目前处理染料废水常用的方法有物理处理法、化学处理法和微生物处理法。物理处理法包括吸附法[4~7]、膜分离法[8,9]和磁分离法[10];化学处理法包括混凝沉淀法[11]、氧化法[12]、还原法、电化学法和焚烧法。吸附法操作方便且分离效果好,其本质是一种界面现象。常用的吸附剂有活性炭[13]、石墨烯[14]、碳纳米管[15,16]、活性碳纤维[17]、沸石[18]、分子筛[19]和活性氧化铝等[20,21]。Cai等[22]制备的核-壳结构碳材料,对锥虫蓝染料的吸附容量达到115.6 mg/g,优于粒状碳。Veerakumar等[23]合成的含酸性官能团的介孔碳,对铬黑T的最大吸附容量为117.0 mg/g,远高于其他吸附剂。Kong等[24]将带正电荷的CTS和带负电荷的CMC溶液在静电相互作用下混合搅拌,制备的中空胶囊对亚甲基蓝、甲基橙和酸性蓝有良好的去除性能。
吲哚又称苯并吡咯,存在于茉莉、水仙和苦橙等植物的花油中,是一种资源丰富且易得的含氮杂化化合物。酸性橙74是含多个芳环的直链结构染料,随印染工业排放至水体中,威胁水生动植物和人类的健康。用传统的水处理方法难以处理这种抗光、抗化学氧化的污染物。用吲哚制备的炭材料吸附酸性离子,有较好的应用前景。本文使用吲哚为碳源和氮源、以CaO为模板、以KOH为扩孔剂制备掺氮多孔炭,研究其对酸性橙74的吸附性能并阐明其吸附机理。
1 实验方法
1.1 实验用试剂和仪器
吲哚、氧化钙、氢氧化钾和酸性橙74,均为分析纯;去离子水。
250型X射线光电子能谱(XPS),扫描范围为0~1350 eV;Nanosem430型扫描电子显微镜(SEM);3H-2000PS1型自动氮吸附仪(BET);JEM-2100型透射电镜(TEM);JH752型紫外分光光度计;GSL-1600X型高温真空管式炉。
1.2 HPCs的制备
将质量比为2∶3∶5的吲哚、纳米氧化钙和氢氧化钾放入在乳钵中研磨,混合均匀后置于刚玉舟并装入管式炉中。通入流速为40 mL/min的氩气,气体后加热。以5℃/min的速率将管式炉加热至150℃,恒温30 min后以相同的升温速率加热至终温(800~900℃),恒温60 min后在保持气流畅通的情况下自然降温至室温。将得到的样品研磨成粉末后加入2 mol/L盐酸酸洗,搅拌10 min后超声30 min,重复多次以确保完全洗去氧化钙模板。再加入热蒸馏水洗涤至滤液成中性,然后置于温度为110℃的鼓风干燥箱干燥24 h。将干燥好的样品研磨成细粉后过325目筛,得到产物HPCT。其中T代表活化终温。
1.3 吸附剂性能的测定
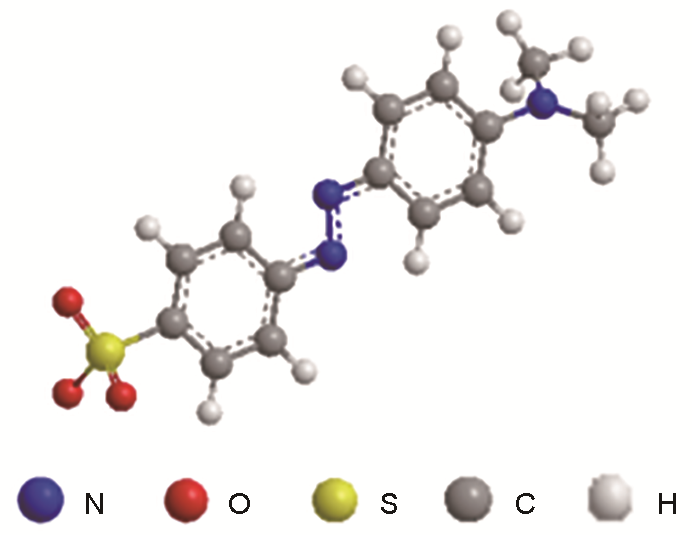
阴离子染料酸性橙74溶于水后显橙色,还溶于乙醇,微溶于溶纤素。其球棍模型结构如图1所示。
图1
使用紫外分光光度计在476 nm波长下测定酸性橙74的标准曲线,由拟合结果得到染料的标准曲线方程为y=-0.83+110.08x,相关性系数R2为0.998,说明相关性非常好。
吸附等温线:在室温条件下,在不同浓度等量酸性橙74溶液中添加10 mg HPCT,震荡搅拌6 h后测量吸附后上清液的浓度,探究炭材料吸附等温线。HPCT对酸性染料的吸附量qt(mg·g-1)为
式中qt为吸附时间t时单位质量吸附剂的吸附量(mg·g-1);C0为初始状态下吸附质的浓度(mg·L-1);Ct为吸附时间t时吸附质的浓度(mg·L-1);V为吸附质的体积(mL);W为吸附质的质量(mg)。
为了更好的了解酸性染料在HPCT上的吸附过程达到平衡与两相浓度间的关系,用Langmuir吸附等温方程
和Freundlich吸附等温方程
进行拟合。式中Ce为吸附质在溶液中的浓度(mg·L-1);qe为单位质量吸附剂的吸附量(mg·g-1);Q为吸附达到平衡时,单层的最大吸附量(mg·g-1);KL为表面吸附亲和性常数(L·mg-1);KF为表面吸附亲和。
吸附动力学:在室温条件下将10 mg的HPCT装入盛有200 mg/L的酸性橙74溶液中,每隔一定的时间测定酸性橙74的即时浓度。为了研究吸附、脱附速度及各种影响因素,使用准一级动力学
和准二级动力学模型
拟合吸附数据。式中qe为平衡时吸附量(mg·g-1);qt为t时刻的吸附量(mg·g-1);K1为拟一级吸附速率常数(min-1);K2为拟二级吸附速率常数(g·mg-1·min-1);t为吸附时间(min)。
2 结果和讨论
2.1 FESEM和TEM分析
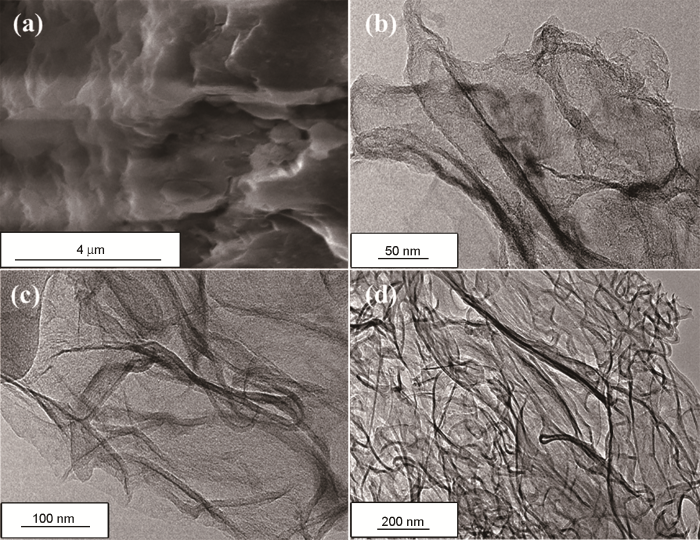
图2
图2
HPC900的扫描电镜照片、HPC800、HPC850和HPC900的透射电镜照片
Fig.2
FESEM images of HPC900 (a) and TEM images of HPC800 (b), HPC850 (c) and HPC900 (d)
2.2 孔结构
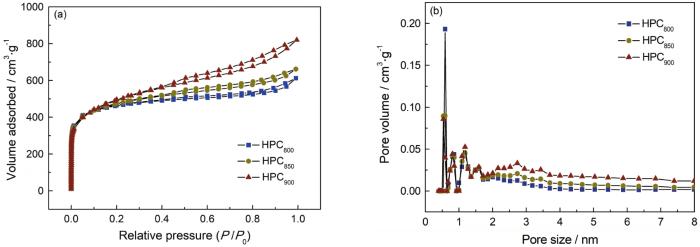
图3
图3
HPCT的氮气吸脱附曲线和孔径分布图
Fig.3
Nitrogen adsorption-desorption isotherms (a) and pore size distribution (b) of HPC T
表1 HPCT样品的比表面积及孔结构参数
Table 1
| Sample | Dap/nm | SBET/m2·g-1 | Smic/m2·g-1 | Vt/cm3·g-1 | Vmic/cm3·g-1 | Non-Vmic/Vt |
|---|---|---|---|---|---|---|
| HPC800 | 2.60 | 1458 | 914 | 0.95 | 0.47 | 0.51 |
| HPC850 | 2.70 | 1520 | 773 | 1.02 | 0.40 | 0.61 |
| HPC900 | 3.12 | 1629 | 495 | 1.27 | 0.26 | 0.79 |
2.3 XPS分析
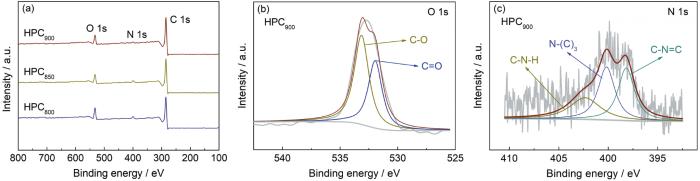
图4
图4
HPCT的XPS全谱图、HPC900的O 1s图和HPC900的N 1s图
Fig.4
XPS spectra of HPCT (a), O 1s spectra of HPC900 (b) and N 1s spectra of HPC900 (c)
表2 制备分级多孔炭中元素及官能团含量
Table 2
| Samples | C 1s | N1s | C-N=C | N-(C)3 | C-NH2 | O 1s | C=O | C-OH |
|---|---|---|---|---|---|---|---|---|
| HPC800 | 82.59 | 5.89 | 2.39 | 2.82 | 0.68 | 11.52 | 2.39 | 2.82 |
| HPC850 | 85.43 | 5.06 | 2.27 | 2.05 | 0.74 | 9.51 | 2.27 | 2.05 |
| HPC900 | 87.09 | 4.66 | 1.67 | 1.71 | 1.28 | 8.25 | 1.67 | 1.71 |
2.4 吸附性能
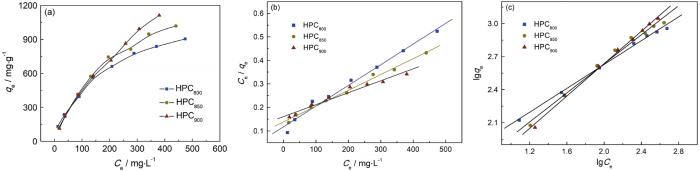
2.4.1 吸附等温线
图5
图5
HPCT吸附酸性橙74的吸附等温线、Langmuir拟合和Freundich拟合
Fig.5
Adsorption isotherms of acid orange 74 onto HPCT (a), Langmuir model fitting (b) and Freundich model fitting (c)
表3 HPCT的等温线拟合参数
Table 3
| Samples | Langmuir | Freundich | ||||
|---|---|---|---|---|---|---|
| qmax/mg·g-1 | KL | R2 | n | KF | R2 | |
| HPC800 | 1136 | 0.007 | 0.99 | 1.85 | 35.49 | 0.99 |
| HPC850 | 1485 | 0.005 | 0.99 | 1.51 | 21.19 | 0.99 |
| HPC900 | 1966 | 0.003 | 0.98 | 1.36 | 15.84 | 0.99 |
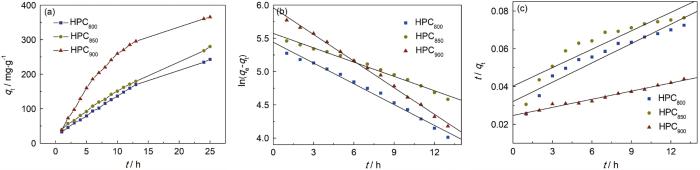
2.4.2 吸附动力学
图6a给出了在室温下酸性橙74吸附量随时间的变化。可以看出,HPCT加入染料溶液中立即表现出良好的吸附性能。其中HPC900的吸附量增加的最快,因为HPC900中的非微孔数量最多,酸性橙74通过2~3 nm的中孔通道快速扩散,到达微孔吸附位点。HPC900表面含氮官能团C-NH2的含量最高,这也是吸附更快、吸附能力更强的重要原因。随着吸附的进行吸附位点被逐渐地占据而使吸附缓慢,吸附24 h后吸附幅度变小,基本上达到平衡状态。图6b、c给出了动力学模型的拟合曲线,表4列出了动力学参数。从表4可见,拟一级动力学模型的qe值和实验值更接近,更加有说服力,表明吸附是化学吸附与物理吸附共同作用,但是以物理吸附作为控速步骤。
图6
图6
HPCT吸附酸性橙74的吸附量随时间变化曲线与吸附动力学拟合曲线
Fig.6
Adsorption capacity of acid orange 74 versus time relationship (a), the fitting curves of pseudo-first-order (b) and pseudo-second-order (c)
表4 HPCT的动力学拟合参数
Table 4
| Samples | Pseudo-first order | Pseudo-second order | ||||
|---|---|---|---|---|---|---|
| qe/mg·g-1 | K1 | R2 | qe | K2 | R2 | |
| HPC800 | 231 | 0.11 | 0.99 | 293 | 3.63×10-4 | 0.95 |
| HPC850 | 263 | 0.07 | 0.99 | 308 | 2.62×10-4 | 0.92 |
| HPC900 | 387 | 0.13 | 0.99 | 699 | 8.29×10-5 | 0.99 |
2.4.3 与其他吸附剂性能的比较
表5 不同吸附剂对酸性橙74染料的吸附
Table 5
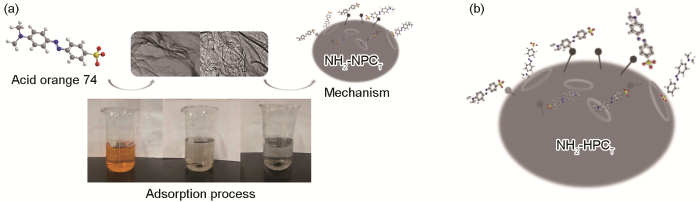
2.5 吸附机理
图7给出了HPCT对酸性橙74的吸附机理示意图。根据上文的表征分析和吸附实验结果,HPCT极高的比表面积能提供大量对酸性橙74的吸附位点。HPCT内部分布着与酸性橙74尺寸相当的孔径,使染料充分结合吸附位点从而使染料分子进入HPCT的孔隙内完成物理吸附。酸性橙74偶氮结构与磺酸基相连使苯环上的电子云密度升高,整体成为强吸电子结构,染料分子内部的电子共轭和电子云密度的变化使分子呈现吸电子状态。本文制备的HPCT分布着许多-NH2官能团,氮元素上的孤对电子能够与给出电子对,与染料分子形成共价键。于是,经过化学吸附形成的供-受电子体系更加稳定,进一步提高了HPCT对酸性橙74的吸附能力。这表明,吸附过程是比表面积和含氮官能团C-NH2共同作用的结果,与动力学分析结果相符。
图7
图7
HPCT对酸性橙74的吸附机理
Fig.7
Mechanism of the adsorption process of HPCT (a), specific adsorption mode (b)
3 结论
以吲哚为碳源和氮源、氧化钙为模板耦合KOH活化并调节活化终温,可制备表面掺氮的层状分级多孔炭(HPCT)。随着活化温度的提高其比表面积增大,活化终温为900℃制得的HPC900比表面积高达1629 m2/g。这种炭材料具有相互连接的层状结构,且随着活化温度的提高炭壁层变薄。这种炭材料表面含氮官能团C-NH2丰富,随着活化温度的提高C-NH2的含量随之提高。C-NH2官能团与酸性橙74发生π-π堆积效应或静电相互作用,有利于对酸性橙74的吸附。Freundlich模型能很好地描述HPCT对染料的吸附过程,在平衡浓度为50 mg/L条件下HPC900对废水中酸性橙74的吸附量超过270 mg/g;拟一级动力学方程能更好的描述HPCT对酸性橙74的吸附过程,物理吸附为控速步骤。
参考文献
Efficient water pollution abatement
[J].
Advanced porous materials for sensing, capture and detoxification of organic pollutants toward water remediation
[J].
Degradation of anthraquinone dyes from effluents: a review focusing on enzymatic dye degradation with industrial potential
[J].
Nickel nanoparticles encapsulated in porous carbon and carbon nanotube hybrids from bimetallic metal-organic-frameworks for highly efficient adsorption of dyes
[J]. J.
Review on recent advances of carbon based adsorbent for methylene blue removal from waste water
[J].
Adsorption of a cationic methylene blue dye on an Algerian palygorskite
[J].
Enhanced adsorption of Congo red dye by functionalized carbon nanotube/mixed metal oxides nanocomposites derived from layered double hydroxide precursor
[J].
Biophenol extracts from olive oil mill wastewaters by membrane separation and adsorption resin
[J].
Membrane adsorption with polyacrylonitrile prepared with superfine powder-activated carbon, case study: separation process applied in water treatment containing diclofenac
[J].
Preparation and property of Fe3O4/P(St-co-MMA) micro-nano composites
[J].
磁性Fe3O4/P(St-co-MMA)微纳米复合物的制备和性能
[J].
Study on oil separation from oil/water emulsion via coagulation and ultrafiltration
[J]. J.
利用混凝-超滤膜法研究乳化油水的分离过程
[J].
Enhanced Ozonation of trace organic contaminants in municipal wastewater plant effluent by adding a preceding filtration step: comparison and prediction of removal efficiency
[J].
Preparation of efficient carbon-based adsorption material using asphaltenes from asphalt rocks
[J].
Graphene-like porous carbon nanostructure from Bengal gram bean husk and its application for fast and efficient adsorption of organic dyes
[J].
Carbon nanotube-based adsorbents for the removal of dyes from waters: A review
[J].
Performance of lithium-ion capacitors using pre-lithiated multi-walled carbon nanotube composite anode
[J].
预嵌锂多壁碳纳米管的性能
[J].
Adsorption properties of metal-organic framework material MIL-53(Al)-F127 for Bisphenol A
金属有机框架材料MIL-53(Al)-F127对双酚A的吸附性能
[J].
Adsorption and kinetic studies of methylene blue on modified HUSY zeolite and an amorphous mixture of γ-alumina and silica
[J].
Adsorption characteristics of anionic azo dye onto large α-alumina beads
[J].
Adsorption of textile dyes using an activated carbon and crosslinked polyvinyl phosphonic acid composite
[J].
Conversion of water-insoluble aluminum sources into metal-organic framework MIL-53(Al) and its adsorptive removal of roxarsone
[J].
水不溶性铝源合成金属有机骨架MIL-53(AL)及其对洛克沙胂的吸附
[J].
Core-shell granular activated carbon and its adsorption of trypan blue
[J].
Preparation of millimeter-sized chitosan/carboxymethyl cellulose hollow capsule and its dye adsorption properties
[J].
Oxygenated monolayer carbon nitride for excellent photocatalytic hydrogen evolution and external quantum efficiency
[J].
KOH etching graphitic carbon nitride for simulated sunlight photocatalytic nitrogen fixation with cyano groups as defects
[J]. J.
Production of hierarchically porous carbon from natural biomass waste for efficient organic contaminants adsorption
[J].
Synthesis of new low-cost organic ultrafiltration membrane made from Polysulfone/Polyetherimide blends and its application for soluble azoic dyes removal
[J].
Post-KOH activation of nitrogen-containing porous carbon with ordering mesostructure synthesized through a self-assembly
[J].
Decolourization of acid orange 74 aqueous solutions in presence of multi-walled carbon nanotubes under ultrasound irradiation
[J].
Removal of acid orange 74 from wastewater with TiO2 nanoparticle
[J].
Modeling the mechanism, equilibrium and kinetics for the adsorption of acid orange 8 onto surfactant-modified clinoptilolite: the application of nonlinear regression analysis
[J].
Removal of acid orange 7 and remazol black 5 reactive dyes from aqueous solutions using a novel biosorbent
[J].
Brilliant green and acid orange 74 dyes removal from water by Pinus roxburghii leaves in naturally benign way: an application of green chemistry
[J].