从上世纪九十年代至今相继开发了各种块体金属玻璃(BMG),其中CuZr基BMG因其较强的非晶形成能力和优异的力学性能而备受关注。例如,Cu64Zr36二元BMG的玻璃转变温度为640 K,过冷液相区宽度(△Tx)达到46 K,压缩断裂强度达到2.0 GPa[1];Cu50Zr50二元BMG的断裂强度为1.79 GPa,塑性变形达到了7.9%[2]。微合金化能改善BMG的性能,掺杂不同原子尺寸的元素可提高体系原子排列的混乱度,从而提高合金的非晶形成能力。例如,在CuZr二元BMG中添加Al元素可制备具有更高热稳定性、强度和塑性的CuZrA1三元金属玻璃[3],且成本较低。其中Cu47.5Zr47.5Al5的塑性应变达到18.0%,断裂强度达到2.265 GPa[4,5]。随着Al含量(原子分数,下同)的提高Cu54.5-xZr45.5Alx(x=3、5、7)金属玻璃体系的热稳定性先增大后减小,当Al含量为5 %时体系的非晶形成能力最好,其压缩和断裂强度都随着Al含量的提高而提高,Al含量为7%的试样断裂强度达到最大[6]。Cu-Zr-Al-Ni体系也具有较高的玻璃形成能力和热稳定性[7]。同时,Cu-Zr-Al金属玻璃还可用作涂层材料,其较高的硬度、弹性模量、耐磨性以及耐蚀性可改善镁合金的综合性能[8]。
以CuZrA1体系为基体添加重金属元素或稀土元素,可进一步改善其热力学性能和非晶形成能力。例如,在CuZrA1合金中添加Ag制备的直径为15 mm的Cu40Zr44Al8Ag8和直径为25 mm的Cu36Zr48Al8Ag8 BMG[9,10],以及在CuZrA1合金中添加Pd制备的直径为30 mm的Cu34Zr48Al8Ag8Pd2块体BMG[11,12],都具有良好的热稳定性和力学性能。上述研究结果表明,添加适量的Ag和Pd元素能显著提高CuZrA1合金体系的非晶形成能力。添加微量Fe元素能显著增大Cu44Zr48Al7体系的过冷液相区宽度,并提高其塑性变形能力[13,14]。在Cu45Zr48Al7中添加稀土元素替代相同原子含量的Zr,可制备出一系列的Cu46Zr47-xAl7Mx(M为稀土元素)BMG[15]。Ce和Pr的添加量(原子分数)为4%时合金的非晶形成能力最佳,之后随着Ce和Pr含量的提高其非晶形成能力反而逐渐降低;但是,添加Tb对该合金的非晶形成能力影响不大[16,17]。
1 实验方法
将纯度为99.99%的金属Cu、Zr、Al和Dy按照设计的合金名义成分Cu50-xZr46Al4Dyx(x=0~4)(原子分数,下同)配料,在高纯氩气保护下用HM630型电弧炉反复熔炼出均匀的合金锭子,再采用铜模吸铸工艺制备成直径为1.5~5.0 mm、长度为60 mm的合金棒。
使用XRD-6100型X射线衍射仪表征合金试样的结构。使用PE-DSC8000型热分析仪对试样进行差示扫描量热(DSC)测试,保护气为氮气,升温速率为20 K/min。使用STA8000型综合热分析仪进行差热分析(DTA)测试,升温速率为20 K/min。使用RGM-300型电子万能试验机完成试样的室温准静态力学测试,应变速率为1×10-4 s-1。用JSM-7800F型扫描电子显微镜观测试样的断裂形貌,使用MHVD-1000/S型多功能显微硬度计测试试样的硬度。
2 结果和讨论
2.1 Dy对Cu50-xZr46Al4Dyx合金非晶形成能力的影响
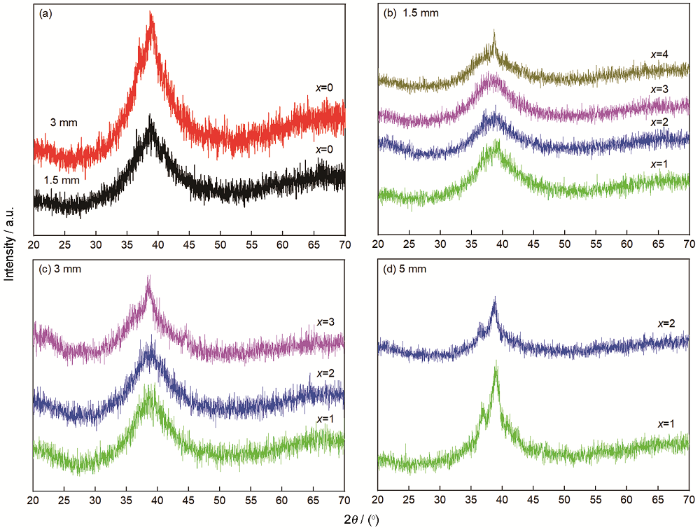
在Cu50Zr46Al4中添加不同含量的Dy元素替代相同摩尔比例的Cu元素,制备出不同直径的Cu50-xZr46Al4Dyx(x=0,1,2,3,4)棒状试样。图1给出了这种试样的XRD图谱。从图1a可以看出,在直径为1.5 mm的Cu50Zr46Al4试样的XRD图谱中只有漫散射峰,没有出现尖锐的晶化峰,表明试样具有较好的非晶结构。直径增大到3.0 mm的试样,其衍射图谱中探测到微弱的晶化峰,说明样品含有部分晶化相。图1b、c和d分别给出了不同直径的Cu50-xZr46Al4Dyx(x=0~4)棒状试样的XRD图谱。可以看出,在直径为1.5 mm、Dy添加量为1%~3%试样的XRD图谱中只出现与非晶相对应的漫散射峰(图1b),证明试样为完全非晶结构。在Dy含量为4%试样的XRD图谱中出现了明显的晶化峰。图1c给出了直径为3.0 mm试样的XRD图谱,可见Dy含量为1%~2%的试样为完全非晶结构。Dy含量为3%的试样,其XRD图谱中开始出现少许晶化峰。直径为5.0 mm的所有试样,其XRD图谱中均出现明显的晶化衍射峰(图1d)。这些结果表明,Dy含量为1%~2%时,可以制备出相对于Cu50Zr46Al4更大尺寸的Cu50-xZr46Al4Dyx系列非晶态合金,具有更佳的非晶形成能力。表1给出了不同直径Cu50-xZr46Al4Dyx(x=0~4)系列合金的结构状态。
图1
图1
不同直径Cu50-xZr46Al4Dyx(x=0~4)试样的XRD图谱
Fig.1
XRD patterns of Cu50-xZr46Al4Dyx(x=0~4)specimens with different diameters
表1 不同直径Cu50-xZr46Al4Dyx(x=0,1,2,3,4)试样的结构
Table 1
| Specimen | d=1.5 mm | d=3.0 mm | d=5.0 mm |
|---|---|---|---|
| Cu50Zr46Al4 | A | A+C | / |
| Cu49Zr46Al4Dy1 | A | A | A+C |
| Cu48Zr46Al4Dy 2 | A | A | A+C |
| Cu47Zr46Al4Dy 3 | A | A+C | / |
| Cu46Zr46Al4Dy 4 | A+C | / | / |
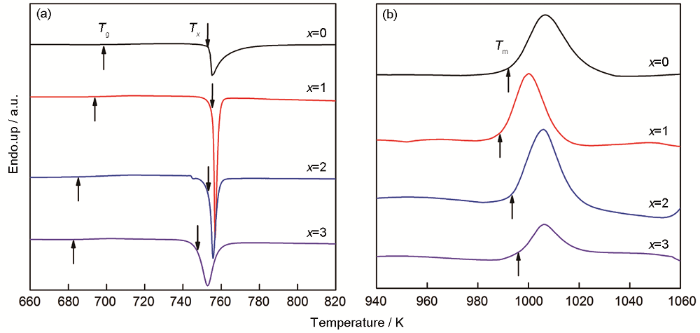
根据试样的相关热学参数可分析合金的热稳定性和非晶形成能力。图2a和b给出了直径为1.5 mm的Cu50-xZr46Al4Dyx(x=0~3)试样的DSC和DTA测试结果。其中Cu50Zr46Al4试样的玻璃转变温度Tg=698.5 K、晶化温度Tx=753.7 K、熔化温度Tm=995.6 K;其过冷液相区宽度△Tx=Tx-Tg=55.2 K,表征非晶形成能力的参数γ=Tx/(Tg+Tm)=0.445。在CuZr体系中添加Al可提高合金的液相稳定性,从而提高其非晶形成能力[3]。Zr/Al的原子尺寸比为1.119,Al/Cu的原子尺寸比为1.117,表明Al元素的加入提高了合金原子排列的复杂性,从而有利于形成非晶态无序结构,为在Cu50Zr46Al4体系中添加重稀土元素Dy提供了实验依据。表2归纳总结了Cu50-xZr46Al4Dyx(x=0~3)系列试样的相关热学参数,其中Cu50-xZr46Al4Dyx(x=1,2,3)的玻璃转变温度Tg和晶化温度Tx分别为693.4、685.3、683.1 K和755.6、753.5、747.1 K;熔化温度Tm分别为988.5、993.4、996.6 K。其过冷液相区宽度△Tx分别为66.2、68.2、64.0 K,γ系数为0.449、0.449、0.445。可以看出,Dy元素的添加会降低Cu50Zr46Al4合金的玻璃转变温度,并扩宽过冷液相区宽度。Dy元素的引入会引起合金组成元素间键合性质的变化,而玻璃转变温度和晶化温度是元素间键合变化的宏观表现[19]。与Cu50Zr46Al4相比,Dy含量为1%~2%的Cu50-xZr46Al4Dyx合金的△Tx和γ参数都提高了,说明添加Dy能提高Cu50Zr46Al4金属玻璃的热稳定性,增强其非晶形成能力。而添加3%的Dy却不能进一步提高过冷液相区宽度和非晶形成能力,说明Dy含量的最佳范围为1%~2%。
图2
图2
直径为1.5 mm的Cu50-xZr46Al4Dyx(x=0,1,2,3)的合金样品升温速率为20 K/min时的DSC和DTA曲线
Fig.2
Curves of (a) DSC and (b) DTA of Cu50-xZr46Al4Dyx(x=0, 1, 2, 3) BMG with a diameter of 1.5 mm under the heating rate of 20 K/min
表2 直径为1.5 mm的Cu50-xZr46Al4Dyx(x=0~4)BMG样品的热学参数
Table 2
| Specimen | Tg/K | Tx/K | Tm/K | △Tx/K | γ |
|---|---|---|---|---|---|
| Cu50Zr46Al4 | 698.5 | 753.7 | 995.6 | 55.2 | 0.445 |
| Cu49Zr46Al4Dy1 | 693.4 | 755.6 | 988.5 | 62.2 | 0.449 |
| Cu48Zr46Al4Dy2 | 685.3 | 753.5 | 993.4 | 68.2 | 0.449 |
| Cu47Zr46Al4Dy3 | 683.1 | 747.1 | 996.6 | 64.0 | 0.445 |
上述实验结果表明,Cu50-xZr46Al4Dyx(x=0~4)合金具有良好的热稳定性和非晶形成能力。负混合焓是形成金属玻璃的有利条件[20],而Cu-Dy和Al-Dy的混合焓分别为-22 kJ/mol和-39 kJ/mol[19],即Dy元素的引入使其与Cu和Al之间产生了较大的负混合焓,从而更加有利于Cu50-xZr46Al4Dyx体系形成金属玻璃合金。另一方面,Dy元素与体系中主要元素的原子尺寸差都大于23%(Cu、Zr、Al和Dy原子半径分别为0.128、0.160、0.143和0.177 nm),Dy原子与周围其他原子的失配使其周围环境产生大幅度畸变,从而提高体系的无序度,使合金的非晶形成能力提高[21]。
2.2 添加Dy对Cu50Zr46Al4合金力学性能的影响
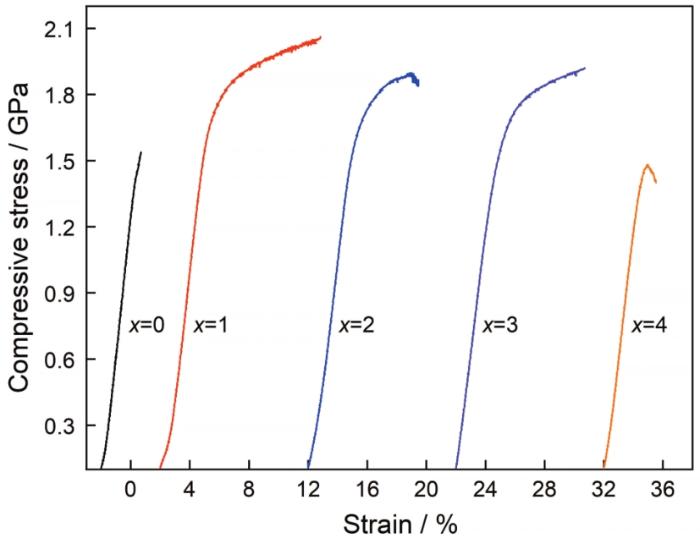
图3给出了直径为1.5 mm的Cu50-xZr46Al4Dyx(x=0~4)试样的室温压缩应力-应变曲线。表3总结了不同Dy含量BMG的屈服强度、断裂强度、弹性应变和塑性应变的数值。结果表明,Cu50Zr46Al4合金试样的断裂强度为1.538 GPa,塑性应变仅为0.49%,没有明显的塑性变形过程。而Dy含量为1%~3%的合金试样的强度和塑性均显著提高,表现出明显的屈服过程和塑性变形过程。其中最明显的Cu49Zr46Al4Dy1试样,其断裂强度和塑性分别达到2.062 GPa和7.62%。断裂强度比Cu50Zr46Al4合金试样提高了34%,塑性变形能力提高了约15倍。由此可见,添加适量的Dy元素可显著提高Cu50Zr46Al4合金的强度和塑性。
图3
图3
1.5 mm直径Cu50-xZr46Al4Dyx(x=0~4)BMG试样的室温压缩应力-应变曲线
Fig.3
Compressive stress-strain curves of Cu50-xZr46Al4Dyx(x=0~4)BMGs with a diameter of 1.5 mm at room temperature
表3 直径为1.5 mm的Cu50-xZr46Al4Dyx(x=0~4)BMG的室温压缩力学参数
Table 3
| Specimens | σy / GPa | σmax / GPa | εy / % | εf / % |
|---|---|---|---|---|
| Cu50Zr46Al4 | 1.375 | 1.538 | 2.21 | 0.49 |
| Cu49Zr46Al4Dy1 | 1.622 | 2.062 | 2.11 | 7.62 |
| Cu48Zr46Al4Dy2 | 1.512 | 1.866 | 2.14 | 4.52 |
| Cu47Zr46Al4Dy3 | 1.316 | 1.921 | 2.24 | 6.36 |
| Cu46Zr46Al4Dy4 | 1.293 | 1.401 | 2.15 | 1.29 |
图4给出了直径为1.5 mm的Cu50-xZr46Al4Dyx(x=0~4)合金压缩试样的断裂面形貌,可见所有试样均表现为典型的非晶断口形貌[22]。在压缩受力过程中蓄积的弹性变形能以绝热方式迅速释放,使材料局部软化,形成粘滞流变层,在断口出现大量清晰可见的脉状纹络[23]。越是脆性金属玻璃其脉状纹络越细小光滑,因此粗大的脉状纹络是韧性金属玻璃压缩断裂的主要特征[24]。Cu46Zr46Al4Dy4试样虽然已有部分晶化,但其断口形貌依然呈现出典型非晶断裂形貌特征。对比各试样的断口形貌,Cu49Zr46Al4Dy1试样断口脉状纹络的密度和宽度最大(图4b),大量清晰可见,周期排布的脉状纹路反映了合金较好的塑性变形能力。其原因可能是,添加微量的Dy有利于剪切带的增生而非直接形成裂纹发生脆断[25~27],在形变过程中能形成更多的剪切带,进而提高合金的塑性。Cu49Zr46Al4Dy1试样的断口形貌,表明其具有较高的压缩强度和塑性变性能力。Qiao等[19]的研究结果也指出,添加Dy会在一定程度上修正体系的塑性变形能力和动力学弛豫过程。此外,添加Dy元素使Cu50Zr46Al4合金具有更加无序的原子结构,增加体系的自由体积,从而有利于合金塑性的提高。添加适量Dy的合金,在压缩过程中表现出明显的屈服过程和加工硬化行为。这种屈服过程、加工硬化和塑性变形行为,可能是合金强度提高的原因。
图4
图4
直径1.5 mm的Cu50-xZr46Al4Dyx(x=0~4)BMG的压缩断裂形貌
Fig.4
Morphology of compressive fracture surfaces of Cu50-xZr46Al4Dyx(x=0~4) BMGs with a diameter of 1.5 mm
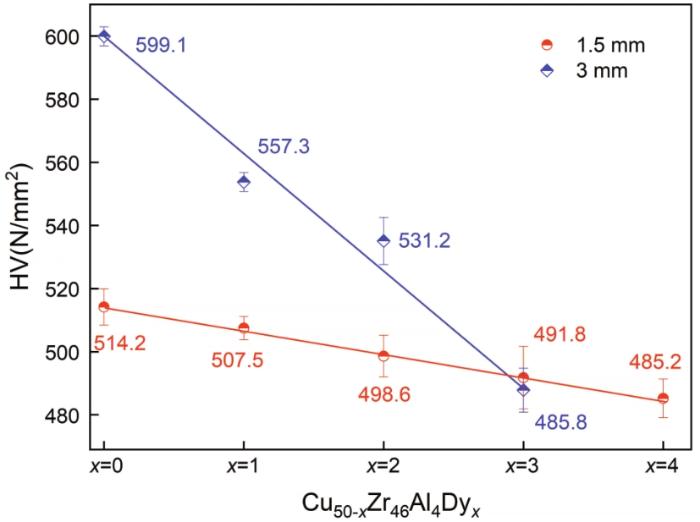
图5给出了直径为1.5 mm和3.0 mm的Cu50-xZr46-Al4Dyx(x=0~4)系列合金的维氏硬度随Dy含量的变化趋势。由图5可见,随着Dy含量的提高Cu50-xZr46-Al4Dyx合金的硬度逐渐降低。添加Dy元素使Cu50Zr46Al4基体具有更加无序的原子结构,从而增大体系的自由体积。这个结果,与Stolpe 等提出的非晶合金自由体积的改变与其硬度变化的关系吻合[28~30]。此外,相同Dy含量直径为1.5 mm的试样其硬度均低于直径为3.0 mm的试样(仅考虑完全非晶试样)。其原因是,在吸铸过程中直径为1.5 mm的试样比直径为3.0 mm的试样具有更高的冷却速率,使体系包含了更多的自由体积,使合金的硬度较低。另一方面,添加Dy元素也会改变体系中原子间化学键的强度,从而影响体系的力学性能。结果表明,直径为1.5 mm的Cu50-xZr46Al4Dyx合金,其硬度随着Dy含量的提高而下降的幅度为约5%,而直径增大到3.0 mm的试样其硬度下降的幅度达到20%。综上所述,适量的Dy元素可提高三元Cu50Zr46Al4金属玻璃的强度和塑性,同时使其硬度有所降低。
图5
图5
直径为1.5 mm和3 mm的Cu50-xZr46Al4Dyx(x=0~4)BMG试样的维氏硬度随Dy含量的变化
Fig.5
Vickers hardness values of Cu50-xZr46Al4Dyx(x=0~4) BMGs with diameters of 1.5 mm and 3 mm as a function of Dy concentration
3 结论
在Cu50Zr46Al4中添加不同含量的Dy元素替代相同摩尔比例的Cu元素,用电弧熔炼铜模吸铸法可制备出不同直径的Cu50-xZr46Al4Dyx(x=0~4)棒状合金试样。添加1%~2%的Dy元素可显著提高合金体系的热稳定性和非晶形成能力。添加Dy元素提高了合金原子结构的无序度,有利于剪切带的增生,能减缓压缩过程中主裂纹的产生和扩展,从而提高合金的压缩断裂强度和塑性变形能力。同时,添加Dy元素使合金体系的硬度降低。
参考文献
Bulk metallic glass formation in binary Cu-rich alloy series-Cu100-xZrx (x=34, 36, 38.2, 40 at.%) and mechanical properties of bulk Cu64Zr36 glass
[J].
“Work-Hardenable” ductile bulk metallic glass
[J].
CuZr-base bulk metallic glasses with good glass-forming ability prepared by Al addition
[J].
具有优良玻璃形成能力添加Al的CuZr基大块金属玻璃
[J].
Ductile metallic glasses in supercooled martensitic alloys
[J].
Effects of Al/Cu co-doping on crystal structure and chemical composition of Nd-rich phases in Nd-Fe-B sintered magnet
[J].
Formation and properties of centimeter-size Zr-Ti-Cu-Al-Y bulk metallic glasses as potential biomaterials
[J].
The electron concentration-constant and atomic size-constant criterion in Zr-based bulk metallic glasses
[J].
Zr基大块非晶合金成分的等电子浓度和等原子尺寸判据
[J].
Laser cladding amorphous composite coating of Cu-Zr-Al on magnesium alloy surface
[J].
镁合金表面激光熔覆Cu-Zr-Al非晶复合涂层
[J].
New Cu-Zr-based bulk metallic glasses with large diameters of up to 1.5 cm
[J].
Synthesis and mechanical properties of new Cu-Zr-based glassy alloys with high glass-forming ability
[J].
Fabrication of new Cu34Pd2Zr48Ag8Al8 bulk glassy alloy with a diameter of 30 mm
[J].
Thermal stability and non-isothermal crystallization kinetics of Ti41.5Cu42.5Ni7.5Zr2.5Hf5Si1 bulk metallic glass
[J]. J
Effects of Y addition on structural and mechanical properties of CuZrAl bulk metallic glass
[J].
The effect of microalloying on mechanical properties in CuZrAl bulk metallic glass
[J].
Formation of bulk metallic glasses in Cu45Zr48-xAl7REx(RE=La, Ce, Nd, Gd and 0≤x≤5at.%)
[J].
Non-isothermal crystallization transformation kinetics analysis and isothermal crystallization kinetics in super-cooled liquid region (SLR) of (Ce0.72Cu0.28)90-x-Al10Fex(x=0, 5 or 10) bulk metallic glasses
[J].
Crystallization, high temperature deformation behavior and solid-to-solid formability of a Ti-based bulk metallic glass within supercooled liquid region
[J].
Enhancement of glass-forming ability and plasticity of Cu-rich Cu-Zr-Al bulk metallic glasses by minor addition of Dy
[J].
Understanding of micro-alloying on plasticity in Cu46Zr47-xAl7Dyx(0≤x≤8) bulk metallic glasses under compression: based on mechanical relaxations and theoretical analysis
[J].
Excellent glass-forming ability in simple Cu50Zr50-based alloys
[J]. J
Intermetallics and formation of bulk glassy alloys
[J].
金属间化合物与大块玻璃合金的形成
[J].
Deformation mechanisms of the Zr40Ti14Ni10Cu12Be24 bulk metallic glass
[J].
Formation and mechanical properties of bulk Cu-Ti-Zr-Ni metallic glasses with high glass forming ability
[J].
The instability of liquid surfaces when accelerated in a direction perpendicular to their planes. I
[J].
Glass formation mechanism of minor yttrium addition in CuZrAl alloys
[J].
Ductile Bulk Metallic Glass
[J].We report on experimental evidence of pronounced global plasticity measured in monolithic Pt57.5Cu14.7Ni5.3P22.5 bulk metallic glass under both bending and unconfined compression loading conditions. A plastic strain of 20% is measured, never before seen in metallic glasses. Also, permanent deformation and a strain exceeding 3% before failure is observed during bending of 4 mm thick samples. To date, no monolithic metallic material has exhibited such a combination of high strength, extensive ductility, and high elastic limit. The large plasticity is reflected in a high Poisson ratio of 0.42, which causes the tip of a shear band to extend rather than initiate a crack. This results in the formation of multiple shear bands and is the origin of the observed large global ductility and very high fracture toughness, approximately 80 MPa m(-1/2).
Effect of minor Sn and Nb additions on the thermal stability and compressive plasticity of Zr-Cu-Fe-Al bulk metallic glass
[J].
微量添加Sn和Nb对Zr-Cu-Fe-Al块体非晶合金热稳定性和塑性的影响
[J].
Correlation between internal states and plasticity in bulk metallic glass
[J].
Free-volume-induced enhancement of plasticity in a monolithic bulk metallic glass at room temperature
[J].
Evolution of shear bands, free volume and hardness during cold rolling of a Zr-based bulk metallic glass
[J].