目前临床上常用的药物中25%的水溶性差,影响了药物的生物利用度、清除率、疗效和毒性。同时,在药物研发过程中筛选出的候选药物约有50%的水溶性差,影响其成药性[1,2]。近年来,两亲性聚合物胶束广泛用于疏水性药物的增溶和靶向输送[3],其中基于天然高分子多糖构建的胶束具有良好的生物相容性、生物可降解性和较低的免疫原性[4]。天然多糖来源广和结构多变,可使用各种单糖用不同的连接方式将其构成分子量各异的线性或多支结构[5]。此外,多糖中的羟基、氨基和羧基等基团赋予多糖不同的电荷性质,而且提供了大量的可修饰位点,有利于多糖的改性、接枝和修饰[6,7]。目前,构建多糖胶束载药系统常用的多糖,有普鲁兰多糖[8~10]、纤维素[11~13]、葡聚糖[14~16]、壳聚糖[17~19]、肝素[20,21]和透明质酸[22~24]等;常用于构建多糖胶束疏水核心或微区的疏水基团,有胆固醇[25]、胆酸[26]、脱氧胆酸[27]、脂肪酸[28]和聚酯[29]等。
植物淀粉(Starch)的颗粒中支链淀粉(Amylopectin)约占80%,其分子量较大(107~109),高度分支;直链淀粉(Amylose)约占20%,是由葡萄糖单体以α-(1,4)糖苷键连接成的近线性聚合物,分子量较小(105),分子结构规整,容易形成氢键作用力较强的结晶区,因此难溶于水[30]。此外,天然淀粉的多羟基结构使其有许多作用力较大的微晶区域,难以熔融塑化,限制了其应用。目前常用交联、酯化、醚化和接枝等方法对其进行化学改性,以拓展其应用范围。
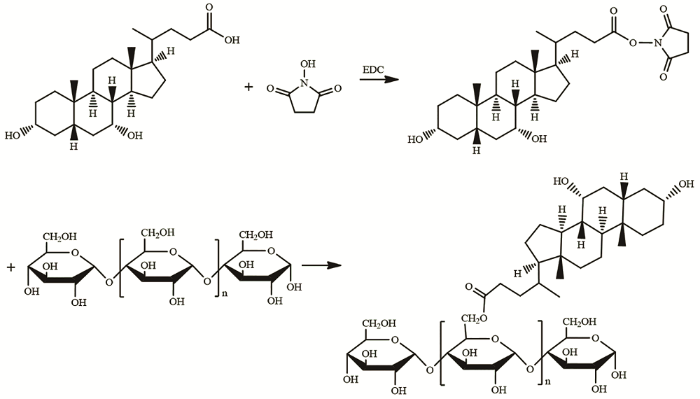
脱氧胆酸(Deoxycholic acid)是胆汁酸的主要成分,是一种具有表面活性的两亲性生物分子,可用于修饰亲水性多糖构建自组装纳米胶束[31]。NHS/EDC称为零长度交联剂,其条件温和,毒性低,生物相容性好,反应效率高。本文使用EDC/NHS交联剂将鹅去氧胆酸(Chenodeoxycholic acid,CDCA)通过酯键接枝在直链淀粉骨架上,用透析法在水中将得到的两亲性聚合物组装成球形胶束。
1 实验方法
1.1 实验用仪器和试剂
实验用仪器:Tecnai G2 F30型场发射透射电子显微镜,Nano ZS90 型激光粒度仪,VERTEX 70傅立叶变换红外光谱仪,DD2 400-MR 400MHZ核磁共振波谱仪,LS55型荧光分光光度计,UV-5200PC型紫外可见分光光度计,miVac QUATTRO型真空离心浓缩仪。
实验用试剂:直链淀粉(来源于玉米)、脱氧胆酸(纯度≥98%)、尼罗红(纯度≥95%)、透析袋(MWCO7000)、N-羟基琥珀酰亚胺(NHS)(纯度≥99.8%)、1-乙基-3-(3-二甲基氨丙基)-碳化二亚胺盐酸盐(EDC)(纯度≥99. 8%),甲醇、丙酮、DMSO等其他试剂均为分析纯。
1.2 AMY-CDCA接枝聚合物的合成
进行酯化反应将脱氧胆酸的羧基接枝到直链淀粉骨架的羟基上,合成路线如图1所示。先将0.370 g直链淀粉加入30 mL DMSO中,在45℃油浴搅拌使其充分溶解。然后将0.394 g CDCA溶解于15 mL DMSO中,加入0.230 g EDC后在室温反应15 min,再加入0.069 g NHS在室温反应30 min,得到羧基活化的CDCA。最后加入直链淀粉的DMSO溶液,在45℃油浴搅拌反应48 h。反应结束后将反应混合液装入透析袋(MWCO=7kDa),先用甲醇/水(体积比为4:1)的混合溶液透析72 h,再用去离子水透析48 h,冷冻干燥后得到AMY-CDCA聚合物。
图1
1.3 AMY-CDCA接枝聚合物的表征
将AMY-CDCA聚合物冻干粉与KBr共混压片,使用傅立叶变换红外光谱仪在4000~400 cm-1波数范围内进行扫描分析。
将30 mg AMY-CDCA聚合物冻干粉溶于600 μL DMSO-d6,使用DD2 400-MR 400MHZ核磁共振波谱仪(美国 Agient)对聚合物的1H NMR进行测试(内标为四甲基硅烷)。
用光度法测定CDCA的接枝度。将10 mg AMY-CDCA溶于0.5 mL DMSO中,加入0.5 mL乙酸溶液(60%)和9.0 mL 水/硫酸(体积比为65:50),混匀后70℃水浴30 min,冷却至室温,测定380 nm的吸光值,以不含聚合物并经上述相同处理的溶液做调零。分别称取0.625 mg、1.25 mg、2.5 mg、5 mg和10 mg CDCA,经上述相同处理并测定380 nm的吸光值,拟合吸光值关于CDCA浓度的标准曲线。CDCA接枝度DS(mol%,即直链淀粉中平均每100个葡萄糖单元所接枝的CDCA分子数)为
式中,w为根据CDCA标准曲线计算出的AMY-CDCA聚合物中CDCA的质量百分比;16200为100个葡萄糖单元的分子量;392.57为CDCA的分子量。
1.4 用透析法制备AMY-CDCA聚合物胶束
将50 mg AMY-CDCA聚合物溶于5 mL DMSO,在搅拌条件下用微量注射器将聚合物溶液缓缓滴加到10 mL去离子水中,将得到的溶液封入透析袋(MWCO=7kDa),用去离子水透析72 h后得到聚合物胶束溶液。
1.5 AMY-CDCA聚合物胶束的表征
将覆碳铜网置于滤纸上,取20 μL AMY-CDCA聚合物胶束滴加到铜网上,静置5 min后用滤纸条沿边缘吸干铜网上的液体,晾干后进行透射电镜(TEM)观察,设置不同电压以得到不同的分辨率。
取1 mL上述AMY-CDCA聚合物胶束,用激光粒度仪测量其粒径,测定温度设定为25 ℃,平衡时间3 min,测试光源为波长633 nm的He-Ne激光。
1.6 AMY-CDCA聚合物胶束CMC值和对尼罗红的增溶作用的测定
用芘荧光法测定AMY-CDCA聚合物胶束的CMC值。分别将30 μL浓度为1.2×10-4 mg/mL的芘丙酮溶液加到若干试管中并吹干,然后分别向每只试管中加入10倍梯度稀释的胶束溶液,超声震荡10 min并静置过夜后使用荧光分光光度计分别测定333 nm和338 nm处的荧光强度。设置发射波长为393 nm,狭缝5 nm,温度25℃。以荧光强度I333/I338对胶束浓度的对数作图,拐点对应的胶束浓度即为CMC。
分别将20 μL浓度为5×10-2 mg/mL的尼罗红丙酮溶液放入7支试管中并用氮气吹干,然后分别向每只试管中加入10倍梯度稀释的胶束溶液1.5 mL,超声震荡10 min并静置过夜后使用荧光分光光度计扫描580~700 nm处的发射光谱,设置激发波长为550 nm,狭缝10 nm。
2 结果和讨论
2.1 AMY-CDCA聚合物的结构表征
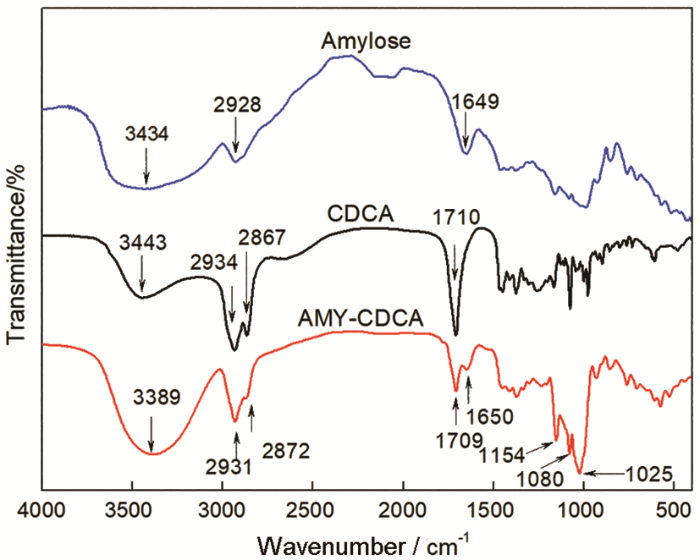
配合使用EDC/NHS活化CDCA分子上的羧基,通过酯键将CDCA接枝到直链淀粉的羟基上。FTIR和1H NMR验证了酯键的生成和CDCA分子接枝的成功。在图2直链淀粉的FTIR谱图中,1649 cm-1处可能是淀粉中水的吸收峰,2928 cm-1处3434 cm-1处的特征峰分别归属于糖环上O-H和C-H伸缩振动的吸收峰。在CDCA谱图中,3443 cm-1和2657 cm-1处的特征峰分别归属于游离O-H和氢键缔合O-H伸缩振动的吸收峰;2934 cm-1和2867 cm-1处的特征峰归属于C-H伸缩振动的吸收峰;1710 cm-1处的特征峰归属于C=O伸缩振动的吸收峰。在AMY-CDCA谱图中,O-H氢键缔合的特征峰消失,游离O-H伸缩振动的特征峰位于3389 cm-1,且强度变弱;2931 cm-1和2872 cm-1处的特征峰归属于C-H伸缩振动的吸收峰;1709 cm-1处的特征峰归属于C=O伸缩振动的吸收峰;1650 cm-1处可能是样品中残存水的吸收峰;1154 cm-1、1080 cm-1和1025 cm-1处的峰归属于C-O伸缩振动的吸收峰。这些变化说明,CDCA已经接枝到了直链淀粉骨架上。
图2
图2
Amylose、CDCA和AMY-CDCA的红外光谱图
Fig.2
FTIR spectra of Amylose, CDCA and AMY-CDCA
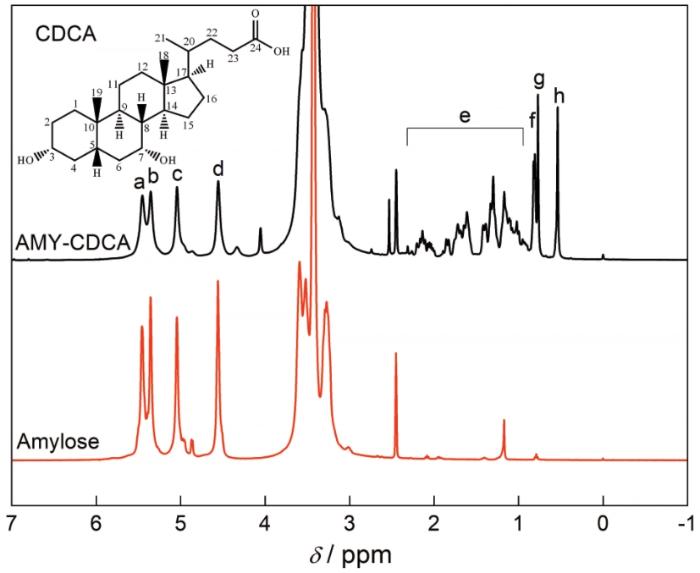
图3给出了Amylose和AMY-CDCA溶于DMSO-d6的1H-NMR谱图,两者的谱图中在4.5~5.5 ppm之间均出现了直链淀粉糖环上的质子峰,分别为C2-OH(a,5.46 ppm),C3-OH(b,5.35 ppm),C1-H(c,5.10 ppm)和C6-OH(d,4.60 ppm)。在AMY-CDCA的谱图中,0.5~2.4 ppm之间出现了脱氧胆酸的特征峰,分别为18-CH3(h,0.54 ppm),19-CH3(g,0.77 ppm),21-CH3(f,0.81 ppm)和甾环上的亚甲基和次甲基氢(e,0.95~2.31 ppm)。这些结果表明,脱氧胆酸已经成功接枝到直链淀粉上[32]。
图3
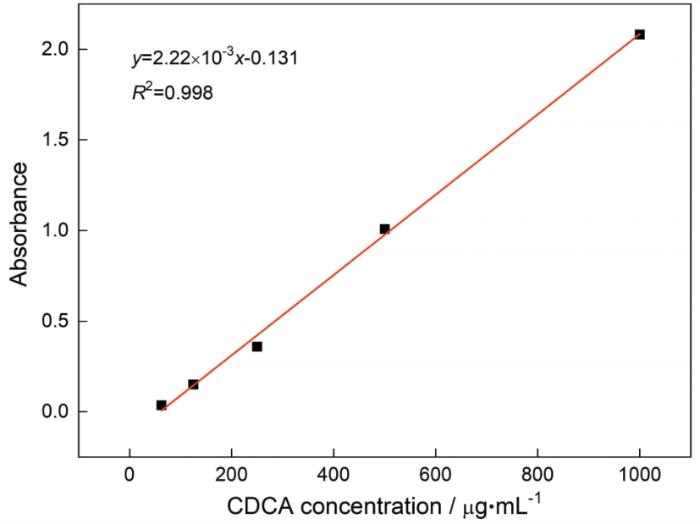
图4表明,CDCA的紫外吸收值和浓度呈良好的线性相关,标准曲线方程为y=2.22×10-3x-0.131,线性良好,样品和标准品经酸做相同处理,水解释放出CDCA,将其吸光值带入标准曲线计算得出样品中CDCA的含量为0.77(质量比),再带入公式计算出AMY-CDCA中CDCA的接枝度为138.15/100个葡萄糖单元。
图4
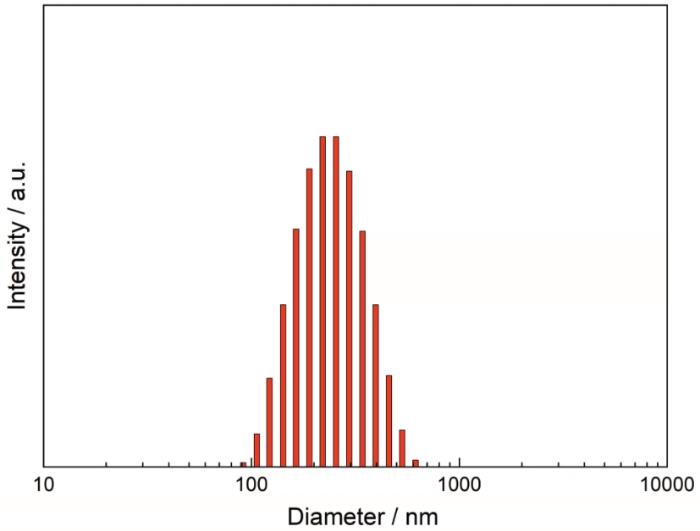
2.2 AMY-CDCA聚合物胶束的的粒径分布和形貌
图5
图5
AMY-CDCA聚合物胶束的的粒径分布
Fig.5
Particle size distribution of AMY-CDCA polymeric micelles
图6
2.3 AMY-CDCA聚合物胶束的自组装行为
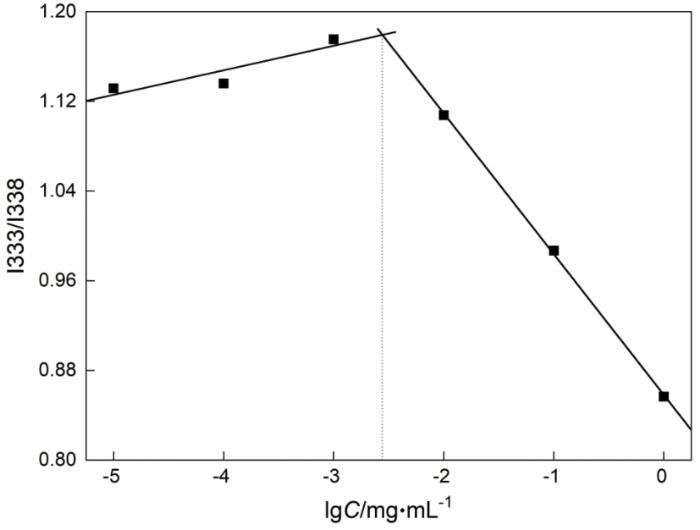
使用疏水性荧光探针芘可检测出两亲性分子在水中自聚集形成胶束的阈浓度,即临界胶束浓度(Critial micelle concentration,CMC)。当AMY-CDCA分子在水中浓度逐渐增大聚集成胶束时,芘逐渐由水相转移至胶束的疏水核心,芘的发射光谱中第一峰(I333)和第三峰(I338)的比值I333/I338逐步降低(图7),突降的拐点对应于CMC值,为2.8×10-3 mg/mL,明显低于一些小分子表面活性剂,如C12H25SO4-Na+(CMC=2.4 mg/mL,25℃)和C16H33(CH3)3N+Br-(CMC=0.36 mg/mL,25℃)[33]。这些结果表明,AMY-CDCA聚合物在较低的浓度下即可组装成胶束。
图7
图7
不同浓度的AMY-CDCA胶束溶液对芘的激发光谱中I333/I338 的影响
Fig.7
Intensity ratios (I333/I338) from pyrene excitation spectra as a function of AMY-CDCA micelle concentration
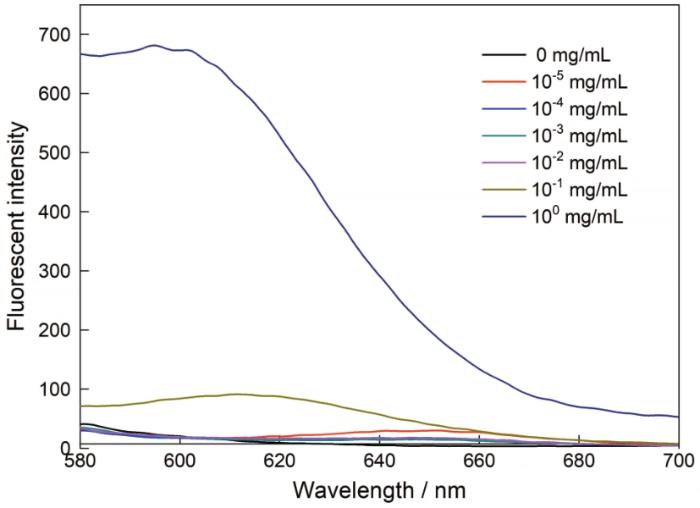
疏水荧光探针尼罗红在水溶液中的荧光非常弱,在疏水环境中可发出强荧光,表现出对极性环境的强依耐性,作为分子探针广泛应用于微环境的表征和主客体包结过程的研究。用尼罗红研究了AMY-CDCA胶束的组装行为和对疏水分子的增溶作用。由图8可以看出,在水溶液中,随着AMY-CDCA浓度的提高尼罗红的荧光强度逐渐增强,当AMY-CDCA浓度在CMC值以下时荧光强度很弱,当AMY-CDCA浓度增加到CMC值以上时荧光强度大幅度增强。这些结果表明,AMY-CDCA在水中聚集成了胶束,其疏水内核有效包裹了尼罗红。
图8
图8
不同浓度的AMY-CDCA胶束溶液中尼罗红的荧光发射光谱
Fig.8
Fluorescence emission spectra of Nile Red in aqueous solutions at different concentrations of AMY-CDCA micelles (λex=550 nm)
3 结论
用NHS/EDC活化鹅去氧胆酸的羧基并将其接枝到直链淀粉骨架上,可构建一种两亲性聚合物AMY-CDCA。CDCA的接枝度为138.15/100个葡萄糖单元。用透析法可将聚合物制备成直径为120~150 nm分散均匀的球形胶束,其平均流体力学直径为224 nm, CMC值为2.8×10-3 mg/mL。这种胶束能增溶尼罗红。
参考文献
Recent advances in polymeric micelles for anti-cancer drug delivery
[J].Block co-polymeric micelles receive increased attention due to their ability to load therapeutics, deliver the cargo to the site of action, improve the pharmacokinetic of the loaded drug and reduce off-target cytotoxicity. While polymeric micelles can be developed with improved drug loading capabilities by modulating hydrophobicity and hydrophilicity of the micelle forming block co-polymers, they can also be successfully cancer targeted by surface modifying with tumor-homing ligands. However, maintenance of the integrity of the self-assembled system in the circulation and disassembly for drug release at the site of drug action remain a challenge. Therefore, stimuli-responsive polymeric micelles for on demand drug delivery with minimal off-target effect has been developed and extensively investigated to assess their sensitivity. This review focuses on discussing various polymeric micelles currently utilized for the delivery of chemotherapeutic drugs. Designs of various stimuli-sensitive micelles that are able to control drug release in response to specific stimuli, either endogenous or exogenous have been delineated.
Polysaccharide-based micelles for drug delivery
[J].
Polysaccharide-based nano-particles: a versatile platform for drug delivery and biomedical imaging
[J].Polysaccharide-based nanoparticles have attracted interest as carriers for imaging and therapeutic agents because of their unique physicochemical properties, including biocompatibility and biodegradability. In addition, the functional groups of the polysaccharide backbone allow facile chemical modification to develop nanoparticles with diverse structures. Some polysaccharides have the intrinsic ability to recognize specific cell types, facilitating the design of targeted-drug delivery systems through receptor-mediated endocytosis. The main objective of this review is to provide an overview of various polysaccharide-based nanoparticles and to highlight the recent efforts that have been made to improve the characteristics of polysaccharide-based nanoparticles for drug delivery and biomedical imaging.
Polysaccharide-based nanoparticles for gene delivery
[J].
Review of polysaccharide particle-based functional drug delivery
[J].
Biocompatible cationic pullulan-g-desoxycholic acid-g-PEI micelles used to co-deliver drug and gene for cancer therapy
[J].
Galactosylated pullulan-curcumin conjugate micelles for site specific anticancer activity to hepatocarcinoma cells
[J].
Novel bifunctional pullulan-based micelles with good hemcompatibility for efficient co-delivery of cancer suppressor (Gene P53) and doxorubicin in cancer cells
[J].
Nano micelles of cellulose-graft-poly (l-lactic acid) anchored with epithelial cell adhesion antibody for enhanced drug loading and anti-tumor effect
[J].
Novel thermo-responsive micelles prepared from amphiphilic hydroxypropyl methyl cellulose-block-JEFFAMINE copolymers
[J].
Self-assembled micelles based on hydrophobically modified quaternized cellulose for drug delivery
[J].
Amphipathic dextran-doxorubicin prodrug micelles for solid tumor therapy
[J].
Intercellular pH-responsive histidine modified dextran-g-cholesterol micelle for anticancer drug delivery
[J].
Multifunctional dextran micelles as drug delivery carriers and magnetic resonance imaging probes
[J].
In vitro drug release and biological evaluation of biomimetic polymeric micelles self-assembled from amphiphilic deoxycholic acid-phosphorylcholine-chitosan conjugate
[J].
Multifunctional quercetin conjugated chitosan nano-micelles with P-gp inhibition and permeation enhancement of anticancer drug
[J].
Targeting delivery of tocopherol and doxorubicin grafted-chitosan polymeric micelles for cancer therapy: In vitro and in vivo evaluation
[J].
Heparin conjugated polymeric micelle for long-term delivery of basic fibroblast growth factor
[J].Heparin conjugated amphiphilic block copolymer, Tetronic-PCL-heparin (TCH), was developed and its polymeric micelles (PMs) were prepared as an injectable vehicle for long-term delivery of bFGF, which is one of the heparin-binding growth factors (HBGF). TCH PMs were fabricated by a single emulsion and solvent evaporation method. The structural properties of TCH were confirmed by (1)H NMR, FT-IR and GPC. The contents of bound heparin were 0.44 micro g/micro g and the heparin activity by APTT assay was 43.6% when compared to free heparin. The critical micelle concentration (CMC) of TCH PMs was approximately 0.11 g/l. The diameter of TC micelle was approximately 25 nm and its size after conjugation of heparin was increased to 114 nm due to the heparin molecules on the shell of the micelle. The bFGF loading amount of TCH PMs was considerably higher than that of TC, caused by specific interactions between heparin and bFGF. In vitro study, bFGF was released from TCH PMs in a controlled manner over 2 months. The results demonstrated that TCH PMs become a novel candidate for the long-term delivery of various growth factors with heparin-binding domain in tissue engineering.
Heparin modification enhances the delivery and tumor targeting of paclitaxel-loaded N-octyl-N-trimethyl chitosan micelles
[J].
Hyaluronic acid based micelle for articular delivery of triamcinolone, preparation, in vitro and in vivo evaluation
[J].
Hyaluronic acid-shelled acid-activatable paclitaxel prodrug micelles effectively target and treat CD44-overexpressing human breast tumor xenografts in vivo
[J].The therapeutic efficacy of nanoscale anticancer drug delivery systems is severely truncated by their low tumor-targetability and inefficient drug release at the target site. Here, we report the design and development of novel endosomal pH-activatable paclitaxel prodrug micelles based on hyaluronic acid-b-dendritic oligoglycerol (HA-dOG-PTX-PM) for active targeting and effective treatment of CD44-overexpressing human breast cancer xenografts in nude mice. HA-dOG-PTX-PM had a high drug content of 20.6 wt.% and an average diameter of 155 nm. The release of PTX was slow at pH 7.4 but greatly accelerated at endosomal pH. MTT assays, flow cytometry and confocal experiments showed that HA-dOG-PTX-PM possessed a high targetability and antitumor activity toward CD44 receptor overexpressing MCF-7 human breast cancer cells. The in vivo pharmacokinetics and biodistribution studies showed that HA-dOG-PTX-PM had a prolonged circulation time in the nude mice and a remarkably high accumulation in the MCF-7 tumor (6.19%ID/g at 12 h post injection). Interestingly, HA-dOG-PTX-PM could effectively treat mice bearing MCF-7 human breast tumor xenografts with little side effects, resulting in complete inhibition of tumor growth and a 100% survival rate over an experimental period of 55 days. These results indicate that hyaluronic acid-shelled acid-activatable PTX prodrug micelles have a great potential for targeted chemotherapy of CD44-positive cancers.
Synthesis and characterization of low molecular weight hyaluronic acid-based cationic micelles for efficient siRNA delivery
[J].AbstractThe aim of this work was to design a new non-viral carrier for gene delivery. This carrier was equipped with low molecular weight hyaluronic acid (LMHA) as ligand, which was conjugated by covalent attachment of hydrophobic amines (fatty amines) with different chain lengths and spermine as cationic segments. Their chemical structure and self-association behavior of the LMHA conjugates were investigated using 1H NMR, dynamic light scattering, fluorescence spectroscopy, and TEM. From the results, it was observed that polymeric micelles were spherical in shape. The micellar particle sizes and CMCs of the conjugates were significantly dependent on the degree of substitution of hydrophobic groups and the chain length. The CMCs of the conjugates were as low as 40–140 mg/L. The zeta potentials and agarose gel electrophoresis assays suggest that LMHA micelles encapsulating siRNA can be used as novel gene carriers for biomedical applications.]]>
Self-assembled pH-sensitive cholesteryl pullulan nanogel as a protein delivery vehicle
[J].A self-assembled nanogel, derived from an acid-labile cholesteryl-modified pullulan (acL-CHP), was prepared by grafting vinyl ether-cholesterol substituents onto a 100 kD pullulan main chain polymer backbone. Stable nanogels are formed by acL-CHP self-assemblies at neutral pH. The hydrodynamic radius of the nanogels, observed to be 26.5 +/- 5.1 rim at pH 7.0, increased by similar to 135% upon acidification of the solution to pH 4.0. SEC analysis of the acL-CHP nanogel at pH 4.0 showed that the grafts were nearly 80% degraded after 24 h, whereas little or no degradation was observed over the same time period for a pH stable analog (acS-CHP) at pH 4.0 or the acL-CHP at pH 7.0. Complexation of BSA with the acL-CHP nanogel was observed at pH 7.0 with subsequent release of the protein upon acidification. These findings suggest that stimuli-responsive, self assembled nanogels can release protein cargo in a manner that is controlled by the degradation rate of the cholesterol-pullulan grafting moiety.
In vitro evaluation of polymeric micelles based on hydrophobically-modified sulfated chitosan as a carrier of doxorubicin
[J].Four types of doxorubicin (DOX)-loaded polymeric micelles based on hydrophobically-modified sulfated chitosan (SCTS) were prepared. The hydrophobic group was composed of glycyrrhetinic acid (GA), cholic acid, stearic acid (SA) or lauric aldehyde. DOX encapsulation depended on several parameters, including the degree of substitution of the sulfate group and the hydrophobic group, and the type of hydrophobic group. Of these micelles, GA-SCTS micelles had the best capability to solubilize DOX. In addition, GA-SCTS micelles had the ability to target HepG(2) cells, and the IC50 for DOX-loaded GA-SCTS micelles was 54.7 ng/mL, which was much lower than that of the other micelles. Further studies on the DOX-loaded GA-SCTS micelles showed that they were stable in salt and protein solutions, in cell culture media, and during long-term storage (6 months). Based on these results, these micelles may be a promising DOX-encapsulated formulation, particularly, GA-SCTS as a potential vehicle for liver-targeted delivery.
Redox-sensitive micelles self-assembled from amphiphilic hyaluronic acid-deoxycholic acid conjugates for targeted intracellular delivery of paclitaxel
[J].
Effective antitumor gene therapy delivered by polyethylenimine-conjugated stearic acid-g-chitosan oligosaccharide micelles
[J].
Fabrication of cationic nanomicelle from chitosan-graft-polycaprolactone as the carrier of 7-ethyl-10-hydroxy-camptothecin
[J].
Starch-based nano-biocomposites
[J].
Deoxycholic acid-modified chitooligosaccharide/mPEG-PDLLA mixed micelles loaded with paclitaxel for enhanced antitumor efficacy
[J].Poly(ethylene glycol) (PEG) as a block in polymeric micelles can prolong circulation life and reduce systemic clearance but decrease the cellular uptake. To overcome this limitation, a mixed micelle composed of deoxycholic acid-modified chitooligosaccharide (COS-DOCA) and methoxy poly(ethylene glycol)-polylactide copolymer (mPEG-PDLLA) was designed to load paclitaxel (PTX). The PTX-loaded mixed micelles was prepared by nanoprecipitation method with high drug-loading efficiency of 8.03% and encapsulation efficiency of 97.09% as well as small size ( approximately 40 nm) and narrow size distribution. COS-DOCA/mPEG-PDLLA mixed micelles exhibited the sustained release property. Due to the positive charge and bioadhesive property of COS-DOCA, the cellular uptake of PTX in mixed micelles was higher in cancer cells but lower in macrophage cells compared to the mPEG-PDLLA micelles. The systemic toxicity of PTX in mixed micelles was much lower than Taxol using zebrafish as a toxicological model. Furthermore, the PTX-loaded COS-DOCA/mPEG-PDLLA mixed micelles can prolong the blood circulation time of PTX and enhance the antitumor efficacy in A549 lung xenograft model. Our findings indicate that COS-DOCA/mPEG-PDLLA mixed micelles could be a potential vehicle for enhanced delivery of anticancer drugs.
Physicochemical characterization of amphiphilic nanoparticles based on the novel starch-deoxycholic acid conjugates and self-aggregates
[J].Novel amphiphilic polymers (starch-deoxycholic acid, St-DCA) were firstly synthesized on the basis of starch (St) as a hydrophilic segment and deoxycholic acid (DCA) as a hydrophobic segment. Hydrophobically modified starch contained 5.4-8.9 deoxycholic acid groups per 100 anhydroglucose units of starch. Self-aggregates of St-DCA conjugates were formed in the PBS media. Physicochemical characterizations of St-DCA conjugates were investigated. The mean sizes of self-aggregates decreased with the degree of substitution (DS) and pH increasing. Zeta potential indicated that nanoparticles were covered with negatively charged starch shells from -5.4 to -23 mV. TEM images demonstrated that nanoparticles were of spherical shape. The critical aggregation concentrations (cac) were dependent on the DS and pH in the range of 0.0185-0.0441 mg/mL. Thus, the study suggested that self-aggregated nanoparticles of St-DCA conjugates could have good pH-responsive and potential application in pharmaceutical and biomedical fields as the delivery of anti-tumor drugs.