在新型清洁能源的研发中,电解水制氢是一种极有应用前景的能量转换方式之一。
共轭微孔聚合物(CMPs)具有的独特π共轭骨架结构[17],可使较大孔隙率的材料具有优异的半导体特性促进电子在其表面的传输[18, 19]。同时,CMPs还能改变其构筑分子的官能团和合成方法,从而精准调控材料的微观结构,使制备出的CMPs材料更适合作为二硫化钼的生长基底[20, 21]。Qiao等用原位生长方法将MoS2负载在CTFs的一维孔道中合成出MoS2@CTFs复合材料,具有比纯MoS2优异的析氢性能(过电势为93 mV,Tafel 斜率为 43 mV dec-1)[22]。Li等将MoS2负载在用煅烧野生芹菜制备的碳管(BCTM)中进行电化学析氢反应,得到聚合物的过电势为176 mV,Tafel斜率为51 mV·dec-1 [23, 24]。这表明,提高二硫化钼复合材料的导电性能可提高体系的电催化析氢性能[25]。
1 实验方法
1.1 共轭微孔聚合物的制备
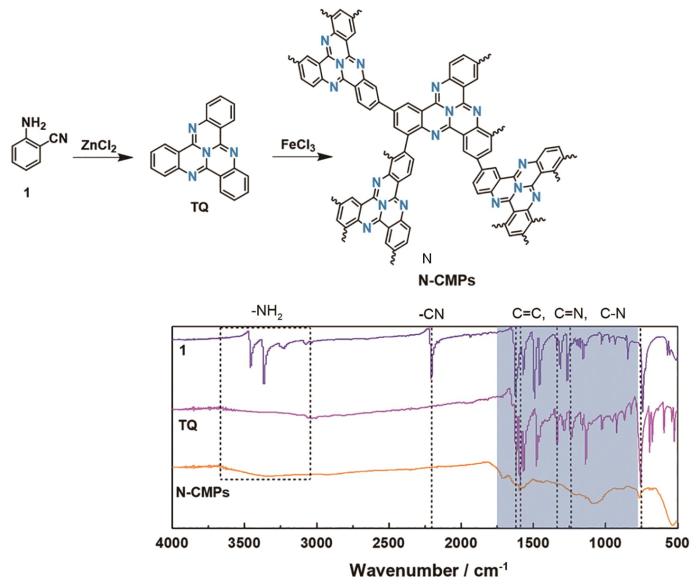
在氮气保护下,将邻氨基苯甲腈(分析纯)(化合物1)(100 mg,0.85 mmol)和无水氯化锌(116 mg,0.85 mmol)置于安瓿瓶中,将其抽真空后进行加热密封。将密封后的安瓿瓶转移到管式炉中加热至350℃,反应24 h后冷却至室温,得到黄色固体。将黄色固体物研磨后依次用盐酸(1 mol/L)和超纯水洗涤,将过滤后的固体溶于氯仿并加入无水硫酸钠以去除水份。将其过滤后将滤液进行旋蒸以除去溶剂,将得到的三环喹唑啉固体产物放入80℃真空烘箱中干燥12 h。
将三环喹唑啉(100 mg,0.31 mmol)溶于氯仿(30 mL)后缓慢滴入由三氯化铁(778 mg,4.8 mmol)和氯仿(20 mL)组成的悬浮液中,在氮气保护下在室温搅拌72 h。向反应液中加入甲醇(100 mL)后过滤,然后将沉淀依次用甲醇和超纯水洗涤并离心分离。将沉淀在装有甲醇的索氏提取器中萃取48 h后在80℃真空干燥12 h。得到的黑色产物即为共轭微孔聚合物TQ-CMPs@MoS2。
1.2 TQ-CMPs@MoS2 和MoS2 的制备
将0.178 mmol的四水合钼酸铵(分析纯)和5.24 mmol的硫脲(分析纯)溶于超纯水(10 mL)中搅拌,然后将所得均相溶液和0.4 g的共轭微孔聚合物一并加入到15 mL的水热反应釜中,在室温搅拌20 min后加热到200°C反应18 h。反应结束后冷却至室温,将产物TQ-CMPs@MoS2 2∶1(2∶1为TQ-CMPs与MoS2的质量比)过滤后依次用超纯水和无水乙醇充分洗涤,最后在60℃真空干燥14 h[30]。得到的产物,即为TQ-CMPs@MoS2 2∶1。
在上述制备过程中不加入共轭微孔聚合物,即得纯MoS2。
1.3 性能表征
用傅里叶红外光谱(FT-IR)仪测试样品的红外光谱。用在77K测定样品的N2吸附和脱附等温线。用X射线粉末衍射仪(D/MAX‒2500(铜靶)型)测试样品的XRD谱。用扫描电子显微镜(Hitachi S-4800)和透射电子显微镜(JEM-2100)观察样品的形貌。
使用电化学工作站(Zennium Pro型)测试TQ-CMPs@MoS2 2∶1的电化学析氢性能:采用三电极体系(对电极,工作电极以及参比电极)测试电化学性能,使用铂丝为对电极,玻碳电极(直径为4 mm)为工作电极,饱和Ag/AgCl (sat.KCl)为参比电极。以标准氢电极(E(RHE)=E(Ag/AgCl)+0.197 V+0.0591×pH)为标准电势。以5 mV·s-1的速率进行伏安线性扫描(LSV),电流密度为10 mA·cm-2对应的电势为过电势结果。测量电化学阻抗谱图(EIS)的频率为0.01~100 KHz,振幅为5 mV。用循环伏安法(CV)验证电化学的稳定性,扫描速率为50 mV·s–1,-0.4~0.1 V的电势范围内循环3000圈。测量CV曲线(0.1~0.35 V vs. RHE)时扫描速率为40~200 mV·s-1,根据不同扫描速率下的电流密度(ΔJ|Ja–Jc|/2)曲线的斜率计算双层电容的数值[31]。
在充分研磨的4 mg催化剂样品中滴入30 µL的5% Nafion溶液和1 mL的乙醇溶液(
2 实验结果
2.1 样品的化学组成
聚合物TQ-CMPs与其对应的化合物1和构筑模块(TQ)的傅里叶红外光谱(FT-IR),如图1所示。谱中位于2210 cm-1和3140~3480 cm-1附近的特征峰分别为化合物1产生的–CN和–NH2键的伸缩振动峰,与TQ中–CN和–NH2键有关的特征峰几乎消失,而在1620、1590、1330和1230 cm-1波数附近产生了新的特征峰,分别对应TQ结构中的C=C、C=N、C-C和C-N键,表明化合物1在ZnCl2的催化下进行了三聚反应。在TQ-CMPs的红外光谱中观察到与TQ一致的特征峰,表明TQ-CMPs中存在TQ的化学结构。
图1
根据在77K测定的N2吸附和脱附等温线(图2),计算出TQ-CMPs聚合网络的孔隙率。根据IUPAC的分类标准,TQ-CMPs具有I型吸附等温线(图2a)。TQ-CMPs在较低的相对压力范围内(P/P0<0.01)具有较强的N2吸附量,表明其空隙结构中存在大量的微孔结构。同时,随着相对压力的提高TQ-CMPs的氮气吸附量迅速增大,表明聚合物中有大量的介孔且其孔径分布较宽。由Brunauer-Emmett-Teller (BET)方程计算出TQ-CMPs的比表面积为781.0 m2·g-1。根据QS-DFT理论计算出TQ-CMPs的孔径分布在1.1~13.1 nm,空隙主要由微孔和介孔组成,与TQ-CMPs的N2等温吸附线的结果一致。随着MoS2的负载,BET的比表面积从781.0 m2·g-1减小到695.2、487.5、299.2、233.5 m2·g-1。与纯MoS2相比,TQ-CMPs@MoS2 2∶1没有出现堆积现象。
图2
图2
在77K测定的N2吸附和脱附等温线(a)和孔隙宽度分布(b)
Fig.2
N2 adsorption and desorption isotherms at 77K (a) and pore width distribution (b)
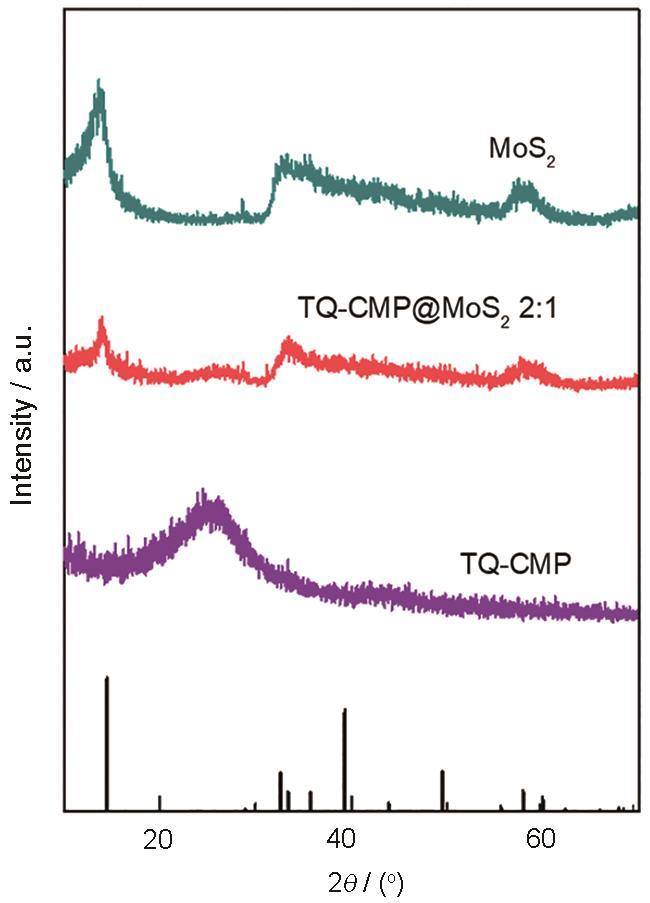
2.2 样品的XRD谱分析
图3
图3
MoS2、TQ-CMPs@MoS2和TQ-CMP的XRD谱
Fig.3
XRD pattern of MoS2, TQ-CMPs@MoS2 and TQ-CMP
2.3 SEM、TEM分析
图4a表明,刚性的构筑单元TQ在动力学控制的生长条件下在溶剂相中很快地发生偶联堆积,形成一种具有较大孔隙的三维拓扑结构。这种孔隙率较大的π共轭骨架有利于在电催化反应中传质和电子输送。图4b给出了TQ-CMPs@MoS2的SEM照片,可见花瓣状的二硫化钼晶体结构整齐地附着在TQ-CMPs的表面,更有利于电子在TQ-CMPs和二硫化钼之间传导。还可以看出,TQ-CMPs@MoS2边缘二硫化钼的片状结构。在高倍TEM图像中可清晰地观察到二硫化钼的晶格条纹,条纹的间距为0.27 nm(图4e)和0.95 nm(图4f),分别对应二硫化钼晶体的(100)和(002)晶面。还可见明显的晶格缺陷,与XRD的结果一致。
图4
图4
TQ-CMPs(a)和TQ-CMPs@MoS2 2∶1(b)的SEM图;TQ-CMPs(c)和TQ-CMPs@MoS2 2∶1(d)的TEM图;TQ-CMPs @MoS2 2∶1(e, f)的HRTEM图
Fig.4
SEM images of TQ-CMPs (a) and TQ-CMPs@MoS2 2∶1 (b). TEM images of TQ-CMPs (c) and TQ-CMPs@MoS2 2∶1 (d). HRTEM images of TQ-CMPs@MoS2 2∶1 (e, f)
2.4 电催化析氢性能
在0.5 mol·L-1的硫酸溶液中复合材料(TQ-CMPs@MoS2 2∶1)显示出最佳的析氢效果,其过电势为71 mV,Tafel斜率为52 mV·dec-1,而纯二硫化钼的Tafel斜率为467 mV·dec-1,可见其催化性能明显提高[33]。
图5给出了TQ-CMPs、纯MoS2、20%Pt/C以及不同硫化钼负载比例的TQ-CMPs@MoS2的电化学析氢性能。图5a中的LSV曲线表明,电流密度为10 mA·cm-2条件下,TQ-CMPs@MoS2 2∶1的过电势为71 mV,纯二硫化钼的过电势为467 mV。其原因是,TQ-CMPs的孔隙率较高,且由三环喹唑啉组成的共轭骨架增强了电子的离域效应,使电子在其表面快速迁移。这就为MoS2提供了更多的负载空间。从TEM照片可见,MoS2负载在TQ-CMPs后暴露出较多的晶格缺陷,为电催化析氢性能提供了更多的活性位点。为了更深入的了解TQ-CMPs@MoS2在电催化析氢的动力学,根据LSV曲线计算出各催化剂的Tafel斜率。结果表明,TQ-CMPs@MoS2 2∶1的Tafel斜率最小,为52 mV·dec-1,说明其具有较高的催化反应速率,催化活性更高。电化学阻抗图谱,表征了催化剂析氢过程中电极表面和电解液之间电子转移的阻力。从图5c可见,TQ-CMPs@MoS2 2∶1的半圆直径最小,表明复合材料表面的电子迁移阻力最小,反应速率更高,与其较低的Tafel斜率相对应。从图5d可见,经过3000圈的循环测试后与其初始的过电势相比产生的差异可以忽略,表明TQ-CMPs@MoS2 2∶1在析氢过程中具有很高的催化稳定性。电化学活性面积(ECSA)是表征在析氢反应中催化活性的另一个重要参数,并且与双层电容(Cdl)有直接的线性关系。从图5e可见,复合材料TQ-CMPs@MoS2 2∶1的循环伏安曲线在非法拉第电流区域(0.1~0.35 V vs. RE)均呈矩形形状。据此计算出各催化剂的Cdl数值为4.5,4.1,3.2,2.8,0.23 mF·cm–2,分别对应着TQ-CMPs@MoS2 2∶1,TQ-CMPs@MoS2 1∶1,TQ-CMPs@MoS2 1∶2,TQ-CMPs@MoS2 5∶1,TQ-CMPs。从这些结果可以看出,TQ-CMPs@MoS2材料比纯TQ-CMPs有较高密度的反应活性位点,有利于促进电荷的有效转移,使其具有较高的催化活性。但是,过高的MoS2负载量容易使结晶颗粒团聚,部分活性位点不能充分利用降低了Cdl而影响催化效率。
图5
图5
催化剂的电化学性能(a)0.5 mol·L‒1 H2SO4中的LSVs,(b)由LSVs计算得到的Tafel斜率,(c)催化剂的Nyquist图,TQ-CMPs@MoS2 2∶1的稳定性(d)和循环伏安曲线(e),催化剂的双层电容(f)
Fig.5
Electrochemical performances of all catalysts (a) LSV in 0.5 mol·L‒1 H2SO4, (b) Tafel slope calculated from LSVs, (c) Nyquist plots of all catalysts, (d) Stability and (e) Cyclic voltammetry curves of TQ-CMPs@MoS2 2∶1, (f) The double-layer capacitance (Cdl) of all catalystsv (■: Pt/C 20%;◆: TQ-CMPs@ MoS2 5∶1; ▲: TQ-CMPs@ MoS2 2∶1; ●: TQ-CMPs@MoS2 1∶1; ▼: TQ-CMPs@MoS2 1∶2; ◀: TQ-CMPs; ▶: MoS2)
3 结论
在基于三环喹唑啉的共轭微孔聚合物TQ-CMPs的共轭骨架表面可原位生长二硫化钼晶体颗粒。TQ-CMPs内部的孔道结构能为H+/e-提供运输通道,从而提高二硫化钼纳米薄片的导电性。TQ-CMPs与MoS2质量比为2∶1的复合材料具有优异的电催化活性,电流密度为10 mA·cm-2时其过电势为71 mV,Tafel斜率为52 mV·dec-1。
参考文献
Synthesis of ultrafine and highly dispersed metal nanoparticles confined in a thioether-containing covalent organic framework and their catalytic applications
[J].Covalent organic frameworks (COFs) with well-defined and customizable pore structures are promising templates for the synthesis of nanomaterials with controllable sizes and dispersity. Herein, a thioether-containing COF has been rationally designed and used for the confined growth of ultrafine metal nanoparticles (NPs). Pt or Pd nanoparticles (Pt NPs and Pd NPs) immobilized inside the cavity of the COF material have been successfully prepared at a high loading with a narrow size distribution (1.7 ± 0.2 nm). We found the crystallinity of the COF support and the presence of thioether groups inside the cavities are critical for the size-controlled synthesis of ultrafine NPs. The as-prepared COF-supported ultrafine Pt NPs and Pd NPs show excellent catalytic activity respectively in nitrophenol reduction and Suzuki-Miyaura coupling reaction under mild conditions and low catalyst loading. More importantly, they are highly stable and easily recycled and reused without loss of their catalytic activities. Such COF-supported size-controlled synthesis of nanoparticles will open a new frontier on design and preparation of metal NP@COF composite materials for various potential applications, such as catalysis and development of optical and electronic materials.
Non-noble metal-based carbon composites in hydrogen evolution reaction: fundamentals to applications
[J].
Highly active, nonprecious electrocatalyst comprising borophene subunits for the hydrogen evolution reaction
[J].Developing nonprecious hydrogen evolution electrocatalysts that can work well at large current densities (e.g., at 1000 mA/cm: a value that is relevant for practical, large-scale applications) is of great importance for realizing a viable water-splitting technology. Herein we present a combined theoretical and experimental study that leads to the identification of α-phase molybdenum diboride (α-MoB) comprising borophene subunits as a noble metal-free, superefficient electrocatalyst for the hydrogen evolution reaction (HER). Our theoretical finding indicates, unlike the surfaces of Pt- and MoS-based catalysts, those of α-MoB can maintain high catalytic activity for HER even at very high hydrogen coverage and attain a high density of efficient catalytic active sites. Experiments confirm α-MoB can deliver large current densities in the order of 1000 mA/cm, and also has excellent catalytic stability during HER. The theoretical and experimental results show α-MoB's catalytic activity, especially at large current densities, is due to its high conductivity, large density of efficient catalytic active sites and good mass transport property.
A molecular molybdenum-oxo catalyst for generating hydrogen from water
[J].
Aqueous platinum(ii)‐cage‐based light‐harvesting system for photocatalytic cross‐coupling hydrogen evolution reaction
[J].
General formation of monodisperse IrM (M = Ni, Co, Fe) bimetallic nanoclusters as bifunctional electrocatalysts for acidic overall water splitting
[J].
Preparation of MoS2/Y zeolite microbial electrolysis cell cathode materialand its electrochemical properties
[J].
MoS2/Y分子筛微生物电解池阴极材料制备及其电化学性能
[J].
Recent advances in heterogeneous electrocatalysts for the hydrogen evolution reaction
[J].
Nitrogen-rich covalent triazine frameworks as high-performance platforms for selective carbon capture and storage
[J].
Conductive molybdenum sulfide for efficient electrocatalytic hydrogen evolution
[J].
Recent development of molybdenum sulfides as advanced electrocatalysts for hydrogen evolution reaction
[J].
MOFs and COFs for batteries and supercapacitors
[J].
Covalent organic frameworks: design, synthesis, and functions
[J].Covalent organic frameworks (COFs) are a class of crystalline porous organic polymers with permanent porosity and highly ordered structures. Unlike other polymers, a significant feature of COFs is that they are structurally predesignable, synthetically controllable, and functionally manageable. In principle, the topological design diagram offers geometric guidance for the structural tiling of extended porous polygons, and the polycondensation reactions provide synthetic ways to construct the predesigned primary and high-order structures. Progress over the past decade in the chemistry of these two aspects undoubtedly established the base of the COF field. By virtue of the availability of organic units and the diversity of topologies and linkages, COFs have emerged as a new field of organic materials that offer a powerful molecular platform for complex structural design and tailor-made functional development. Here we target a comprehensive review of the COF field, provide a historic overview of the chemistry of the COF field, survey the advances in the topology design and synthetic reactions, illustrate the structural features and diversities, scrutinize the development and potential of various functions through elucidating structure-function correlations based on interactions with photons, electrons, holes, spins, ions, and molecules, discuss the key fundamental and challenging issues that need to be addressed, and predict the future directions from chemistry, physics, and materials perspectives.
Covalent organic framework with frustrated bonding network for enhanced carbon dioxide storage
[J].
Triazine-based covalent organic polymers: design, synthesis and applications in heterogeneous catalysis
[J].
Electrochemical stimuli-driven facile metal-free hydrogen evolution from pyrene-porphyrin-based crystalline covalent organic framework
[J].
Preparation, characterization and electrochemical performance in H2SO4 electrolyte of PbSO4/AC
[J].
PbSO4/活性炭复合材料的制备和电化学性能
[J].
Hydrogen evolution and oxidation: mechanistic studies and material advances
[J].
Probing size and substrate effects on the hydrogen evolution reaction by single isolated Pt atoms, atomic clusters, and nanoparticles
[J].We report the catalytic activity of a single, isolated Pt deposit on Bi and Pb supports to probe the size and substrate effects on the electrochemical hydrogen evolution reaction (HER). Deposits were made electrolytically by an atom-by-atom method in a controlled plating; we prepared an individual Pt deposit on Bi and Pb ultramicroelectrodes (UMEs) such as a single isolated atom, clusters containing one to five Pt atoms, and nanoparticles to about 10 nm radius. A steady-state voltammogram on the single Pt deposits is observed by electrocatalytic amplification of the HER, with a negligible contribution by the HER at the substrate UME. A single Pt atom can act as an electrode for the HER, showing a diffusion-limiting current plateau in the voltammogram that can be used to estimate the radius of a single deposit. We simulated the voltammograms of the individual deposits, assuming the Volmer step of the HER is appropriate for a Pt cluster deposit, to obtain kinetic parameters for each deposit. The HER kinetics increases as the particle radius increases from ∼0.2 to ∼4 nm for Bi and Pb substrates and then reaches a limiting plateau. The limiting kinetics on the Bi substrate approaches that of bulk Pt while that on the Pb substrate is much smaller.
Conjugated microporous polymers: design, synthesis and application
[J].Conjugated microporous polymers (CMPs) are a class of organic porous polymers that combine π-conjugated skeletons with permanent nanopores, in sharp contrast to other porous materials that are not π-conjugated and with conventional conjugated polymers that are nonporous. As an emerging material platform, CMPs offer a high flexibility for the molecular design of conjugated skeletons and nanopores. Various chemical reactions, building blocks and synthetic methods have been developed and a broad variety of CMPs with different structures and specific properties have been synthesized, driving the rapid growth of the field. CMPs are unique in that they allow the complementary utilization of π-conjugated skeletons and nanopores for functional exploration; they have shown great potential for challenging energy and environmental issues, as exemplified by their excellent performance in gas adsorption, heterogeneous catalysis, light emitting, light harvesting and electrical energy storage. This review describes the molecular design principles of CMPs, advancements in synthetic and structural studies and the frontiers of functional exploration and potential applications.
Advances in Conjugated Microporous Polymers
[J].
Pore surface engineering of covalent triazine frameworks@MoS2 electrocatalyst for the hydrogen evolution reaction
[J].
Micrometer-scale biomass carbon tube matrix auxiliary MoS2 heterojunction for electrocatalytic hydrogen evolution
[J].
Unveiling the layer-dependent catalytic activity of PtSe 2 atomic crystals for the hydrogen evolution reaction
[J].
Stable rhodium (IV) oxide for alkaline hydrogen evolution reaction
[J].
Synthesis of porous covalent quinazoline networks (CQNs) and their gas sorption properties
[J].
Reaction mechanism for the hydrogen evolution reaction on the basal plane sulfur vacancy site of MoS2 using grand canonical potential kinetics
[J].
Unsaturated sulfur edge engineering of strongly coupled MoS2 nanosheet-carbon macroporous hybrid catalyst for enhanced hydrogen generation
[J].
Revealing the contribution of individual factors to hydrogen evolution reaction catalytic activity
[J].
Ultrafine Co nanoparticles encapsulated in carbon-nanotubes-grafted graphene sheets as advanced electrocatalysts for the hydrogen evolution reaction
[J].
Oxidative R1-H/R2-H Cross-coupling with hydrogen evolution
[J].
Enhancing hydrogen evolution activity of Au(111) in alkaline media through molecular engineering of a 2D polymer
[J].