尼龙66(PA66)分子中的极性酰胺基团(-CONH-)和两端的活性羧基(-COOH)和氨基(-NH2)结构,使其具有较高的熔点(250~260℃)和较高的力学性能[1,2]。但是,PA66分子中的极性基团使其极易吸水和变形,影响制品的尺寸稳定性。因此,在实际应用中添加玻纤(GF)或石墨等无机填料进行改性。改性不仅降低了复合材料的成本,还使其力学性能显著提高[3]。同时,纯PA66的极限氧指数(LOI)为24%,在燃烧过程中出现明显的熔融滴落,带走大量的热使材料自熄。纯PA66在UL-94测试中只达到V-2级别(属于易燃材料),因为尼龙燃烧时产生的带火熔滴和大量浓烟极易引燃其它可燃物而使火焰蔓延[4,5]。
本文用热重分析法[10]测定PA66和两种GF/PA材料的热分解过程并计算其热分解反应的活化能和反应级数,研究GF的加入对PA66热分解性能的影响。
1 实验方法
实验用材料:尼龙66,两种33%玻纤增强PA66复合材料(记为GF/PA-1和GF/PA-2)。
将3~5 mg样品(精确到0.1 mg)置于同步热分析仪(Labsys.Evo)的瓷坩埚中,在Ar气氛中以一定的升温速率从30℃升至650℃,记录其热分解过程。测试前将样品真空干燥2 h。
2 结果和讨论
2.1 PA66和GF/PA66的热分解
图1
图1
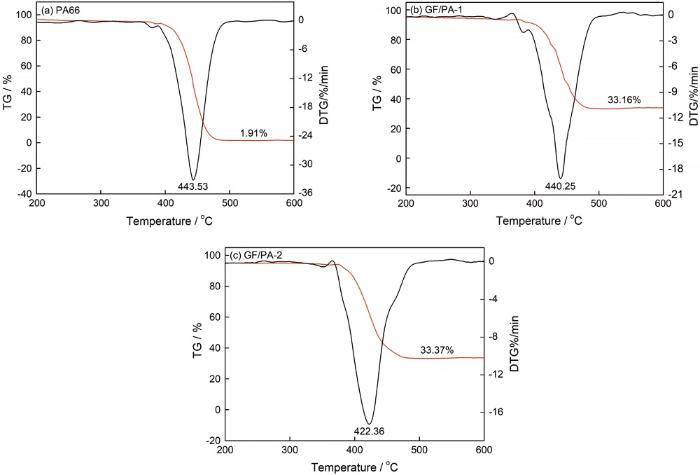
PA66、GF/PA-1和GF/PA-2的TG和DTG曲线
Fig.1
TG and DTG curves of PA66 (a), GF/PA-1 (b) and GF/PA-2 (c)
表1 PA66和GF/PA的热分解数据
Table 1
| Sample | T5%/℃ | T20%/℃ | Tmax/℃ | Tover/℃ | Residue remaining/% |
|---|---|---|---|---|---|
| PA66 | 304.4 | 423.3 | 443.5 | 486.8 | 1.91 |
| GF/PA-1 | 213.6 | 422.1 | 440.3 | 489.9 | 33.16 |
| GF/PA-2 | 227.5 | 405.5 | 422.4 | 486.9 | 33.37 |
由图1a~c可知,PA66和GF/PA热分解曲线均只有一个失重台阶,为一步分解反应。表1可见,在15℃/min升温速率条件下,PA66分解5%的温度为304.4℃,分解20%的温度为423.3℃,当温度达到443.5℃时分解速率最大,486.8℃分解完全,残渣余量为1.91%;GF/PA-1和GF/PA-2分解5%的温度分别为213.6℃和227.5℃,分解20%的温度分别为422.1℃和405.5℃,GF/PA-1在440.3℃时分解速率最快,489.9℃时趋于平衡,残渣余量33.16%;而GF/PA-2在422.4℃时分解速率最快,486.9℃时趋于平衡,残渣余量33.37%,可见,加入玻纤后,复合材料的分解温度均比纯PA的降低,但残渣余量提高。
2.2 热分解动力学
2.2.1 理论依据
PA66和GF/PA热分解时的失重率为
其中M失为样品在某个时间段的失重,M总为样品的总失重。
按照质量作用定律[11]
其中k为分解速率常数,符合Arrhenius方程[12]
将
式中n为反应级数。
设样品的升温速率为
则
将
测定不同温度下样品的失重率α,则用Kissinger法和Crane法可计算出热分解反应的活化能E和级数n。
2.2.2 用Kissinger法计算热分解活化能[13]
Kissinger法,是将
因为热分解速率最大时
其中Tp为最大分解温度。Kissinger法认为,
将
可见
图2
图2
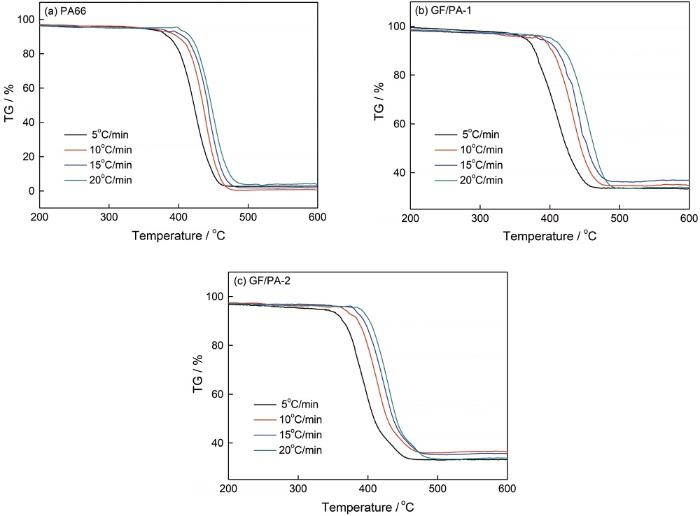
PA66、GF/PA-1和GF/PA-2不同升温速率的TG曲线
Fig.2
TG curves at different heating rates of PA66 (a), GF/PA-1 (b) and GF/PA-2 (c)
图3
图3
PA66、GF/PA-1和GF/PA-2不同升温速率的DTG曲线
Fig.3
DTG curves at different heating rates (a) PA66; (b) GF/PA-1; (c) GF/PA-2
表2 升温速率不同时PA66和GF/PA的Tp和相应的计算值
Table 2
β /K·min-1 | Tp/℃ | (1/Tp)/×10-3 K-1 | ln(β/Tp2)/K-1·min-1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PA66 | GF/PA-1 | GF/PA-2 | PA66 | GF/PA-1 | GF/PA-2 | PA66 | GF/PA-1 | GF/PA-2 | |
| 5 | 423.5 | 410.7 | 391.5 | 1.44 | 1.46 | 1.50 | -11.48 | -11.45 | -11.39 |
| 10 | 438.4 | 433.7 | 413.4 | 1.41 | 1.41 | 1.46 | -10.83 | -10.82 | -10.76 |
| 15 | 443.5 | 440.3 | 422.4 | 1.40 | 1.40 | 1.44 | -10.44 | -10.43 | -10.38 |
| 20 | 448.5 | 454.9 | 428.0 | 1.39 | 1.37 | 1.43 | -10.17 | -10.18 | -10.11 |
图4
图4
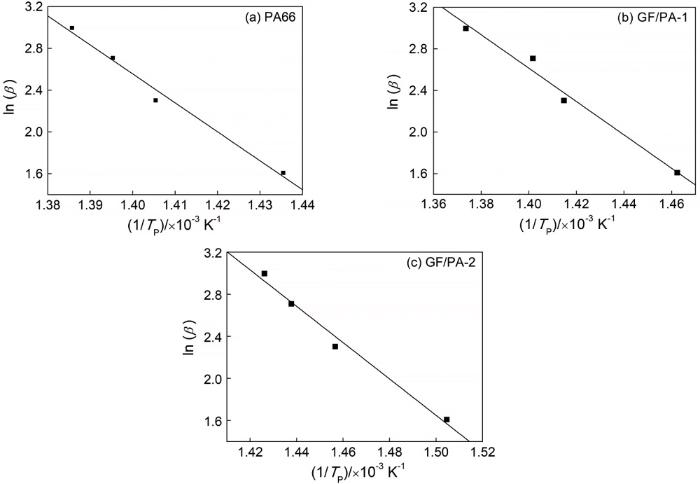
PA66、GF/PA-1和GF/PA-2的ln(β/Tp2)~1/Tp线性拟合曲线
Fig.4
Linear fitting curves of ln(β/Tp2)~1/Tp (a) PA66; (b) GF/PA-1; (c) GF/PA-2
2.2.3 用Crane法计算热分解反应级数
在已知热分解反应活化能E的条件下,用Crane方程可计算PA66、GF/PA-1和GF/PA-1热分解反应级数n。
Crane方程[16]为
图5
图5
PA66、GF/PA-1和GF/PA-2的lnβ~1/Tp线性拟合曲线
Fig.5
Linear fitting curve of lnβ~1/Tp (a) PA66; (b) GF/PA-1; (c) GF/PA-2
直线的斜率为-E/nR,于是可求出热分解反应级数,其中nPA66=0.949,nGF/PA-1=0.912和nGF/PA-2=0.921,说明热分解反应近似为一阶反应,与热分解曲线TG和DTG给出的结果一致。
3 结论
PA66和GF/PA的热分解都属于一步分解反应。加热速率越高热分解曲线的右移越明显,在加热速率相同的条件下两种GF/PA达到最大分解速率时的温度都比PA66的低。PA66、GF/PA-1和GF/PA-2的热分解活化能分别为218.65 kJ/mol、121.81 kJ/mol和132.23 kJ/mol,反应级数分别为0.949、0.912和0.921。这些结果表明,玻纤的加入使PA66的热分解活化能显著降低,但是反应级数没有变化。
参考文献
Sequence distribution, crystallization behavior and copolymerization kinetics of nylon 6/66 copolymer
[D].
尼龙6/66原位共聚物的序列分布、结晶行为及其反应动力学研究
[D].
The research on essential flame retarded PA66 and PA66 composite materials
[D].
本质阻燃PA66及其复合材料的研究
[D].
The mechanical properties of PA66 composites reinforced by glass fiber
[J].
玻纤增强PA66复合材料的力学性能
[J].
Study on the synthesis of phosphorus-containing flame retardant copolyamide66 and its properties
[D].
含磷阻燃共聚PA66的合成及性能研究
[D].
Study on toughened and reinforced nylon66 with Non-halogen flame retardants
[D].
无卤阻燃增韧增强PA66的研究
[D].
An efficient interfacial flame-resistance mode to prepare glass fiber reinforced and flame retarded polyam‐ide 6 with high performance
[J].
Preparation, properties and characterizations of halogen-free nitrogen-phosphorous flame-retarded glass fiber rein‐forced polyamide 6 composite
[J].
Study on properties of aluminum diethylphosphinate retardent system on glass fiber reinforced flame retardent copolyamide 66
[D].
二乙基次磷酸铝阻燃体系对玻纤增强阻燃共聚尼龙66的性能研究
[D].
Study on glass-fiber reinforced poly (butylene terephthalate) preparation technology and synergistic flame retardant modification
[D].
玻纤增强聚对苯二甲酸丁二醇酯的制备工艺探讨及协同阻燃改性研究
[D].
Study on the thermal stability of polyamide 66 fiber
[J].
聚酰胺66纤维的热稳定性研究
[J].
Kinetics of thermal decomposition of domestic heterocyclic aromatic polyamide fibers
[J].
国产含杂环的芳香族聚酰胺纤维的热分解动力学
[J].
Study on thermal degradation kinetic of brominated polystyrene in synchronizing antimony trioxide flame-retarded PA66
[J].
溴化聚苯乙烯协同三氧化二锑阻燃PA66热分解动力学研究
[J].