国内外学者对碳纳米填料对聚合物热学性能的影响,进行了大量研究。2006年Juan Li等[6]通过热重分析研究了多壁碳纳米管(MWNTs)/聚酰胺6(PA6)复合材料的热降解行为,并用Kissinger法计算出纯PA6、p-MWNTs/PA6和f-MWNTs/ PA6复合材料在空气和氮气中的降解活化能。结果表明,MWNTs的存在明显提高了PA6在空气中的稳定性,但是对PA6在氮气中的热降解行为影响不大;2009年Mi Wang等[7] 研究了聚硅氧烷接枝多壁碳纳米管/聚碳酸酯复合材料的性能,发现大量相互连接的碳纳米管形成了能阻止热量传递的碳层屏障,从而提高了底层聚合物材料的热稳定性;2017年Yamamoto T[8]通过静电相互作用将PMMA颗粒吸附在碳纤维表面,促进了碳纤维在PMMA中的分散、扩散和界面粘合,从而提高了PMMA材料的机械性能。但是目前使用的材料大多是价格昂贵的碳纳米管和碳纤维填料,且制作工艺复杂,难以在批量生产中应用。
碳黑填充剂具有良好的经济性、在聚合物基体中具有良好的分散特性以及对聚合物材料优异的增强作用。鉴于此,本文研究不同含量的碳黑对PMMA的热稳定性的影响,并在固态反应动力学的基础上求解和验证不同碳黑含量的PMMA的热降解动力学模型。
1 实验方法
实验用材料为PMMA(ALTUGLAS® V040)。不同碳黑含量的PMMA由纯PMMA母粒与不同质量分数的碳黑粉末(0%~0.25%,质量分数)共混注塑而成。共混成型的PMMA主要物理性能参数,列于表1。
表1 PMMA的物理性能
Table1
| Material | Density/kg∙(m3)-1 | Molar specific heat capacity/J∙(kg∙K)-1 | Thermal conductivity /W∙(m∙K)-1 | Viscous flow temperature/℃ |
|---|---|---|---|---|
| PMMA | 1190 | 1470 | 0.21 | 220 |
用于热重分析实验的样品为5~10 mg不同碳黑含量的PMMA板。进行热重分析实验前,先将试样清洗20 min,然后在100~120℃干燥箱中干燥2 h;用Q500型热失重分析仪测试样品在程序升温过程中的热失重,测试温度范围为室温-600℃,升温速率5~20℃/min,氮气流速40 mL/min。所有TGA实验均进行两次,以确保实验结果的准确性。
2 热降解动力学求解
固态物质的热降解反应过程动力学,可表示为[19]
其中α为转化率;k为速率常数;f(α)为反应模型,是反应动力学机理函数关于转化率α的代数式,该反应模型通常是描述固态动力学反应的物理模型。
转化率α可表示为
其中W0为试样初始质量;Wt为试样在t时刻的质量;Wf为试样最终质量。本文用转化率预测PMMA的热降解。
可用Arrhenius(阿伦尼乌斯)方程表示速率常数
其中A为指前因子,min-1;E为活化能,kJ/mol;R为摩尔气体常数,由于实验采用氮气气氛,故这里取8.314 J/(mol·K);T为热力学温度,K。
将
TGA实验采用多个升温速率升温,升温速率可表示为
将
Li等[20]的研究结果表明:对于可用一步反应表征的固体热降解过程,可用n阶模型描述整个反应过程。因此,本文使用n阶模型表征PMMA的热降解过程,其热降解动力学机理函数为
其中n为反应级数。
Friedman法是一种微分等转化率法,其动力学方程为
在TGA曲线上截取不同升温速率β下相同转化率α时dα/dt-T的值,绘制
FWO法是一种积分等转化率法。对
式中G(α)为f(α)的积分式。在TGA曲线截取不同升温速率β下相同转化率α时T的值,绘制lgβ和1/T数据点并进行线性拟合,得到的直线其斜率即为-0.4567E/R,进而可计算出活化能E。
KAS法是一种积分等转化率法,其动力学方程为
在TGA曲线上截取不同升温速率β下相同转化率α时T的值,绘制ln(β/T2)和1/T数据点并进行线性拟合,得到的直线其斜率即为-E/R,进而可计算出活化能E。
Freeman-Carroll法是一种微分等转化率法,其动力学方程为
在TGA曲线上截取不同升温速率β下相同转化率α时dα/dt-T的值,绘制
使用得到的活化能,可计算Arrhenius方程中的指前因子A,
其中Tm为DTG峰值温度。
3 实验结果和分析
3.1 热重分析
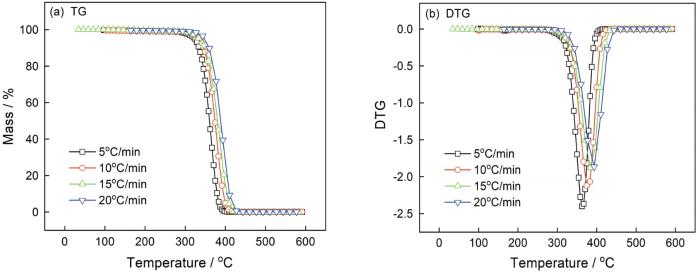
图1给出了不同升温速率下PMMA/0% CB的TG/DTG曲线。热降解的起始温度范围为280~325℃,热降解的最高速率出现在350~390℃,高于405~440℃热降解基本完成。随着升温速率的提高,不同碳黑含量PMMA的起始分解温度、主降解阶段分解温度及终止分解温度均向高温侧移动。其原因是,试样相同的温度,升温速率越大经历的反应时间越短,反应程度越低。同时,升温速率还影响测点与试样、外层试样与内部试样间的传热温差和温度梯度,使热滞后现象加重和曲线向高温侧移动。
图1
图1
在不同升温速率下PMMA/0% CB的热稳定性曲线
Fig.1
Thermal stability of PMMA/0% CB with different heating rates (a) TG curves, (b) DTG curves
图2a~c给出了不同碳黑含量的PMMA在升温速率为5℃/min下的TG曲线。可以看出,不同碳黑含量PMMA的热降解过程都只有一个失重阶段,碳黑含量的变化不影响热失重曲线的形状,都为倒“S”型;随着碳黑含量的提高PMMA的热分解温度Tm 先提高后降低,并在碳黑含量为0.1%时达到峰值。碳黑含量小于0.1%时,提高碳黑含量可提高PMMA的热稳定性[7,21~26]。其原因是,碳黑是一种支链形式的无定形碳,是大量碳粒子通过碳晶体层相互点缀的结果[23]。因此,碳黑易于形成难以打破的三维网状碳层。碳黑纳米填料在PMMA外部形成了一层稳定的网状碳层,有效保护了底层聚合物材料,减轻了热降解程度,从而提高聚合物的热稳定性能[27,28];当碳黑含量高于0.1%时PMMA的热稳定性开始降低,因为碳黑颗粒极易在其一级和二级结构中团聚[29,30]。当碳黑含量过高时碳黑填料在聚合物基质中的分散和分布较差,使施加热载荷时填料和聚合物基体之间的热量耗散差[7,30],从而降低了材料的热稳定性,其对应的分子解释模型如图2d所示。
图2
图2
升温速率为5℃/min掺碳黑PMMA的热稳定性
Fig.2
TG curves of PMMA with different CB contents at a heating rate of 5℃/min (a) from 25 to 600℃, (b) from 350 to 390℃, (c) from 450 to 500℃, and (d) the molecular model
对降解产物质量的测量结果表明,残余物的质量变化与碳黑含量变化基本一致。这表明,掺有碳黑的PMMA并没有完全降解,不同碳黑含量的PMMA残余量随着碳黑含量的提高而增加,进而判断这些残余物是碳黑;纯PMMA的降解率接近100%,没有降解残余物。
3.2 热降解动力学
图3
图3
PMMA/0% CB四种等转化率曲线
Fig.3
Four iso-conversional rate curves of PMMA/0% CB (a) Friedman, (b) FWO, (c) KAS, (d) Freeman-Carroll
表2 Friedman、FWO、KAS方法下不同碳黑含量PMMA的活化能E
Table 2
| CCB/% | Friedman | FWO | KAS | |||
|---|---|---|---|---|---|---|
| E/kJ·mol-1 | R2 | E/kJ·mol-1 | R2 | E/kJ·mol-1 | R2 | |
| 0 | 159.608 | 0.9981 | 166.755 | 0.9834 | 164.620 | 0.9813 |
| 0.05 | 161.461 | 0.9927 | 164.789 | 0.9898 | 162.575 | 0.9885 |
| 0.10 | 177.371 | 0.9979 | 175.778 | 0.9994 | 174.124 | 0.9994 |
| 0.15 | 169.740 | 0.9976 | 167.471 | 0.9870 | 165.371 | 0.9853 |
| 0.20 | 160.212 | 0.9894 | 158.353 | 0.9881 | 155.796 | 0.9864 |
| 0.25 | 157.274 | 0.9945 | 159.349 | 0.9970 | 156.827 | 0.9965 |
表3 Freeman-carroll法下不同碳黑含量下PMMA的反应级数n
Table 3
| β/℃·min-1 | Freeman-Carroll | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCB/% | ||||||||||||
| 0 | 0.05 | 0.1 | 0.15 | 0.2 | 0.25 | |||||||
| n | R2 | n | R2 | n | R2 | n | R2 | n | R2 | n | R2 | |
| 5 | 0.91 | 0.9998 | 0.62 | 0.9988 | 1.40 | 1.0000 | 1.38 | 1.0000 | 1.11 | 1.0000 | 0.38 | 0.9978 |
| 10 | 0.92 | 0.9998 | 1.07 | 1.0000 | 1.44 | 1.0000 | 1.37 | 1.0000 | 0.58 | 0.9990 | 0.95 | 0.9996 |
| 15 | 1.13 | 1.0000 | 1.52 | 0.9998 | 1.28 | 1.0000 | 1.44 | 1.0000 | 1.91 | 0.9986 | 1.20 | 1.0000 |
| 20 | 1.53 | 0.9996 | 0.95 | 0.9998 | 1.52 | 0.9996 | 1.60 | 0.9998 | 1.50 | 0.9996 | 1.45 | 0.9998 |
| Average value | 1.12 | 0.9998 | 1.04 | 0.9996 | 1.41 | 0.9999 | 1.45 | 1.0000 | 1.27 | 0.9993 | 1.00 | 0.9993 |
用Friedman、FWO、KAS三种方法求解出的活化能值十分接近,但是其变化趋势不完全相同。Friedman法求解的PMMA的活化能随着碳黑含量的提高先增大后减小,用FWO和KAS法求解的活化能大小波动,其共同点是都在碳黑含量为0.1%时达到活化能峰值。用Friedman法求解出的不同碳黑含量的PMMA活化能变化趋势与前文中热分解温度的变化规律相同,因此取Friedman法求解值为最终活化能值。活化能,是分子从常态转变为容易发生化学反应的活跃状态所需要的能量,活化能值越高反应越难发生。这表明,碳黑含量的提高改变了PMMA发生化学反应需要的活化能,间接证实了碳黑能在一定程度上提高PMMA基材的热稳定性。
将活化能E值与热分解温度Tm 代入
表4 不同碳黑含量PMMA的指前因子A
Table 4
| β/℃·min-1 | A | |||||
|---|---|---|---|---|---|---|
| CCB/% | ||||||
| 0 | 0.05 | 0.1 | 0.15 | 0.2 | 0.25 | |
| 5 | 2.8856 | 4.0788 | 87.2426 | 1.9197 | 20.8827 | 3.2088 |
| 10 | 2.8823 | 4.2802 | 88.1820 | 1.9237 | 21.2166 | 3.1756 |
| 15 | 2.7539 | 4.2433 | 94.9790 | 1.9435 | 20.1800 | 2.8766 |
| 20 | 2.7710 | 4.1112 | 82.3381 | 1.7478 | 19.1612 | 3.3272 |
| Average value | 2.8232 | 4.1784 | 88.1854 | 1.8837 | 20.3601 | 3.1471 |
3.3 热降解动力学模型的验证
使用MATLAB 软件,将上述求出的不同碳黑含量的PMMA热降解动力学参数带入
图4给出了不同碳黑含量的PMMA在升温速率为5℃/min下转化率的模拟值与实验值的对比,其中矩形黑线是由TG实验结果变形而来的转化率曲线,圆形红线是由热降解动力学模型模拟出的转化率曲线。可以看出,实验值与模拟值二者大体上吻合。因此使用n阶模型和Friedman、Freeman-Carroll、直接求解A值法得到的不同碳黑含量的PMMA的动力学参数,能用于表征其热降解过程。
图4
图4
不同碳黑含量的PMMA热降解模型验证,升温速率为5℃/min
Fig.4
Validation of PMMA thermal degradation model with different CB contents at a heating rate of 5℃/min. (a) PMMA/0% CB, (b) PMMA/0.05% CB, (c) PMMA/0.10% CB, (d) PMMA/0.15% CB, (e) PMMA/0.20% CB, (f) PMMA/0.25% CB
4 结论
(1) 利用Friedman、Freeman-Carroll、直接求解A值法求解的热降解反应动力学模型,能再现不同碳黑含量的PMMA在氮气中的热重实验结果。
(2) 随着碳黑含量的提高PMMA的活化能先增大后减小,并在0.1%CB达到峰值。掺有碳黑PMMA的活化能,比纯PMMA提高了17.76 kJ·mol-1。碳黑能在一定程度上提高PMMA的热稳定性。
参考文献
Kinetic and volatile products study of micron-sized PMMA waste pyrolysis using thermogravimetry and Fourier transform infrared analysis
[J].Much attention has been devoted to disposing traditional-sized poly(methyl methacrylate) (PMMA) waste by pyrolysis for methyl methacrylate (MMA). The pyrolysis of micron-sized PMMA waste, which may be different from that of traditional-sized PMMA waste, received little concern. The present study investigated the kinetics and volatile products of micron-sized PMMA waste pyrolysis in inert atmosphere using thermogravimetry and Fourier transform infrared analysis. A global optimization algorithm namely Shuffled Complex Evolution (SCE) was employed to simultaneously optimize the kinetic parameters. Results indicated that one shoulder and one peak occurred in the MLR variations with temperature. The values of the MLR at the shoulder and peak, the average MLR all increased with the heating rate. The optimized kinetic parameters by SCE can be utilized to well reproduce the experimental thermogravimetric data. The values of activation energy and natural logarithm of pre-exponential factor were in the range of 235.95-248.61 kJ/mol and 16.96-28.76 min, respectively. The value of activation energy of micron-sized PMMA waste pyrolysis under the present study was greater than that of the traditional-sized PMMA pyrolysis in the previous studies. MMA and CO were the major volatile products generated from the micron-sized PMMA waste pyrolysis. The volatile products yield at peak was much larger than that at shoulder. The MMA and CO yield were in the range of 87.98-93.54% and 6.46-12.02%, respectively. High MMA yield may be obtained from the pyrolysis of micron-sized PMMA waste in inert atmosphere by appropriately increasing the heating rate adopted in the reactors in the practical applications.Copyright © 2020 Elsevier Ltd. All rights reserved.
Chemical recycling of poly(methyl methacrylate) by pyrolysis. Potential use of the liquid fraction as a raw material for the reproduction of the polymer
[J].
Experimental and modelling studies on the kinetics and mechanisms of thermal degradation of polymethyl methacrylate in nitrogen and air
[J].
Methyl methacrylate (MMA) and alumina recovery from waste artificial marble powder pyrolysis
[J].
Comparison study of carbon black (CB) used as conductive filler in epoxy and polymethylmethacrylate (PMMA)
[J].
Thermal degradation behavior of multi-walled carbon nanotubes/polyamide 6 composites
[J].
Preparation and properties of polysiloxane grafting multi-walled carbon nanotubes/polycarbonate nanocomposites
[J].
Improved mechanical properties of PMMA composites: dispersion, diffusion and surface adhesion of recycled carbon fiber fillers from CFRP with adsorbed particulate PMMA
[J].
Mechanical reinforcement of polymers using carbon nanotubes
[J].
Thermal degradation kinetics of plastics and model selection
[J].
Thermal decomposition kinetics of dynamically vulcanized polyamide 6-acrylonitrile butadiene rubber-halloysite nanotube nanocomposites
[J].
Pyrolysis kinetics of glass fiber/epoxy foam sandwich panel
[J].The thermal decomposition characteristics of glass fiber/epoxy foam sandwich panels was studied via DTG-60(AH) thermogravimetric analyzer by different heating rates and in three atmospheres with different oxygen contents. The results show that the pyrolysis reaction of glass fiber/epoxy foam sandwich panels in air can be differentiated into three stages. As the heating rate increases, the initial reaction temperature, the termination reaction temperature and the maximum mass loss rate temperature of the pyrolysis reaction shifted to the high temperature. The decrease of oxygen content in atmospheres has a greater impact on the third stage of thermal decomposition. The pyrolysis kinetics were analyzed by the Flynn-Wall-Ozawa method and the Starink method to obtain the apparent activation energy.
玻璃纤维/环氧树脂泡沫夹层板的热降解动力学
[J].使用DTG-60(AH)热重分析仪分析了玻璃纤维/环氧树脂泡沫夹层板在不同升温速率和不同氧含量条件下的热分解特性。结果表明,在空气中玻璃纤维/环氧树脂泡沫夹层板的热分解反应可分为三个阶段。随着升温速率的提高,热分解反应的初始反应温度、终止反应温度以及最大质量损失速率温度均向高温方向移动。氧含量的降低对热分解的第三阶段有较大的影响。采用Flynn-Wall-Ozawa法和Starink法进行热解动力学分析,得到玻璃纤维/环氧树脂泡沫夹层板的表观活化能。
Study on pyrolysis kinetics of typical carbon fiber bidirectional sheet
[J].
典型碳纤维编织布的热降解动力学
[J].
Kinetics of thermal degradation of char-forming plastic from themogravimetry. Application to phenolic plastic
[J]. J.
A quick, direct method for the determination of activation energy from themogravimetric data
[J]. J.
Kinetic parameters from thermogravimetric data
[J].
Polymer degradation during contour laser transmission welding
[A].
Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis
[J].
ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data
[J].
Pyrolysis of polyurethane foam: optimized search for kinetic properties via simultaneous K-K method, genetic algorithm and elemental analysis
[J].
One-step amino-functionalization of milled carbon fibre for enhancement of thermophysical properties of epoxy composites
[J].
Click coupled graphene for fabrication of high-performance polymer nanocomposites
[J]. J.
Carbon black reinforced polymethyl methacrylate (PMMA)-based composite particles: preparation, characterization, and application
[J].
Thermal decomposition of polyolefin/carbon black composites
[J].
Thermal ageing of conducting polymeric composites
[J].
Effect of dispersion of carbon black on electrical and thermal properties of poly(ethylene terephthalate)/carbon Black composites
[J]. J.
Emerging trends in poly(methyl methacrylate) containing carbonaceous reinforcements-carbon nanotube, carbon black, and carbon fiber
[J].
Synergistic effects and mechanism of multiwalled carbon nanotubes with magnesium hydroxide in halogen-free flame retardant EVA/MH/MWNT nanocomposites
[J].
Modifications of carbon for polymer composites and nanocomposites
[J].