...
Lead-free BaTiO3-Bi(Zn2/3Nb1/3)O3 weakly coupled relaxor ferroelectric materials for energy storage
1
2016
... 有机电介质即聚合物基电介质具有较高的击穿场强(>400 kV/mm)、较低的介电损耗(<0.01)、较高的储能效率(>60%),但是其介电常数水平很低(<5),且易老化变质[27,28].无机电介质材料的介电常数较高(>30)且范围可调,但是其击穿场强(<15 kV/mm)较低和介电损耗(>0.05)较高[29,30].微晶玻璃是一种新型复合材料,其内部的无孔隙玻璃相击穿场强较高,内部的高极化铁电晶体相的介电常数较高.这表明,介电微晶玻璃兼具高介电常数和高击穿场强的潜质.但是界面效应和制备过程中引起微观结构不均匀性,其内部大量玻璃相和晶体相界面引起的空间电荷使局部电荷集中而降低了整体的击穿场强,从而限制了微晶玻璃电介质整体的储能行为. ...
Silver niobate lead-free antiferroelectric ceramics: enhancing energy storage density by B-site doping
1
2018
... 有机电介质即聚合物基电介质具有较高的击穿场强(>400 kV/mm)、较低的介电损耗(<0.01)、较高的储能效率(>60%),但是其介电常数水平很低(<5),且易老化变质[27,28].无机电介质材料的介电常数较高(>30)且范围可调,但是其击穿场强(<15 kV/mm)较低和介电损耗(>0.05)较高[29,30].微晶玻璃是一种新型复合材料,其内部的无孔隙玻璃相击穿场强较高,内部的高极化铁电晶体相的介电常数较高.这表明,介电微晶玻璃兼具高介电常数和高击穿场强的潜质.但是界面效应和制备过程中引起微观结构不均匀性,其内部大量玻璃相和晶体相界面引起的空间电荷使局部电荷集中而降低了整体的击穿场强,从而限制了微晶玻璃电介质整体的储能行为. ...
Optimization of energy storage density in ANb2O6-NaNbO3-SiO2 (A=[(1-x)Pb, xSr]) nanostructured glass-ceramic dielectrics
1
2012
... Electric property of glass-ceramics
Table 1| No. | Glass-ceramic system | εr | tan δ | E /kV·mm-1 | Ud /mJ·cm-3 | η | Ref. |
|---|
| 1 | PbO-SrO-Na2O-Nb2O5-SiO2 | 427 | 0.022 | 44.2 | 850 | - | [31] |
| 2 | BaO-Na2O-Nb2O5-SiO2 | 312 | 0.016 | 16.3 | 370 | 72 | [7] |
| 3 | BaO-SrO-TiO2-Al2O3-SiO2 | 282 | 0.018 | 26.5 | 178 | 42.3 | [32] |
| 4 | BaTiO3-glass (BaO-Bi2O3-P2O5) | 1750 | 0.088 | 1.5 | 2.3 | 42.3 | [25] |
| 5 | BaO-Na2O-P2O5-Nb2O5-WO3 | 38 | 0.021 | 9 | 25.6 | 73.7 | [26] |
| 6 | NaNbO3-glass (Na2O-Nb2O5- P2O5) | 406 | 0.01 | 6 | 68.4 | 84.8 | [33] |
<strong>3</strong> 结论(1) 用熔融法和烧结法制备的P2O5-Nb2O5-BaO-Na2O-CeO2系磷酸盐微晶玻璃,都由BaNb2P2O11和Nb(PO4)O两个结晶相组成.随着CeO2的添加量从0提高到1%微晶玻璃的结晶度逐渐提高,且其内部结构逐渐致密化. ...
Sintering temperature dependence of energy-storage properties in (Ba, Sr)TiO3 glass-ceramics
1
2011
... Electric property of glass-ceramics
Table 1| No. | Glass-ceramic system | εr | tan δ | E /kV·mm-1 | Ud /mJ·cm-3 | η | Ref. |
|---|
| 1 | PbO-SrO-Na2O-Nb2O5-SiO2 | 427 | 0.022 | 44.2 | 850 | - | [31] |
| 2 | BaO-Na2O-Nb2O5-SiO2 | 312 | 0.016 | 16.3 | 370 | 72 | [7] |
| 3 | BaO-SrO-TiO2-Al2O3-SiO2 | 282 | 0.018 | 26.5 | 178 | 42.3 | [32] |
| 4 | BaTiO3-glass (BaO-Bi2O3-P2O5) | 1750 | 0.088 | 1.5 | 2.3 | 42.3 | [25] |
| 5 | BaO-Na2O-P2O5-Nb2O5-WO3 | 38 | 0.021 | 9 | 25.6 | 73.7 | [26] |
| 6 | NaNbO3-glass (Na2O-Nb2O5- P2O5) | 406 | 0.01 | 6 | 68.4 | 84.8 | [33] |
<strong>3</strong> 结论(1) 用熔融法和烧结法制备的P2O5-Nb2O5-BaO-Na2O-CeO2系磷酸盐微晶玻璃,都由BaNb2P2O11和Nb(PO4)O两个结晶相组成.随着CeO2的添加量从0提高到1%微晶玻璃的结晶度逐渐提高,且其内部结构逐渐致密化. ...
Effect of the Na2O-Nb2O5-P2O5 glass additive on the structure, dielectric and energy storage performances of sodium niobate ceramics
2
2021
... 用于储能电容器的微晶玻璃电介质,目前主要是硅酸盐玻璃基,磷酸盐玻璃基微晶玻璃报道较少,目前只有Bih L.组作了相关报导[25,26,33].表1列出了目前基于两种玻璃体系的介电微晶玻璃的电学性能.可以看出,硅酸盐玻璃基和磷酸盐玻璃基的微晶玻璃的介电常数普遍较高且范围可调,介电损耗可控制在0.022以内.本文制备的磷酸盐微晶玻璃的介电常数为37~43,介电损耗低于0.02,与表1列出的水平相当.从表1还可以看出,基于硅酸盐微晶玻璃的工作场强普遍高于基于磷酸盐的微晶玻璃,是其能量释放密度也普遍偏高.本文制备的磷酸盐微晶玻璃的最高工作场强和能量释放密度与表1给出的磷酸盐的微晶玻璃的相当,也反映出磷酸盐微晶玻璃的最高工作场强和能量释放密度还有待提高.由于储能效率属于多变量参数,与应用场强和电介质类型有关,场强越大弛豫型(或反铁电型)电介质的储能效率随之提高.磷酸盐体系较低的工作场强使本文制备的微晶玻璃的储能效率受到限制.本文制备的添加CeO2为1%的微晶玻璃储能效率最佳,为50.1%. ...
... Electric property of glass-ceramics
Table 1| No. | Glass-ceramic system | εr | tan δ | E /kV·mm-1 | Ud /mJ·cm-3 | η | Ref. |
|---|
| 1 | PbO-SrO-Na2O-Nb2O5-SiO2 | 427 | 0.022 | 44.2 | 850 | - | [31] |
| 2 | BaO-Na2O-Nb2O5-SiO2 | 312 | 0.016 | 16.3 | 370 | 72 | [7] |
| 3 | BaO-SrO-TiO2-Al2O3-SiO2 | 282 | 0.018 | 26.5 | 178 | 42.3 | [32] |
| 4 | BaTiO3-glass (BaO-Bi2O3-P2O5) | 1750 | 0.088 | 1.5 | 2.3 | 42.3 | [25] |
| 5 | BaO-Na2O-P2O5-Nb2O5-WO3 | 38 | 0.021 | 9 | 25.6 | 73.7 | [26] |
| 6 | NaNbO3-glass (Na2O-Nb2O5- P2O5) | 406 | 0.01 | 6 | 68.4 | 84.8 | [33] |
<strong>3</strong> 结论(1) 用熔融法和烧结法制备的P2O5-Nb2O5-BaO-Na2O-CeO2系磷酸盐微晶玻璃,都由BaNb2P2O11和Nb(PO4)O两个结晶相组成.随着CeO2的添加量从0提高到1%微晶玻璃的结晶度逐渐提高,且其内部结构逐渐致密化. ...