磷酸基团具有强大的金属螯合能力,可以与铁、镁、铜等多种金属进行螯合,磷羟基可以与金属表面形成稳定的P-O-Fe/Mg/Cu共价键,形成一层致密的单分子保护膜,提高涂层对金属表面的附着力和防腐蚀性能[6, 7]。在石墨烯中引入磷酸基团,可结合两者的优点,不仅使石墨烯具有优良的分散性和物理屏蔽作用,而且磷酸基团通过共价键的结合生成钝化膜,发挥了限制腐蚀介质扩散的作用[8]。Huang等[9]通过丙烯酸乙烯酯磷酸功能单体对氧化石墨烯(GO)进行功能化再与环氧树脂复合,复合涂层具有优异的耐腐蚀性能。Ding等[10]利用制备的羟基环氧磷酸盐单体增强石墨烯在环氧树脂中的相容性,从而使涂层具有良好的防腐蚀性能。然而,这些报道均是在石墨烯片层表面接枝磷酸基团,磷酸基团的含量相对较少,没有充分发挥出磷酸基团与金属的螯合作用。因此,有必要制备出高含量含磷石墨烯的复合涂层,以充分发挥石墨烯和磷酸酯基团的协同作用,提高涂层的腐蚀防护性能。
本文以含磷酸酯基团丰富的植酸(PhA)为原料,采用热解法制备含磷石墨烯[11],并以硅树脂为成膜物制备了含磷石墨烯/硅树脂(PhA-G/SiR)复合涂层,进而对比研究了复合涂层的耐蚀性能以及含磷石墨烯含量的影响。
1 实验方法
1.1 含磷石墨烯(PhA-G)的制备
将PhA放到鼓风干燥箱中,在150℃加热4 h除去水分,使之形成凝固状态。然后在管式气氛炉氮气中920℃热解2 h,自然冷却到室温。将得到的产物分散于无水乙醇中,700 W超声2 h,离心回收乙醇悬浮液中的固体,40℃真空干燥8 h得到PhA-G。
1.2 硅树脂(SiR)的制备
采用溶胶-凝胶法制备硅树脂。在5 g乙醇和2.5 g水的混合溶液中加入6 g乙烯基三甲氧基硅烷(A151)和1.5 g二甲基二甲氧基硅烷(DMDMS),混合均匀,再加入0.15 g柠檬酸超声使其溶解,在40℃水浴中搅拌反应8 h,结束后静置陈化24 h后密封待用。
1.3 PhA-G/SiR复合涂层的制备
称取一定量的PhA-G于乙醇中超声分散至均匀,再以制备的SiR为成膜基质,将PhA-G的乙醇分散液加入到一定量的SiR中,适当加热以挥发多余溶剂。加入适量固化剂硅烷偶联剂(KH550),采用滚涂法涂于经过表面处理的Q235钢电极(裸露电极面积为1 cm2)或不锈钢片(3 cm×6 cm)表面,放入50℃烘箱中固化。经多次滚涂,将涂层的厚度控制在80~100 μm之间,随后继续在50℃烘箱中固化24 h。通过调整PhA-G的添加量,制备出PhA-G含量分别为1%、2%、3%和4%(质量分数,下同)的PhA-G/SiR复合涂层。同时,为了对比,根据上述方法也制备了纯SiR涂层以及氧化石墨烯/SiR复合涂层(GO/SiR)进行对比研究。其中,涂层的厚度是利用薄膜测厚仪测试的,不同种类涂层的厚度基本保持一致,误差不超过±5 μm。
1.4 PhA-G的表征
采用日本日立S-4300场发射扫描电子显微镜(SEM)观察样品表面微观形貌,电压20 kV。采用HJYlabRAMHR-Evdotion紫外共聚焦拉曼光谱仪,表征样品的结构,采用512 nm激发波长,扫描范围为0~4000 cm-1。采用日本日立H-7650透射电子显微镜(TEM)观察样品微观形貌,电压100 kV。采用美国Thermo公司ESCALAB250Xi型X-射线光电子能谱仪(XPS)分析样品结构,电压100 kV。采用德国Bruker公司NanoScopeV型原子力显微镜(AFM)观察样品表面微观形貌。
1.5 PhA-G/SiR复合涂层性能测试
使用CANY-II-F/NF型测厚仪测量不同涂层的厚度,每个试样测5个点,取平均值。通过德国ZEISS公司AxioScope.A1Apol型偏光显微镜观察复合涂层的表面形貌。
通过接触角和吸水率测试PhA-G/SiR复合涂层的疏水性[12]。以水为测定液体,用静滴法在JY-82型接触角测定仪上测定涂层表面对水的接触角,水滴体积约0.5 μL,每个试样测5个点,取平均值。将涂覆涂层的不锈钢片置于去离子水中浸泡48 h(室温),按
其中,X是吸水率,m0和m1分别是涂层吸水前、后的质量。
用电化学阻抗谱、极化曲线和盐雾实验测试涂层的耐蚀性能[13]。以Ag/AgCl电极作为参比电极,铂柱电极作为辅助电极,涂覆涂层的Q235钢电极作为工作电极,电极面积为1 cm2。使用美国Gamry公司Interface1000电化学工作站测试涂层的电化学阻抗谱和极化曲线。测试介质为3.5%的NaCl水溶液。电化学阻抗谱测试是在开路电位下进行的,频率范围为10-2~105 Hz,交流幅值为10 mV。极化曲线的测试电位范围为-2~1 V(相对开路电位),扫描速率为0.5 mV·s-1。
按
根据GB6458-86,用OL-T-60型盐雾箱测试涂层的耐盐雾腐蚀性能。
2 结果与讨论
2.1 含磷石墨烯的结构与形貌
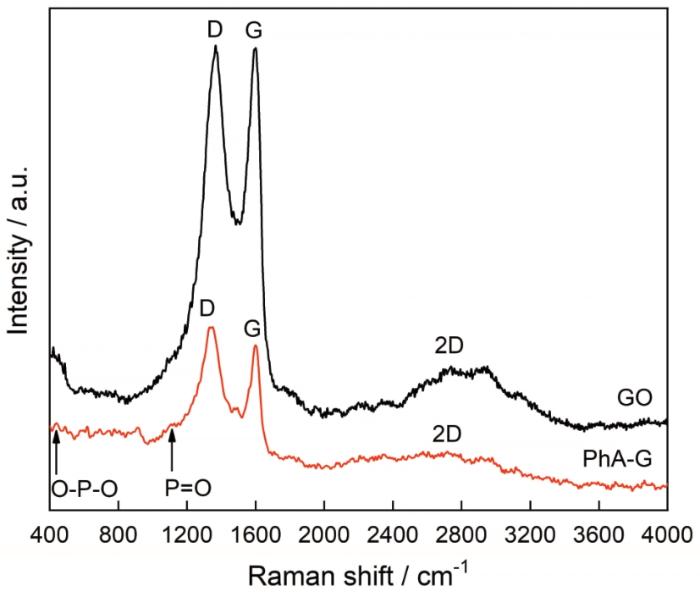
图1为GO和热解制备的PhA-G的拉曼光谱图。如图所示,在PhA-G的拉曼光谱中出现了与GO相同的石墨烯的三个特征峰,分别是2700 cm-1的2D峰、1600 cm-1的G峰和1350 cm-1的D峰,证明了制备的PhA-G具有石墨烯的结构特征。而且,D峰与G峰的相对强度(ID/IG)为1.19,略高于GO的相对强度0.94,说明了PhA-G的石墨烯结构具有较高的无序度。另外,PhA-G在2D峰波段的半峰宽较GO的略宽,表明了PhA-G为多片层结构[14]。同时,在PhA-G的D波段出现一个在1169 cm-1的肩峰,在低频区有一个位于466 cm-1的尖峰,这是由于PhA-G本身的磷酸基团中的O-P-O和P=O弯曲振动造成的[11],表明制备的PhA-G为含磷石墨烯。
图1
图2为GO和PhA-G的XPS谱图。其中,图2a为GO和PhA-G的XPS全谱图,在PhA-G的全谱中可以观察到C1s、O1s、P2s、P2p四个特征峰。其中,284.6 eV处的C1s归属于sp2杂化碳原子的π*峰[15],532.1 eV处的O1s对应着PhA-G表面的剩余含氧官能团,这两个特征峰与GO一致,表明PhA-G具有与GO相似的石墨烯结构。而且,与GO全谱相比,PhA-G的碳元素含量增加,氧元素含量下降,说明PhA-G在热解制备过程中被部分还原。同时,在132.2与191.2 eV处的P2p与P2s表明了磷元素的存在。为了研究C、O、P的成键情况,对各元素精细谱进行了分峰处理。如图2b所示,C1s能谱主要由四个峰组成,分别是284.8 eV的C-C,285.4 eV的C-P,286.9 eV的C-O-P/C-O-C以及290.7 eV的C=O[16]。相对应的,图2c中的O1s能谱出现了531.2 eV的C-O/P-O-C,532.8 eV的C=O/P=O,535.1 eV的O-H三个含氧峰。另外,P2p能谱(图2d)被分为134.6 eV的P-O与133.6 eV的C-P两个峰[17],结合C1s和O1s的分峰结果,可以证明通过高温热解成功制备了含磷石墨烯,其中部分磷原子进入了石墨烯骨架,部分磷原子在石墨烯表面以磷酸基团形式存在。
图2
图3
图4
图5
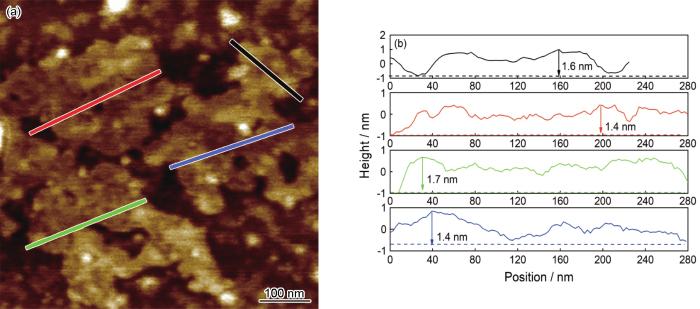
图5
PhA-G样品的AFM图像及厚度分析
Fig.5
AFM image (a) and thickness analysis results of PhA-G sample (b)
2.2 复合涂层的形貌及疏水性
图6
图6
不同PhA-G含量的PhA-G/SiR复合涂层的表面形貌照片
Fig.6
Surface morphologies of PhA-G/SiR composite coatings containing different mass fractions of PhA-G: (a) 1%, (b) 2%, (c) 3%, (d) 4%
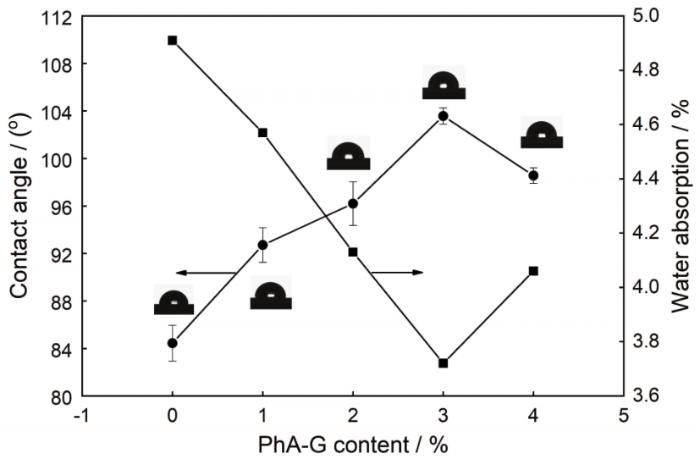
图7给出了PhA-G添加量对复合涂层疏水性能的影响曲线。随着PhA-G添加量的增加,复合涂层的接触角有所增大,而吸水率显著减小;当PhA-G添加量为3%时,涂层的接触角最大为103.5°,吸水率最小为3.72%。继续添加PhA-G达到4%后,涂层接触角降至100˚以下,吸水率超过4%。
图7
图7
PhA-G含量对复合涂层接触角和吸水率的影响
Fig.7
Effects of the content of PhA-G on the contact angle and water absorption of the composite coating
2.3 涂层的耐蚀性能
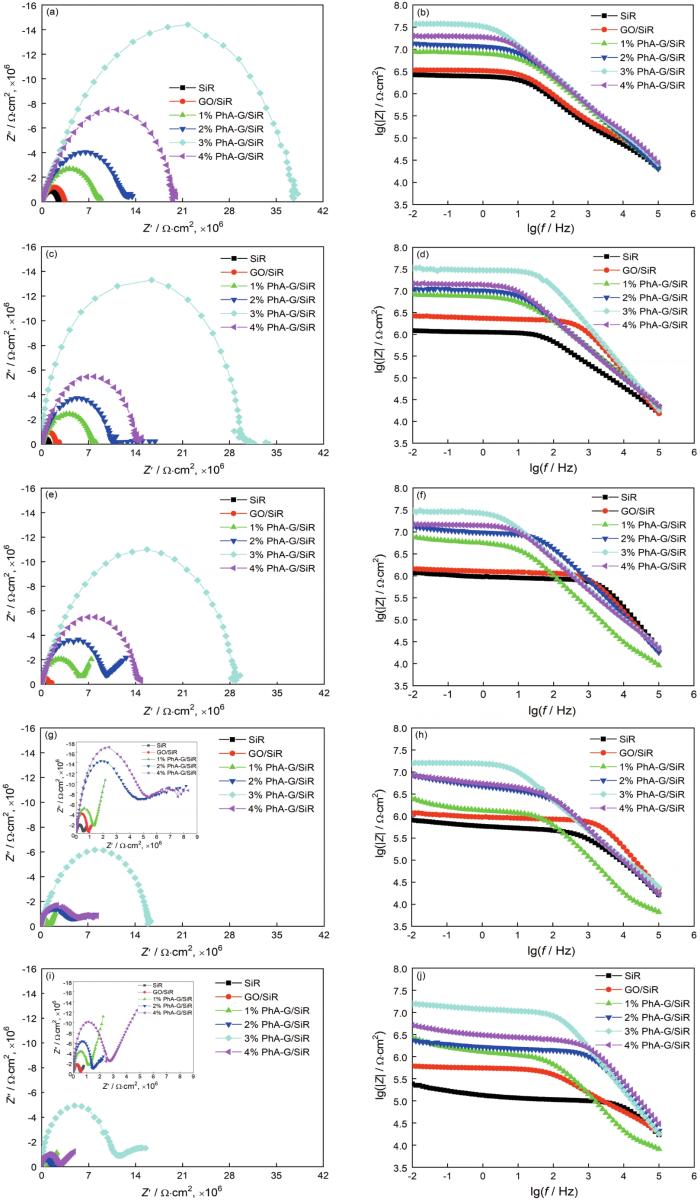
图8为纯SiR涂层、GO/SiR涂层和PhA-G含量分别为1%、2%、3%、4%的PhA-G/SiR复合涂层在3.5% NaCl溶液中浸泡不同时间后的电化学阻抗谱(Nyquist图和Bode图)。从图中可以看出,在浸泡初期(0 h),涂层的Nyquist图是由中高频处的容抗弧组成的。添加GO和PhA-G的复合涂层的容抗弧半径要大于纯SiR涂层的,说明添加GO和PhA-G提高了复合涂层的阻抗。相比之下,PhA-G/SiR复合涂层的容抗弧半径更大。当PhA-G的加入量为3% 时,PhA-G/SiR复合涂层的电化学阻抗值最大,而Bode图中0.01 Hz时的阻抗模值(|Z|)为3.82×107 Ω·cm2,说明此时复合涂层的屏蔽性能最佳。
图8
图8
SiR涂层、GO/SiR涂层和4种PhA-G/SiR复合涂层在3.5% NaCl溶液中浸泡不同时间的Nyquist和Bode图
Fig.8
Nyquist and Bode plots of SiR coating, GO/SiR coating and four PhA-G/SiR coatings after immersion in 3.5% NaCl solution for (a, b) 0 h, (c, d) 48 h, (e, f) 96 h, (g, h) 144 h, (i, j) 192 h
随着浸泡时间的延长,不同涂层的电化学阻抗值均有所减小,而且在低频处出现扩散,说明腐蚀介质逐渐渗透进入涂层,使涂层对Q235钢的防护能力减弱。相比GO/SiR涂层及SiR涂层,PhA-G/SiR复合涂层的电化学阻抗值下降较为缓慢,低频处出现扩散的时间较晚。其中,PhA-G的加入量为3%的PhA-G/SiR复合涂层在浸泡至192 h时,电化学阻抗谱低频处才出现轻微扩散,而且,0.01 Hz时的阻抗模值(|Z|)仍维持在1.55×107 Ω·cm2,说明3%的PhA-G/SiR复合涂层耐久性较好。
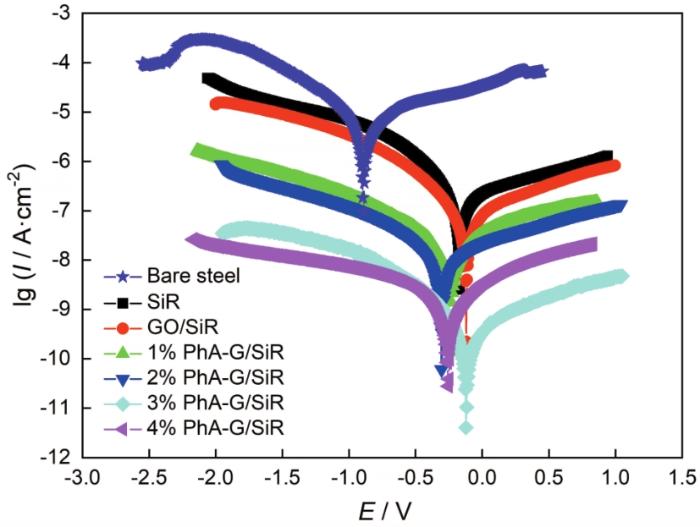
图9为裸钢、SiR涂层、GO/SiR涂层和不同PhA-G含量的PhA-G/SiR复合涂层的极化曲线图,极化曲线拟合数据见表1。从图和表中数据可以看出,Q235钢涂覆涂层之后,腐蚀电流密度(Icorr)均减小,腐蚀电位(E)均正移,说明涂层均起到了腐蚀防护作用。其中,相比GO/SiR涂层及SiR涂层,PhA-G/SiR复合涂层的改善效果更为明显,说明PhA-G的含磷片层结构显著提高了SiR涂层的耐蚀性能。随着PhA-G含量的增加,复合涂层的腐蚀电流密度先减小后增大;当PhA-G的含量为3%时,复合涂层的腐蚀电流密度最小,为3.53×10-10 A·cm-2,腐蚀电位最正,为-0.118 V,说明此时复合涂层的防腐蚀性能最佳。通过计算得到复合涂层的极化电阻(Rp)、腐蚀速率(CR)和腐蚀防护效率(PE),可以发现当PhA-G的含量为3%时,涂层的极化电阻最大,为2.82×108 Ω·cm2;腐蚀速率最小,为2.73×10-6 mm·a-1;涂层的腐蚀防护效率可以达到99.99%。该测试结果与电化学阻抗谱结果一致。
图9
图9
裸钢、SiR涂层、GO/SiR涂层和4种PhA-G/SiR复合涂层的极化曲线
Fig.9
Potentiodynamic polarization curves of bare steel, SiR coating, GO/SiR coating and four PhA-G/SiR coatings
表1 极化曲线拟合数据
Table 1
| Sample | Icorr / A·cm-2 | E / V | ba | bc | CR / mm·a-1 | Rp / Ω·cm2 | PE / % |
|---|---|---|---|---|---|---|---|
| Bare steel | 1.66×10-5 | -0.891 | 3.59 | 0.824 | 1.28×10-1 | 1.75×104 | - |
| SiR | 2.95×10-7 | -0.171 | 3.08 | 0.413 | 2.28×10-3 | 5.36×105 | 98.21 |
| GO/SiR | 1.76×10-7 | -0.120 | 2.92 | 0.645 | 1.36×10-3 | 1.31×106 | 98.93 |
| 1%PhA-G/SiR | 4.78×10-8 | -0.242 | 1.95 | 1.150 | 3.69×10-4 | 6.58×106 | 99.71 |
| 2%PhA-G/SiR | 2.45×10-8 | -0.306 | 2.42 | 0.832 | 1.89×10-4 | 1.10×107 | 99.85 |
| 3%PhA-G/SiR | 3.53×10-10 | -0.118 | 0.77 | 0.327 | 2.73×10-6 | 2.82×108 | 99.99 |
| 4%PhA-G/SiR | 3.58×10-9 | -0.252 | 1.10 | 2.060 | 2.76×10-5 | 8.71×107 | 99.97 |
图10为Q235钢片涂覆SiR涂层、GO/SiR涂层和不同PhA-G含量的PhA-G/SiR复合涂层样品经不同时间盐雾实验后以及去除涂层后Q235钢片的表面宏观形貌照片。可以看出,随着盐雾实验时间的延长,涂层划伤处都有不同程度的腐蚀及扩散。其中,涂覆SiR涂层的Q235钢片腐蚀情况较为严重,在240 h时,划伤处出现腐蚀,且腐蚀向涂层内部扩散;480 h时锈点逐渐扩散直至960 h时扩散了大半个钢片。涂覆GO/SiR涂层和不同PhA-G含量的PhA-G/SiR复合涂层的Q235钢片腐蚀程度减弱,而以PhA-G/SiR复合涂层的防护效果更好。当PhA-G的含量为3%时(图10e),盐雾240 h时涂层划伤处并没有腐蚀;480 h时出现轻微锈点,腐蚀介质还没有扩散;960 h时涂层表面平滑没有鼓包吸水现象,涂层划伤处锈点略有增加,涂层下金属表面略有扩散,说明3% PhA-G/SiR复合涂层的腐蚀防护作用最佳,实现了对涂层下金属的长期防护。
图10
图10
不同涂层经不同时间的盐雾实验后以及去掉涂层后钢片表面的宏观形貌照片
Fig.10
Macro-photos of (a) SiR, (b) GO/SiR, (c) 1% PhA-G/SiR, (d) 2% PhA-G/SiR, (e) 3% PhA-G/SiR, (f) 4% PhA-G/SiR coatings after salt spray test for different time, and steel substrate after removing the corroded coatings
图11
图11
盐雾实验960 h后去掉涂层后钢基体表面的显微形貌照片
Fig.11
Microscope photos of the surfaces of steel substrates after salt spray test for 960 h and then removal of (a) SiR, (b) GO/SiR, (c) 1% PhA-G/SiR, (d) 2% PhA-G/SiR, (e) 3% PhA-G/SiR, (f) 4% PhA-G/SiR surface coatings
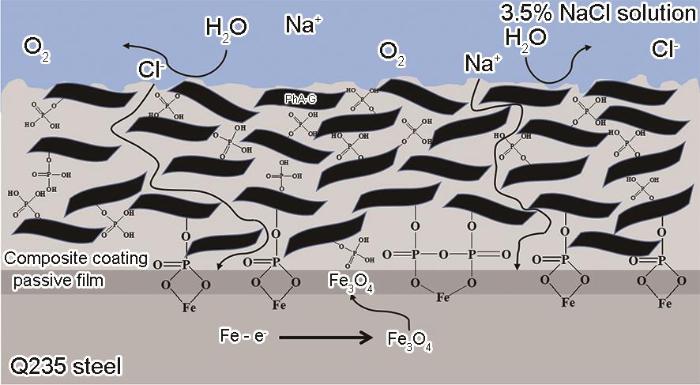
2.4 PhA-G/SiR复合涂层的防腐蚀机理
图12为PhA-G/SiR复合涂层的防腐蚀机理示意图。结合PhA-G的结构表征和PhA-G/SiR复合涂层的防腐蚀性能测试结果,分析认为PhA-G/SiR复合涂层良好的防腐蚀性能主要得益于以下效应:首先,适量的PhA-G均匀分散在涂层中,产生了“迷宫效应”[21],石墨烯片层结构有效的延长了H2O、O2以及Na+、Cl-等腐蚀介质在涂层中的渗透路径,阻隔了腐蚀介质渗透到金属表面对金属造成腐蚀[22];其次,PhA-G中的磷酸酯基团与金属可以发生螯合作用,在金属表面形成钝化膜,对金属起到钝化作用[8];另外,PhA-G可以弥补SiR基体的微孔和缺陷,增加涂层表面的微观粗糙度,进而提高复合涂层的疏水性。综上,PhA-G/SiR复合涂层的防腐蚀效果明显优于SiR涂层和GO/SiR复合涂层,是PhA-G片层的阻隔作用、PhA-G中磷酸酯基团的螯合钝化作用以及PhA-G/SiR复合涂层的疏水作用协同发挥的结果。
图12
图12
PhA-G/SiR复合涂层防腐蚀机理示意图
Fig.12
Schematic diagram of corrosion prevention mechanism of PhA-G/SiR composite coating
阻隔作用、螯合作用和疏水作用的协同发挥,使PhA-G/SiR复合涂层对金属的持久保护作用得到显著提升,而这些作用的发挥很大程度上取决于PhA-G在SiR中的添加量。当PhA-G含量较低(1%)时,PhA-G的阻隔作用和屏蔽作用发挥不理想,涂层表面的疏水能力也较弱,腐蚀介质侵入涂层内部相对容易。另外,PhA-G的含量低使得磷酸酯基团含量较少,与金属表面的钝化作用程度较低。因此,相对于GO其防腐蚀性能改善程度不大。随着PhA-G含量的增加(2%),阻隔作用和屏蔽作用增强,钝化作用也增加,复合涂层的防腐蚀性能提高。当PhA-G含量增加到3%时,适量的PhA-G在SiR涂层中均匀分散,一方面使涂层表面具有一定的粗糙度,从而使涂层表现出较好的疏水性能,减少了水及腐蚀离子的浸入;另一方面提供了良好的阻隔作用和屏蔽作用,进一步延长了腐蚀介质的侵入路径。而且,较多的磷酸酯基团可以加快钝化作用,促进金属表面钝化膜的产生,综合作用使复合涂层的防腐蚀性能达到最佳。当然,继续增加PhA-G含量到4%后,过多的PhA-G在涂层中团聚导致分散性变差,从而降低了屏蔽效率,进而影响了防腐蚀性能[23],但是高含量磷酸酯基团与金属基材的钝化作用比低含量的更充分,所以4%含量PhA-G的复合涂层耐蚀性仍优于1%与2%含量的。
3 结论
(1) 通过高温热解制备了含磷石墨烯PhA-G,其中部分磷原子进入了石墨烯骨架,部分磷原子在石墨烯表面以磷酸酯基团形式存在。PhA-G具有明显的二维片层结构,片层厚度在1.5 nm左右。
(2) PhA-G的添加使制备的PhA-G/SiR复合涂层的防腐蚀效果明显优于SiR涂层和GO/SiR复合涂层。
(3) 当PhA-G添加量为3%时,所制备的PhA-G/SiR复合涂层具有良好的疏水性、防腐蚀性能和耐久性,接触角最大为103.5°,吸水率为3.72%,电化学阻抗值达到3.82×107 Ω·cm2,腐蚀电流密度仅为 3.53×10-10 A/cm2,盐雾实验达到960 h。
参考文献
A corrosion-protective coating based on a solution-processable polymer-grafted graphene oxide nanocomposite
[J].
Application of aminated grap-hite oxide for waterborne anti-corrosion and fireproof coatings
[J].
氨基化氧化石墨烯在水性防腐防火一体化涂料中的应用
[J].
Experimental and computational studies of graphene oxide covalently functionalized by octylamine: electrochemical stability, hydrogen evolution, and corrosion inhibition of the AZ13 Mg alloy in 3.5%NaCl
[J].Recently, carbon allotropes were shown to play a key role in energy harvesting and as hydrophobic coatings on metal alloys. We have designed octylamine-functionalized graphene oxide materials for energy harvesting and as an anti-corrosion coating for metal alloy protection in a 3.5% NaCl medium. The material has been characterized by different techniques to confirm the structure and composition of the modified graphene oxide sheet: FTIR spectroscopy, XRD, Raman spectroscopy, FESEM and TEM. The electrochemical stability and corrosion inhibition efficiency were studied by electrochemical methods. The electrochemical stability increased with an increase in the applied voltage up to 500 mV, and the corrosion inhibition efficiency was shown to be 73%. The coating stability studies showed a long stability time in the corrosion medium.This journal is © The Royal Society of Chemistry.
Synergistic effect of polyvinylpyrrolidone noncovalently modified graphene and epoxy resin in anticorrosion application
[J].
Interesting corrosion inhibition performance and mechanism of two silanes containing multiple phosphate group
[J].
Phosphate-doped polyaniline/Al2O3 nanocomposite coating for protection of stainless steel
[J].
Anticorrosive performance of phosphorylated graphene oxide applied at waterborne epoxy coating
[J].
磷酸化氧化石墨烯对水性环氧涂料防腐增强作用研究
[J].
Modification of graphene oxide with acrylate phosphorus monomer via thiol-Michael addition click reaction to enhance the anti-corrosive performance of waterborne epoxy coating
[J].
A novel hydroxyl epoxy phosp hate monomer enhancing the anticorrosive performance of waterborne Graphene/Epoxy coatings
[J].
Phosphorus-doped graphene as a metal-free material for thermochemical water reforming at unusually mild conditions
[J].P-doped graphene (Phy-G) prepared by pyrolysis of phytic acid at 900 degrees C under inert atmosphere has been evaluated as a metal-free catalyst for the thermochemical water splitting. XPS, solid-state P-31 NMR, and Raman spectroscopy confirm the presence of P atoms bonded to C atoms in the graphene lattice as well as some oxygenated P groups, such as phosphates or phosphonates. HRTEM and AFM images show the characteristic sheet morphology of 2D graphene materials of several micrometers lateral size and exhibiting a high crystallinity with the characteristic hexagonal arrangement of graphenic materials. Phy-G has been submitted to consecutive oxidation/activation thermochemical cycles at 650 and 800 degrees C under H2O-saturated Ar and dry Ar atmospheres, respectively. During the oxidation periods, H-2 evolution up to 21.6 mu mol/min.g was measured. However, no O-2 evolves in the activation steps. Experimental evidence and computational calculations support the formation of P=O bonds during the oxidation steps. The computational calculations suggest that the thermocatalytic H2O splitting occurs on the P atoms of doped graphene through a stepwise process involving an intermediate with a P-OH group and a H attached to a neighboring C atom and subsequent H-2 evolution, leading to the formation of P-O bonds.
Hybrid organosiloxane coatings containing epoxide precursors for protecting mild steel against corrosion in a saline medium
[J].
Curing mechanism, heat resistance, and anticorrosion properties of a furan/methyl phenyl silicone coating
[J].
Raman spectroscopy of graphene
[J].
拉曼光谱在石墨烯结构表征中的应用
[J].石墨烯是sp<sup>2</sup>碳原子紧密堆积形成的二维原子晶体结构,因其独特的结构与性质引起了科学家们的广泛关注. 拉曼光谱是一种快速而又简洁的表征物质结构的方法. 主要综述了拉曼光谱技术在石墨烯结构表征中应用的一些最新进展. 首先,在系统分析石墨烯声子色散曲线的基础上介绍了石墨烯的典型拉曼特征(G’峰、G峰和D峰),讨论了G’峰、G峰和D峰在石墨烯层数的指认和石墨烯边缘与缺陷态分析中的应用;然后,通过对石墨烯拉曼G峰和G’峰的峰位、峰型以及强度的分析,讨论了石墨烯的层间堆垛方式、掺杂、基底、温度和应力等对石墨烯的电子能带结构的影响;最后,介绍了石墨烯中的二阶和频与倍频拉曼特征以及石墨烯的低频拉曼特征(剪切和层间呼吸振动模),并讨论了其对石墨烯结构的依赖性.
Preparation and characterization of phosphorus-doped graphene
[J].
磷掺杂石墨烯的制备与表征
[J].
Boron/phosphorus doping for retarding the oxidation of reduced graphene oxide
[J].
Preparation and structure study of phosphorus-doped porous graphdiyne and its efficient lithium storage application
[J].
Efficient electrocatalytic activity for oxygen reduction reaction by phosphorus-doped graphene using supercritical fluid processing
[J].
Reaction mechanism of N-(4-hydroxyphenyl)ethanamide electrodegradation via phosphorus-graphene prepared from triphenylphosphine: Generation and destruction of the reactive species
[J].
Tribological properties of graphene in PAO base oil
[J].
石墨烯在PAO基础油中的摩擦学性能
[J].
Preparation of graphene/polyaniline nanocomposite by in situ intercalation polymerization and their application in anti-corrosion coatings
[J].
Distinctive roles of silane coupling agents on the corrosion inhibition performance of graphene oxide in epoxy coatings
[J].