1 实验方法
1.1 CNSs-H-D阻燃剂的制备
先用Fenton试剂法[15]制备羟基化碳纳米球(CNSs-OH)。将1 g的CNSs-OH和0.4 g六氯环三磷腈(HCCP,纯度98%)加到100 mL的无水乙醇中,超声震荡30 min后在30℃搅拌反应4 h;将反应产物用无水乙醇抽滤清洗,然后将滤饼在120℃烘干4 h,研磨均匀后得CNSs-HCCP。将1 g 的CNSs-HCCP和0.3 g二氨基二苯砜(DDS,纯度≥99%,广州宏程生物科技有限公司)加入100 mL的无水乙醇中超声震荡30 min,然后在40℃搅拌反应2 h;将反应产物先后用无水乙醇和去离子水抽滤清洗,再将滤饼在120℃烘干4 h,研磨均匀后得到CNSs-H-D。
1.2 CNSs-H-D/EP复合材料的制备
将适量的CNSs-H-D和环氧树脂WSR618加入锥形瓶,搅拌30 min真空脱泡后再加入适量的CYDHD-593固化剂,搅拌5 min后再次真空脱泡。将脱泡后的混合物倒入模具内,室温下固化24 h得到CNSs-H-D/EP复合材料。阻燃环氧复合材料的配方,列于表1。
表1 阻燃环氧复合材料的配方
Table 1
| Samples | E-51/g | CYDHD-593/g | CNSs-H-D/g |
|---|---|---|---|
| EP-0 | 100 | 28 | 0 |
| EP-1 | 100 | 26.72 | 1.28 |
| EP-2 | 100 | 24.16 | 3.84 |
| EP-3 | 100 | 21.6 | 6.4 |
1.3 复合阻燃剂性能的表征
CNSs-H-D的表征:用JSM-6510LA型场发射扫描电镜观察微观形貌,用能谱仪(EDS)测定表面元素,加速电压为5 kV;用Spectrum two傅里叶变换红外光谱仪(FTIR)、溴化钾压片法测定其化学结构,扫描范围4000~400 cm-1;用Perkin-Elmer TGA 4000热重分析仪(TG)研究热分解行为,N2流速60 mL/min,升温速率10 ℃/min,温度范围为30~800℃。
CNSs-H-D/EP的表征:按照GB/T 2406.2-2009 标准用JF-3型数显氧指数仪测试样品的极限氧指数(LOI),样品尺寸为120 mm × 6.5 mm × 3 mm;按照ANSI/UL94-2013标准用CZF-5水平垂直燃烧仪测试样品的垂直燃烧性能,样品的尺寸为130 mm×13 mm×3 mm;用C-1087型锥形量热仪测试燃烧参数,样品的尺寸为100 mm×100 mm×6 mm,辐射照度为50 kW/m2,用Perkin-Elmer TGA 4000测试其热稳定性和成炭性。
残炭的表征:用扫描电镜观察锥形量热仪燃烧后残炭的形貌,用TGA测试其热稳定性,用FTIR测定其化学结构。
2 实验结果
2.1 CNSs-H-D的形貌和热稳定性
图1给出了原CNSs和CNSs-H-D的SEM照片, 可见CNSs和CNSs-H-D均呈规则的球形颗粒状。不同的是,原始CNSs表面光滑,而CNSs-H-D表面粗糙,平均粒径约为80 nm。由EDS谱图可见:纯CNSs中只含有C元素,而CNSs-H-D中还有N、P、S等元素,来自CNSs-H-D中的桥梁HCCP和表面接枝的DDS。
图1
图1
CNSs和CNSs-H-D的SEM照片和EDS能谱
Fig.1
SEM and EDS images of CNSs (a) and CNSs-H-D (b)
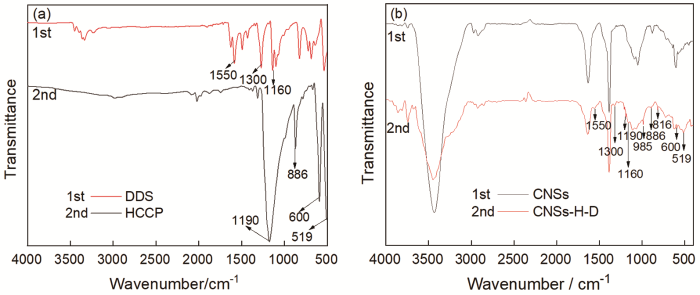
用FTIR进一步表征了CNSs-H-D的结构(图2)。由图2可见,与CNSs的红外光谱相比,在CNSs-H-D的红外光谱816 cm-1处和985 cm-1处新增了特征峰,是CNSs-H-D中CNSs-OH桥联HCCP形成的P-O键的伸缩振动峰;600 cm-1和519 cm-1处的特征峰为HCCP中部分残余P-Cl键的特征峰;886 cm-1处的为HCCP中P-N的特征峰;1190 cm-1处的为HCCP中P=N键的特征峰。同时,与CNSs的红外光谱相比,CNSs-H-D在1160 cm-1处和1300 cm-1处新增的特征峰是DDS中S=O的伸缩振动峰;1550 cm-1处新增的特征峰是DDS中N-H的弯曲振动峰。这些结果表明,在CNSs-H-D阻燃剂中,DDS通过HCCP的桥梁作用接枝在CNSs的表面。
图2
图2
DDS、HCCP以及CNSs、CNSs-H-D的红外光谱
Fig.2
Infrared spectra of DDS, HCCP (a) and CNSs, CNSs-H-D (b)
图3
2.2 阻燃环氧复合材料的阻燃性能
表2 LOI和UL-94的垂直燃烧测试结果
Table 2
| Samples | LOI /% | UL-94 vertical burning test | |||
|---|---|---|---|---|---|
| t1/t2/s | t3/s | Ignitecotton | Rate | ||
| EP-0 | 20.0 | >30 | >60 | Yes | NR |
| EP-1 | 23.0 | 6.8/5.1 | 6.0 | Yes | V-2 |
| EP-2 | 25.8 | 5.5/3.3 | 4.0 | Yes | V-2 |
| EP-3 | 27.5 | 3.9/1.9 | 2.2 | Yes | V-2 |
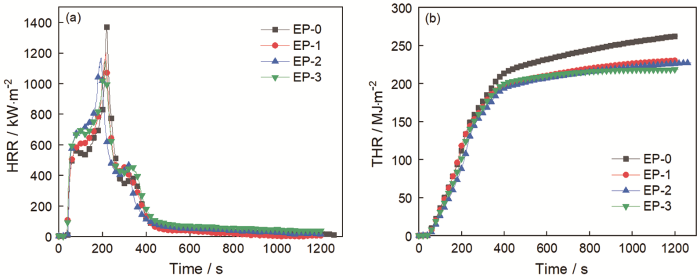
图4和表3给出了锥形量热仪的测试结果。热释放速率(HRR)是表征材料火灾危险性的主要依据。从图4a和表3可见,纯环氧树脂EP-0点燃后热释放速率急剧增大,其峰值热释放速率(pk-HRR)为1372.29 kW/m2。与之相比,阻燃环氧复合材料EP-1、EP-2、EP-3的热释放速率依次降低,其中EP-3的pk-HRR降低到1146.12 kW/m2,比EP-0降低了16.8%,表明CNSs-H-D能有效降低EP的火灾危险性。由图4b中样品的总热释放(THR)曲线和表3可见,EP-0的THR最多为262.45 MJ/m2,而EP-1、EP-2、EP-3的THR与之相比都有所降低。在材料的燃烧过程中释放的热量越多燃烧越充分,表明阻燃性越差[16];这个结果与HRR结果一致,表明抑制热释放是CNSs-H-D的一个重要阻燃特性。
图4
表3 锥形量热仪测试数据
Table 3
| Samples | EP-0 | EP-1 | EP-2 | EP-3 |
|---|---|---|---|---|
| TTI / s | 27 | 29 | 38 | 39 |
| pk-HRR / kW·m-2 | 1372.29 | 1203.01 | 1168.31 | 1146.12 |
| THR / MJ·m-2 | 262.45 | 230.50 | 227.45 | 218.32 |
| pk-HRR/TTI /kW·(m2·s)-1 | 50.83 | 41.48 | 30.75 | 29.39 |
2.3 阻燃机理
2.3.1 阻燃环氧复合材料的热重分析
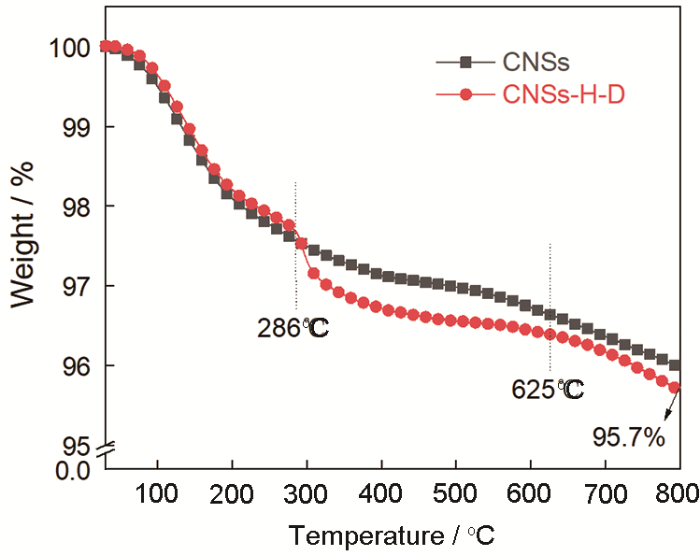
为了研究CNSs-H-D对环氧树脂热分解行为的影响以揭示其阻燃机理,对纯EP(EP-0)和CNSs-H-D/EP(EP-3)进行了TG和DTG分析,结果如图5所示。由图5可见,EP和CNSs-H-D/EP的热分解行为类似,都只有一个质量损失阶段。EP的初始分解温度(Tonset,定义为质量损失5%对应的温度)为187℃,而CNSs-H-D/EP的初始分解温度提高到了227℃,比纯EP提高了40℃,意味着CNSs-H-D的引入显著提高了环氧树脂的热稳定性。同时,纯EP在800℃的残炭量(Char800℃)为4.7%,而CNSs-H-D/EP的Char800℃为11.5%,比纯EP提高了144.7%,表明CNSs-H-D对EP有促进成炭作用。聚合物在高温下生成的残炭越多,则燃烧时发生热分解的部分越少[18]。这是阻燃环氧复合材料热释放速率降低的主要原因之一。结合DTG结果可知,CNSs-H-D/Ep与纯EP的最大热失重温度(Tmax)接近,约为350℃,表明CNSs-H-D的引入促进了EP在热分解中后期的成炭反应。
图5
图5
EP和CNSs-H-D/EP的TG和DTG曲线
Fig.5
TG (a) and DTG (b) curves of EP and CNSs-H-D/EP
2.3.2 对残炭的分析
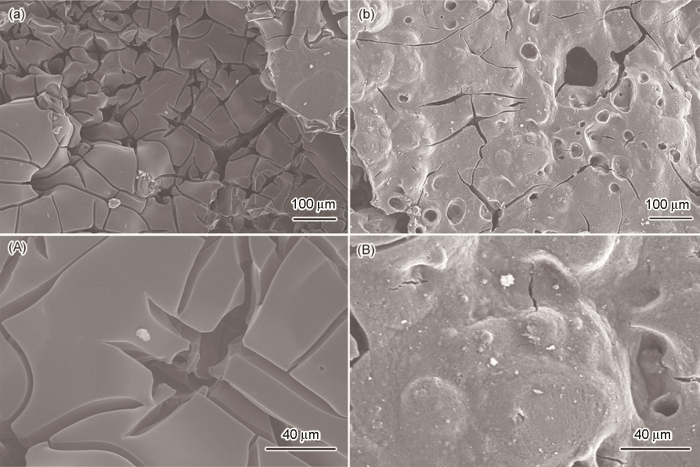
图6给出了残炭的SEM照片。由图6可见,纯EP的炭层破裂程度较高,表面光滑,未见碳质颗粒沉积。相比之下,CNSs-H-D/EP的炭层更加致密连续,表面的裂纹和孔洞减少,还有大量鼓起的未破裂气泡。这种形貌的炭层燃烧时能抑制可燃气体溢出并隔热和隔氧[19, 20],另一方面也有利于将CNSs-H-D受热分解生成的二氧化碳、氨气、氮气、水蒸汽等难燃性气体保留在炭层中,降低燃烧区域内可燃气体的浓度。同时,图6B表明,CNSs-H-D/EP的炭层表面粗糙,明显可见大量绵密的炭质颗粒沉积。这表明,在EP燃烧过程中CNSs-H-D中的CNSs充当了炭质颗粒的沉积骨架,承载并联结了热解产物,从而生成了致密连续的炭层[20]。这个作用,与碳纳米管等碳纳米材料对聚合物的阻燃作用类似。
图6
图6
EP和CNSs-H-D/EP残炭的SEM 照片
Fig.6
SEM images of the char residues of EP (a , A) and CNSs-H-D/EP (b , B)
图7
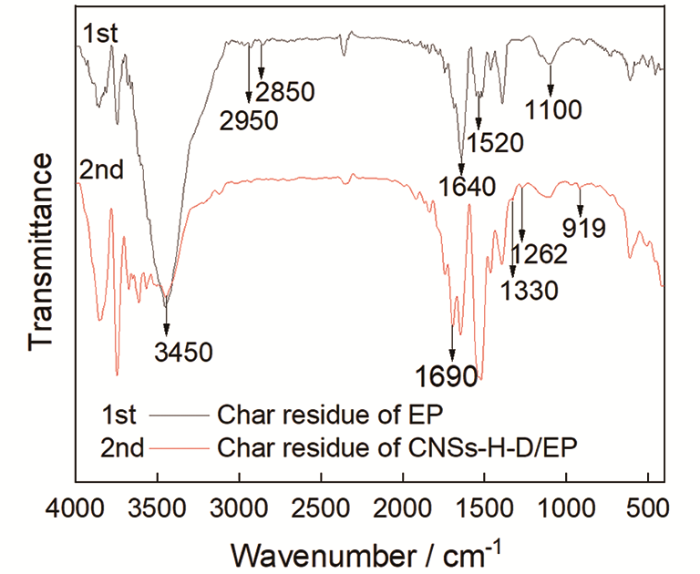
图8给出了残炭的红外光谱。可以看出,EP残炭的成分主要是含有-OH(3450 cm-1)、-CH3(2950~2850 cm-1)、-CH2(1100 cm-1)和苯环(1640 cm-1和1520 cm-1)的化合物。与之相比,CNSs-H-D/EP残炭在1690 cm-1处新增了杂环芳香族化合物中C=N伸缩振动峰;1330 cm-1处新增了DDS中S=O伸缩振动峰;这些新特征峰的出现归因于CNSs-H-D中DDS的分解产物,表明CNSs-H-D的存在使EP在热分解过程中生成了包含以上基团的高热稳定性产物,有利于提高体系的残炭量。同时,CNSs-H-D/EP残炭的红外光谱在1262 cm-1处出现了P=O特征峰,表明其炭层中可能存在磷酸、偏磷酸或聚磷酸,这有利于EP脱水成炭,而这些磷元素来自于CNSs-H-D中的HCCP。以上分析也表明,CNSs-H-D阻燃剂的引入在EP中形成了氮-磷-硫协同阻燃作用。
图8
3 结论
(1) 以CNSs为核、HCCP和DDS为桥梁和接枝剂可制备一种碳纳米球基氮-磷-硫复合阻燃剂CNSs-H-D。CNSs-H-D球形颗粒的粒径约80 nm,热稳定性优异。
(2) CNSs-H-D的引入可显著提高EP的阻燃性、热稳定性和成炭性。CNSs-H-D添加量为5%的CNSs-H-D/EP其LOI从EP的20.0%提高至27.5%,阻燃等级达到V-2级,热释放速率峰值和火灾危险性指数比纯EP分别降低了16.8%和42.2%。CNSs-H-D/EP的初始分解温度比纯EP提高了40℃,高温残炭量比纯EP提高了144.7%。
(3) CNSs-H-D/EP具有典型的凝聚相阻燃机理。与纯EP的炭层相比,CNSs-H-D/EP燃烧生成的炭层的致密性和连续性好,初始失重温度提高了190℃,且在800℃时仍能保持94.5%的剩余质量。其原因是,CNSs-H-D/EP燃烧时CNSs充当了炭质颗粒的沉积骨架,CNSs-H-D阻燃剂在EP中产生了氮-磷-硫协同阻燃效应。
参考文献
Preparation and properties of epoxy resin composites incorporated with optical fiber preform waste
[J].
光棒废料改性环氧树脂复合材料的制备和性能
[J].
Preparation and flame retardancy of intumescent flame-retardant epoxy resin
[J].
新型磷氮膨胀性阻燃剂/OMMT协同阻燃环氧树脂的制备及阻燃性能
[J].
Polypyrrole-interface-functionalized nano-magnetite epoxy nanocomposites as electromagnetic wave absorbers with enhanced flame retardancy
[J].
Vanillin-derived high-performance flame retardant epoxy resins: facile synthesis and properties
[J].
The non-halogen flame retardant epoxy resin based on a novel compound with phosphaphenanthrene and cyclotriphosphazene double functional groups
[J].
Facile synthesis of a novel transparent hyperbranched phosphorous/nitrogen-containing flame retardant and its application in reducing the fire hazard of epoxy resin
[J].
High-efficiency phosphorus/nitrogen-containing flame retardant on epoxy resin
[J].
Sulfur's role in the flame retardancy of thio-ether–linked hyperbranched polyphosphoesters in epoxy resins
[J].
Carbon-family materials for flame retardant polymeric materials
[J].
Synergistic flame retardancy effect of graphene nanosheets and traditional retardants on epoxy resin
[J].
Improving thermal and flame retardant properties of epoxy resin with organic NiFe-layered double hydroxide-carbon nanotubes hybrids
[J].
In situ preparation of reduced graphene oxide/DOPO-based phosphonamidate hybrids towards high-performance epoxy nanocomposites
[J].
A lithium ion-imprinted adsorbent using magnetic carbon nanospheres as a support for the selective recovery of lithium ions
[J].
Core–shell reduced graphene oxide/MnOx@carbon hollow nanospheres for high performance supercapacitor electrodes
[J].
Effect of chemical oxidation on the structure of single-walled carbon nanotubes
[J].
Correlation analysis of cone calorimetry and microscale combustion calorimetry experiments
[J].
Use of cone calorimetry to quantify the burning hazard of apparel fabrics
[J].
Effect of chlorinated phosphate ester based on castor oil on thermal degradation of poly (vinyl chloride) blends and its flame retardant mechanism as secondary plasticizer
[J].
Char barrier effect of graphene nanoplatelets on the flame retardancy and thermal stability of high-density polyethylene flame-retarded by brominated polystyrene
[J].
Flame-retardant effect of a novel phosphaphenanthrene/triazine-trione bi-group compound on an epoxy thermoset and its pyrolysis behaviour
[J].