有致癌、致畸、致突变等毒性的工业废水(如印染废水、造纸废水等),对人类的健康和生态环境构成极大的威胁。基于半导体材料的多相光催化氧化技术,有节能、条件温和无二次污染等优点[1]。目前,光催化降解废水的关键是选择适当的光催化剂。TiO2是一种具有代表性的半导体光催化材料。但是TiO2只能利用紫外光致使其光利用率不高和量子效率不高,因而影响其实际应用[2]。铋基半导体化合物,如Bi2O3[3]、BiOX(X=Cl、Br、I)[4,5,6,7]、BiVO4[8,9,10,11]、Bi2WO6[12,13]、Bi2O2CO3[14,15]、BiOCOOH[16,17]及其它们的复合物,也在光催化降解有机污染物等方面有极大的潜力。
甲酸氧铋(BiOCOOH)是一种新型铋基材料,已用于光催化降解污染物研究。BiOCOOH的结构与Sillen族的典型化合物BiOX(X=Cl、Br、I)相似,在其结构中层状结构的[Bi2O2]2+与片层状的X(Cl-、Br-、I-、COOH-)交替,有利于光催化反应的进行[18]。但是,纯BiOCOOH相的带隙与TiO2相似(~3.3 eV),只响应紫外光[19]。将宽带隙半导体与窄带隙半导体复合,可提高可见光的利用率。制备光催化复合材料,可选取的窄带隙半导体种类繁多,如Bi2S3、Bi2O3、Bi2WO6等等。其中的Bi2O3具有优异的折射率、可见光活性、介电常数、显著的光敏性等特性,备受关注[20,21,22]。Bi2O3的主要晶相有5种,分别为单斜晶相α[23]、四方晶相β[3]、体心立方晶相γ[24]、立方晶相δ和三斜晶相ω。作为一种p型半导体,Bi2O3具有恰当的能带结构和一定氧化性的电极电势,其中α-Bi2O3和β-Bi2O3的带隙分别约为2.8 eV和2.4 eV[25,26]。β-Bi2O3具有较窄的带隙结构[27],地域降解有机化学污染物表现出优异的光催化性能。许多研究者开展了基于Bi2O3的光催化复合材料的研究,如Bi2O3/Bi2MoO6[28,29]、Bi2O3/Bi2WO6[30,31]、Bi2O3/ZrO2[32]、Bi2O3/ZnO[33]、Bi2O3/BiOCl[34]。这些复合材料都表现出显著增强的光催化活性。本文使用BiOCOOH作为自牺牲前驱体,控制热处理温度制备具有同源性铋的Bi2O3/BiOCOOH复合光催化材料,研究热处理温度对复合材料的形成及其可见光光催化性能的影响。
1 实验方法
1.1 实验用试剂
实验用五水硝酸铋(Bi(NO3)3·5H2O)、甲酸钠(HCOONa)、无水乙醇(CH3CH2OH)、二甘醇(C4H10O3)、罗丹明B(C28H31ClN2O3)等试剂均为分析纯,实验用水为二水蒸馏所得的去离子水。
1.2 BiOCOOH和复合光催化剂的制备
BiOCOOH制备:以Bi(NO3)3·5H2O和HCOONa为原料,用水热法制备目标产物BiOCOOH。将1 mmol Bi(NO3)3·5H2O与1 mmol HCOONa加入35 mL二甘醇中,在常温常压下剧烈搅拌1 h后转移到50 mL的聚四氟乙烯水热釜中。将反应釜置于配套的钢套中并放入烘箱,在150℃保温2 h。将产物(沉淀物)自然降温至室温后用去离子水和无水乙醇充分离心洗涤,然后在80℃烘箱中干燥6 h。研磨后的白色粉末,即为预合成的BiOCOOH样品。
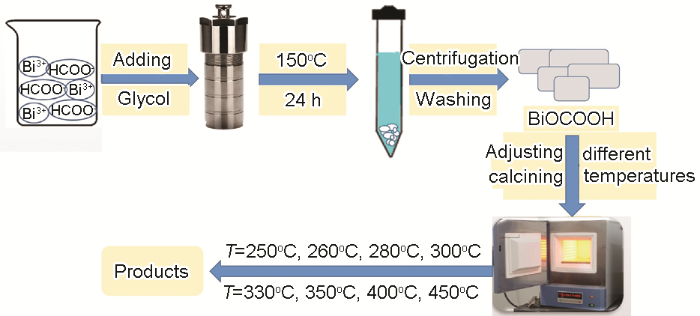
复合光催化剂的制备:使用马弗炉,将适量预合成的BiOCOOH样品在不同温度进行热处理(煅烧)。以平均2℃/min的升温速率由室温分别升到250、260、280、300、330、350、400、450℃,并在各自设定的温度下恒温2 h。待冷却至室温后取出,研磨后即得复合光催化剂样品。制备过程如图1所示。
图1
1.3 性能表征
使用X射线衍射仪(XRD,型号6100,日本岛津)测定预合成样品的XRD谱,采用Cu靶(Kα,λ=0.15406 nm) 辐射源,工作电流和电压分别为40 mA和4 kV,扫描速度为0.2 s/步,2θ角的扫描范围从100º到80º。用扫描电子显微镜(SU8000,Hitachi)观察样品的微观形貌,加速电压5~25 kV,样品室的真空度小于3×10-5 Pa。测试前,对样品进行喷金30 s处理。用Lambda 950型UV-Vis-NIR光谱仪测定固体粉末样品的紫外可见漫反射光谱,BaSO4作为标准参比,检测范围为200~800 nm。用岛津TOCv-cph总有机碳测试仪检测溶液的总有机碳(TOC)。使用ASAP 2020型分析仪在77 K下测试样品的比表面积和孔径分布,分析前将样品在一定温度下脱气至样品室的真空度小于1.33×10-3 Pa。根据吸附等温线吸附量(P/P0=0~0.35)采用多点吸附法计算比表面积,用Barrett-Joyner-Halenda(BJH)方法得到孔径分布。使用M204型电化学工作站测量样品的光电化学性能,使用传统三电极电化学池,Pt片对电极、Ag/AgCl为参比电极和工作电极。工作电极的制备:将10 mg催化剂均匀分散在0.1 mL N,N-二甲基甲酰胺中,超声分散1 h,取20 μL悬浊液均与涂在ITO导电玻璃上,面积约为0.25 cm2,在室温下干燥即得到工作电极。电解液为0.2 mol·L-1 Na2SO4,光源为420 nm的氙灯。所加偏压为0.4 mV,每光照40 s后关灯40 s,连续测试400 s。
光催化性能评价:使用可见光光催化反应装置,光源为300 W的氙灯,使用420 nm的截止滤光片滤掉紫外光。以罗丹明B(初始浓度的质量分数为0.001%)作为模拟降解污染物,考察样品的液相光催化活性。在可见光光催化反应过程中,催化剂用量为0.08 g,罗丹明B溶液体积为80 mL。在光催化反应过程中,将适量的样品投入一定量的模拟污染物的溶液中形成悬浮液,在避光条件下搅拌0.5 h达到吸附-脱附平衡。打开灯源后每隔一定时间取样3.0 mL,离心分离后用Cary-50型紫外可见光谱仪测定溶液的紫外可见吸收光谱的变化,并根据溶液的脱色率评价催化剂的活性。
2 实验结果
2.1 样品的物相
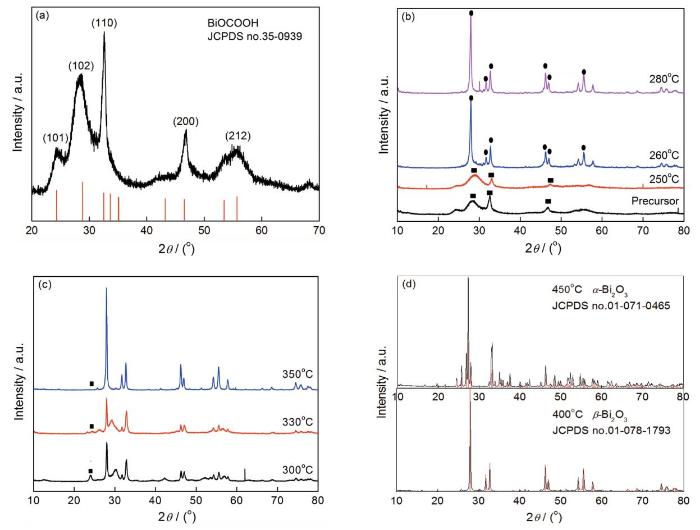
以Bi(NO3)3·5H2O和HCOONa为原料通过水热法合成的白色粉末样品的晶相结构,如图2所示。从图2a可以看出,所得样品的XRD衍射峰与标准谱库中四方晶相结构的BiOCOOH具有很高的匹配度。其中位于24.33°、28.82°、32.52°、46.53°、55.70°的衍射峰分别对应于(101)、(102)、(110)、(200)、(212)晶面衍射峰。在XRD图谱中并未出现其它物质的晶体衍射峰,表明以硝酸铋和甲酸钠为原料进行水热反应得到的样品是纯相BiOCOOH粉末。用水热法得到的BiOCOOH粉末在不同温度热处理后,样品的晶相结构发生了很大的变化。图2b分别给出了BiOCOOH前驱体及BiOCOOH分别在250、260、280℃煅烧后产物的XRD对比谱图。可以看出,前驱物和在250℃处理样品的峰形较宽,衍射峰较弱,表明其结晶程度不高。随着煅烧温度升高至260、280℃谱图中出现了明显不属于BiOCOOH的衍射峰,证实有新物质生成。对比标准谱库证实,新生成的物质为四方晶相的β-Bi2O3。其中最强峰为27.95°位置出现的衍射峰,对应于β-Bi2O3的(201)晶面。此外,BiOCOOH与β-Bi2O3有几个位置的衍射峰重合,如BiOCOOH在32.57°位置出现了归属于(110)晶面衍射峰,β-Bi2O3同样在接近的32.82°和32.69°上分别出现了归属于(220)晶面和(002)晶面的特征衍射峰;BiOCOOH在46.61°有归属于(200)晶面衍射峰,β-Bi2O3在46.20°和46.91°同样有分别归属于(222)和(400)晶面的衍射峰。根据谱图判断,BiOCOOH作为前驱物于260、280℃下煅烧,产物是组成为β-Bi2O3/BiOCOOH的复合材料。
图2
图2
在不同温度热处理样品的XRD谱图
Fig.2
XRD patterns of samples calcined at different temperatures (a) precursor BiOCOOH; (b) samples with heat treatment temperatures from 250℃ to 280℃; (c) samples with heat treatment temperatures from 300℃ to 350℃; (d) samples with heat treatment temperatures of 400℃ and 450℃
逐渐提高煅烧温度,观察产物的组成和结构发生的变化。图2c给出了BiOCOOH前驱体分别在300、330、350℃煅烧后所得产物的XRD谱图。避开衍射峰重叠位置,在24.15°找到专属于BiOCOOH(101)晶面的特征峰,见图2c中黑点处。因为β-Bi2O3在此位置没有衍射峰,可根据该衍射峰的变化来判断。从图中可见,在热处理温度从300℃升至330、350℃的过程中,归属于BiOCOOH的24.15°特征峰逐渐减弱。据此可以推测,在300、330、350℃煅烧的产物仍为BiOCOOH和β-Bi2O3两者的复合物。随着温度的升高,混合物中BiOCOOH的比例逐渐降低。继续升高煅烧温度,所得样品的XRD图谱如图2d所示。可以看出,在400℃煅烧后的产物与四方晶相β-Bi2O3(JCPDS卡号:01-078-1793)的标准衍射峰有很高的匹配度,未能发现归属于BiOCOOH的衍射峰,尤其是专属于BiOCOOH的24.15°特征峰已然消失。这表明,在400℃煅烧的产物中没有BiOCOOH成分,是纯相β-Bi2O3。此外,在450℃热处理后样品的峰已完全不同于400℃处理后的衍射峰,出现了全新的衍射峰。经过与标准谱图比对,可知450℃煅烧产物为单斜相的α-Bi2O3。由此可以推测,随着煅烧温度从400℃升到450℃产物从β-Bi2O3转变为α-Bi2O3。
2.2 样品的形貌
图3给出了不同样品的SEM照片。从图3a,b可以看出,纯相BiOCOOH呈现出由轻盈的、带絮状的不规则薄片组成的多级结构形貌。在330℃热处理后样品的组成为BiOCOOH和β-Bi2O3的复合物,其形貌如图3c, d所示,可见部分带絮状的片状物已经破坏并出现了纳米晶体小颗粒。在400℃的煅烧产物纯相β-Bi2O3的形貌如图3e, f所示,可见与在330℃煅烧得到的复合材料形貌相似,由不规则片状物和纳米晶体颗粒组成。当热处理温度升至450℃产物转变为α-Bi2O3,薄片状逐渐消失,表面有大量的纳米晶体产生,并保留了原始的多级结构形貌。这些结果表明,热处理后BiOCOOH纳米片逐渐转化成β-Bi2O3和α-Bi2O3时,整体的多级形貌结构没有发生很大改变。因此,BiOCOOH前驱体在这一过程中起自牺牲模板的作用。
图3
图3
不同样品的SEM照片
Fig.3
SEM images of BiOCOOH (a) (b), β-Bi2O3/BiOCOOH (c) (d), β-Bi2O3 (e) (f), and α-Bi2O3 (g) (h)
图4
图4
β-Bi2O3/BiOCOOH样品的TEM和HRTEM照片
Fig.4
TEM (a) and HRTEM (b) images of β-Bi2O3/BiOCOOH
2.3 光吸收性能
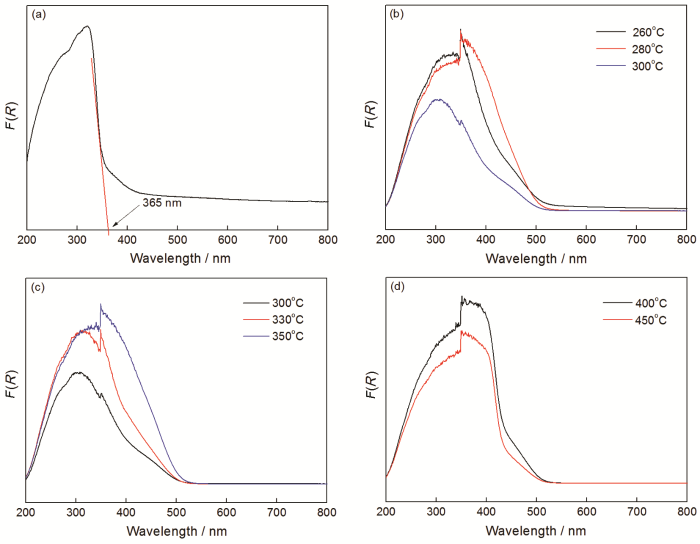
热处理温度的变化,直接影响材料的晶相组成结构和光吸收性能。用紫外可见漫反射仪表征了几个样品进行光吸收性能,结果如图5所示。从图5a可见,纯相BiOCOOH样品的吸收带边约为365 nm,用公式Eg=1240/λg(λg为半导体材料吸收带边值)可估算出BiOCOOH的带隙约为3.39 eV,与文献报道的数值接近[16]。热处理温度分别为260、280、300℃样品的吸收带边与纯相BiOCOOH对比发生了明显的红移,证实热处理后样品在可见光下有了一定的吸收,主要原因是生成了β-Bi2O3。β-Bi2O3是一种具有可见光响应的光催化材料,在可见光下具有一定的吸收性能。从图5c可以看出,在300、330、350℃热处理的这三个样品,其可见光吸收强度随着处理温度的升高在逐渐增加。其主要原因是,具有可见光吸收性能的β-Bi2O3在复合物中的比例随着热处理温度升高而逐渐提高。当处理温度升高至400、450℃时,光吸收曲线与低于400℃处理的样品有所不同,因为物相结构发生了变化。上文的XRD实验证实,当热处理温度升高至400、450℃时,得到的样品粉末分别为β-Bi2O3和α-Bi2O3。因此,热处理高于400℃和450℃的产物其可见光吸收带边分别约为454 nm和444 nm,对应的带隙宽度约为2.73 eV和2.79 eV。
图5
图5
不同样品的紫外可见分光光度图谱
Fig.5
UV-Vis diffuse reflectance spectra of diffenent samples
2.4 样品的比表面
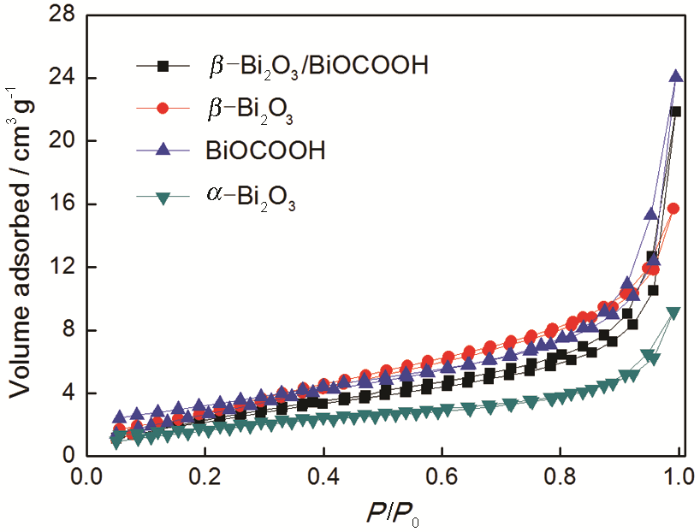
用N2吸-脱附法研究了所得样品的比表面积和孔隙率,结果如图6所示。所有样品的等温线,包括前驱体BiOCOOH、β-Bi2O3/BiOCOOH和β-Bi2O3均与所有样品的等温线,都与IV型的H3回滞环相对应,说明具有介孔特征。与前驱体BiOCOOH的BET比表面积(11.9 m2/g)对比,煅烧后样品的比表面积减小,分别为β-Bi2O3/BiOCOOH(9.1 m2/g)、β-Bi2O3(6.9 m2/g)、α-Bi2O3(5.2 m2/g)。值得注意的是,无论前驱体BiOCOOH还是热处理后的产物,其比表面积的差异都不大。
图6
图6
前驱体BiOCOOH、β-Bi2O3/BiOCOOH、β-Bi2O3和α-Bi2O3的N2吸-脱附等温线
Fig.6
Nitrogen adsorption-desorption isotherms of the BiOCOOH,β-Bi2O3/BiOCOOH,β-Bi2O3 and α-Bi2O3
2.5 光催化性能
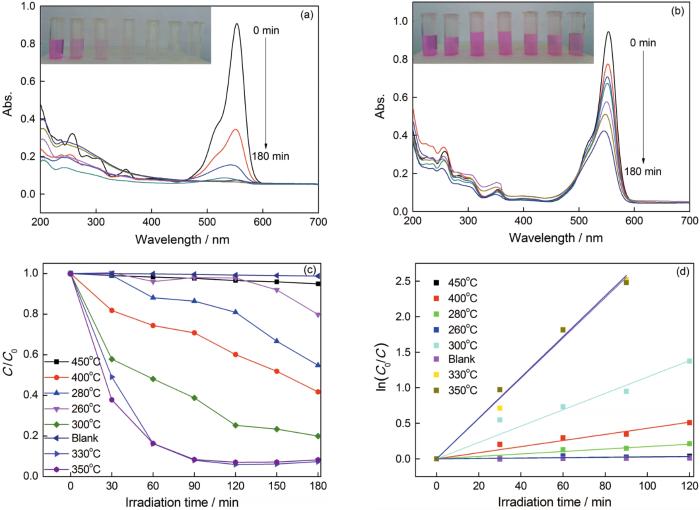
在可见光(λ>420 nm)下以降解染料罗丹明B(RhB)作为光催化探针反应,评价在不同温度热处理产物的光催化性能。图7a、b分别给出了在330℃和450℃热处理后样品对相同浓度RhB溶液在可见光条件下的光催化降解结果。可以看出,在330℃热处理的样品在可见光照90min后对罗丹明B的脱色率达到92%以上,而在450℃热处理的样品在可见光照180 min后对罗丹明B的脱色率仅59%。图7c给出了所有样品的光催化降解结果。可以看出,罗丹明B溶液在可见光条件下的空白降解作用很弱,说明该染料溶液在可见光条件下相对稳定。对比在不同温度热处理样品的光催化活性,连接在330℃和350℃热处理的β-Bi2O3/BiOCOOH复合材料的光催化性能接近,且在所有样品中活性最佳;在400℃热处理的β-Bi2O3对罗丹明B表现出一定的光催化降解性能,但是活性较差;在450℃热处理的样品α-Bi2O3对罗丹明B的可见光降解效率极低,几乎接近于罗丹明B溶液在可见光下的空白降解。因此,光催化性能高低的排序为:β-Bi2O3/BiOCOOH>β-Bi2O3 >α-Bi2O3。针对罗丹明B溶液的光催化降解反应进行了Langmuir-Hinshelwood模型的一级反应动力学拟合,拟合曲线如图7d所示。拟合公式为:ln(Ct/C0)=kappt,其中C0和Ct分别为t=0和t=t时刻罗丹明B的浓度。代入实验数据可计算出表观反应速率常数kapp。热处理温度为450、400、260、280、300、330、350℃,计算出表观反应速率常数kapp分别为0.000283、0.00429、0.000251、0.00172、0.0115、0.0284、0.0287。由此可见,在350℃热处理样品β-Bi2O3/BiOCOOH的反应速率常数分别是450℃热处理样品α-Bi2O3、400℃热处理样品β-Bi2O3反应速度常数的100倍和6.7倍。
图7
图7
在330℃热处理样品β-Bi2O3/BiOCOOH降解RhB溶液、在400℃热处理样品β-Bi2O3降解RhB溶液、在不同温度热处理样品对RhB溶液的降解活性对比以及光催化降解反应的一级反应动力学拟合曲线
Fig.7
(a) The absorption of RhB solution at different irradiation time in the presence of β-Bi2O3/BiOCOOH obtained at 330℃. (b) The absorption of RhB solution at different irradiation time in the presence of β-Bi2O3 obtained at 400℃. (c) Photocatalytic degradation kinetics of RhB over samples calcined at different temperatures. (d) Linear plots of ln(C0/Ct) as a function of degradation time
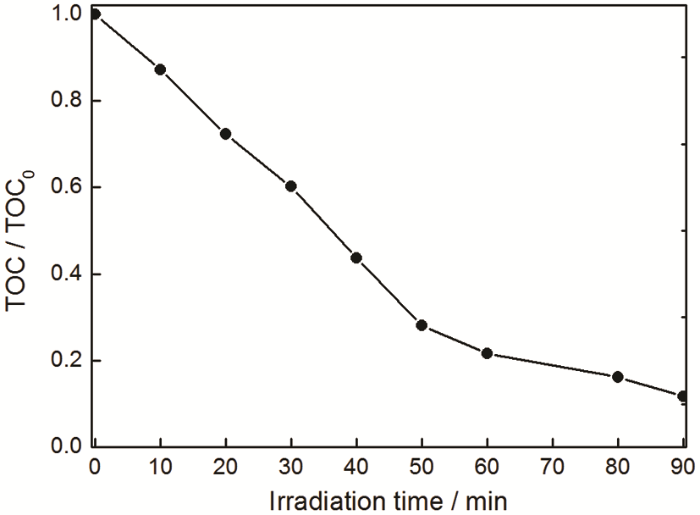
在光催化降解水体中污染物的过程中,除了脱色率,还要监测污染物分子的矿化率。根据检测光催化降解过程中罗丹明B溶液的总有机碳(TOC)值的变化,可计算其矿化率。在350℃热处理样品β-Bi2O3/BiOCOOH的光催化降解过程中,溶液的TOC值随着光照时间的延长而逐渐降低,如图8所示。可以看出,经过90 min可见光光催化反应后,88%左右的罗丹明B溶液彻底矿化分解。
图8
图8
β-Bi2O3/BiOCOOH光催化体系对罗丹明B溶液的TOC去除率
Fig.8
TOC removal of RhB over as-synthesized β-Bi2O3/BiOCOOH photocatalytic system
2.6 光电化学性能
将BiOCOOH在不同温度热处理,三种产物在光催化降解罗丹明B溶液中表现出不同的性能。其光催化性能高低排序为:β-Bi2O3/BiOCOOH(330℃或350℃)>β-Bi2O3(400℃)>α-Bi2O3(450℃)。为了进一步探究复合材料在光诱导下的电荷分离效率,分别测试了β-Bi2O3/BiOCOOH、β-Bi2O3、α-Bi2O3这三种材料的光电流,结果如图9所示。从图9可见,这些反应电极随着可见光灯的开、关表现出快速的、周期性的变化,这种电流变化与三种材料产生有效分离的光生载流子数量有关。在关灯暗态情况下,三种材料未被激发产生光生载流子,因此均未产生明显变化的电流;在开灯光照状态,三种材料均有可见光响应,产生了明显变化的电流。其中纯BiOCOOH的光电流相当低,因为其带隙(3.39 eV)较大在可见光波段的吸收较少;β-Bi2O3在可见光吸收强度比纯BiOCOOH大,但复合效率较高;β-Bi2O3/BiOCOOH的光电流明显高于β-Bi2O3和纯BiOCOOH,表明β-Bi2O3/BiOCOOH在可见光下产生了更多有效分离的光生载流子,这与其异质结复合结构有关。由于β-Bi2O3/BiOCOOH的可见光吸收光谱强度低于β-Bi2O3,β-Bi2O3/BiOCOOH异质结的形成在很大程度上降低了光生电子和空穴的复合速率。三种物质的光电流瞬态响应强弱顺序与其光催化性能高低顺序相一致,证实材料的光催化性能与光生载流子的分离能力有直接的关系。
图9
图9
BiOCOOH、β-Bi2O3和β-Bi2O3/BiOCOOH的光电流瞬态响应
Fig.9
Photocurrent transient responses for BiOCOOH,β-Bi2O3 and β-Bi2O3/BiOCOOH composites
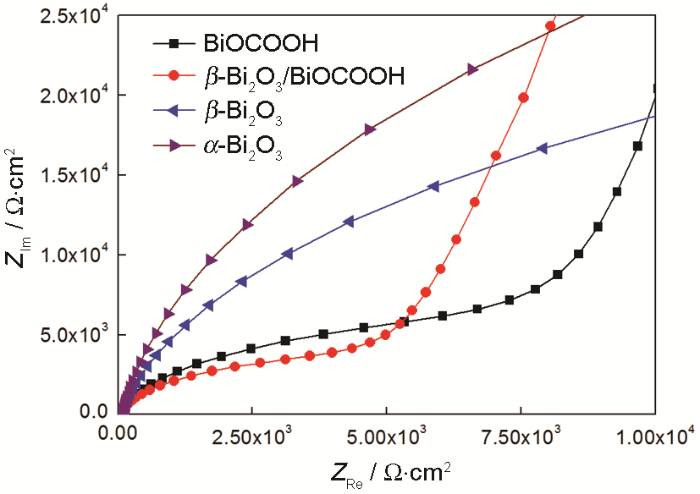
本文的电化学阻抗谱(EIS)的频率范围为4~50 MHz,振幅电压为10 mV,且相对Ag/AgCl电极0偏压情况下测定。图10给出了暗态下四种材料的典型EIS的Nyquist曲线。出现接近半圆的弧线是高频范围下电荷转移的特征过程,且弧线半圆的直径等于电子转移的阻力。与纯相BiOCOOH、β-Bi2O3、α-Bi2O3相比,复合材料β-Bi2O3/BiOCOOH的弧度最小,亦即阻抗最小,证实复合材料在光催化反应时与外界的电荷传递速率或效率更高。其原因可能是,两种材料之间的极化率不同或内建电场的不同引起两种材料界面间电磁场的变化,从而有利于电荷传递。同时,BiOCOOH样品与β-Bi2O3、α-Bi2O3相比,在Nyquist曲线中较早出现上扬,说明BiOCOOH与电解液之间的电荷传递(电子)速率达到了与电解液的电荷(SO42-离子)扩散速率,说明BiOCOOH与电解液之间的电荷传递比β-Bi2O3、α-Bi2O3强。而复合材料β-Bi2O3/BiOCOOH更早出现拐点也说明了这一点,这也印证了高频阻抗的结果。因此,EIS实验结果说明复合材料能促进界面电子转移从而提高电荷的分离效率,最终提高催化剂的光催化性能。
图10
图10
BiOCOOH、β-Bi2O3、α-Bi2O3和β-Bi2O3/BiOCOOH的Nyquist阻抗图
Fig.10
Nyquist impedance plots of BiOCOOH, β-Bi2O3, α-Bi2O3 and β-Bi2O3/BiOCOOH
图11
图11
BiOCOOH和β-Bi2O3的莫特-肖特基曲线
Fig.11
Mott-Schottky curve of BiOCOOH and β-Bi2O3
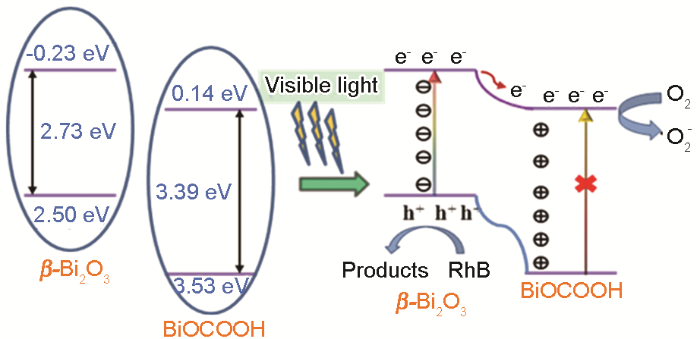
2.7 复合材料的催化机理
图12
图12
β-Bi2O3/BiOCOOH复合材料在可见光下的电子-空穴分离示意图
Fig.12
Schematic diagram of separation of electron-hole pairs over β-Bi2O3/BiOCOOH composites under visible light
3 结论
(1) 用水热法制备多级结构花状的BiOCOOH,将其作为自牺牲前驱体在不同温度热处理可制备出具有多级结构形貌的几种不同材料。热处理温度低于250℃不能得到β-Bi2O3/BiOCOOH复合材料;在260~350℃热处理产物为β-Bi2O3/BiOCOOH;热处理温度达到400℃则BiOCOOH全部转化为β-Bi2O3,热处理温度达到450℃则β-Bi2O3进一步转化为α-Bi2O3。
(2) 经330℃或350℃热处理得到的β-Bi2O3/BiOCOOH复合光催化剂在可见光下降解罗丹明B的效率最高,且其矿化率可达88%。
(3) 复合材料β-Bi2O3/BiOCOOH具有比β-Bi2O3、α-Bi2O3更高的可见光催化活性,因为β-Bi2O3与BiOCOOH紧密结合形成Z-型光催化结构,具有更高的光生载流子的分离效率而实现了光生电荷的有效分离。
参考文献
Environmental applications of semiconductor photocatalysis
[J].
NiFe layered double hydroxide on nitrogen doped TiO2 nanotube arrays toward efficient oxygen evolution
[J].
Facile large-scale synthesis of β-Bi2O3 nanospheres as a highly efficient photocatalyst for the degradation of acetaminophen under visible light irradiation
[J].
Photocatalytic degradation of PCP-Na over BiOI nanosheets under simulated sunlight irradiation
[J].
BiOX (X=Cl, Br, I) photocatalysts prepared using NaBiO3 as the Bi source: Characterization and catalytic performance
[J].In order to reduce fatal self-poisoning legislation was introduced in the UK in 1998 to restrict pack sizes of paracetamol sold in pharmacies (maximum 32 tablets) and non-pharmacy outlets (maximum 16 tablets), and in Ireland in 2001, but with smaller maximum pack sizes (24 and 12 tablets). Our aim was to determine whether this resulted in smaller overdoses of paracetamol in Ireland compared with the UK.
Solvothermal synthesis of flower-like BiOBr microspheres with highly visible-light photocatalytic performances
[J].
Solvothermal synthesis of BiOCl flower-like hierarchical structures with high photocatalytic activity
[J].
Porous olive-like BiVO4: Alcoho-hydrothermal preparation and excellent visible-light-driven photocatalytic performance for the degradation of phenol
[J].
BiVO4 nano-leaves: Mild synthesis and improved photocatalytic activity for O2 production under visible light irradiation
[J].
Preparation and photoelectrochemical study of BiVO4 thin films deposited by ultrasonic spray pyrolysis
[J].
Superior photoelectrochemical properties of BiVO4 nanofilms enhanced by PbS quantum dots decoration
[J].
Bi2WO6 micro/nano-structures: Synthesis, modifications and visible-light-driven photocatalytic applications
[J].
Rapid preparation of Bi2WO6 photocatalyst with nanosheet morphology via microwave-assisted solvothermal synthesis
[J].
Synthesis of flower-like heterostructured β-Bi2O3/Bi2O2CO3 microspheres using Bi2O2CO3 self-sacrifice precursor and its visible-light-induced photocatalytic degradation of o-phenylphenol
[J].
Citrate/Urea/Solvent Mediated Self-Assembly of (BiO)2CO3 hierarchical nanostructures and their associated photocatalytic performance
[J].
BiOCOOH hierarchical nanostructures: Shape-controlled solvothermal synthesis and photocatalytic degradation performances
[J].Different shaped bismuth oxide formate (BiOCOOH), including spherical-like, sponge-like, tremella-like, flower-like hierarchical nanostructures have been successfully synthesized through a facile and template-free solvothermal process in different solvents. The products were characterized by powder X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), UV-vis diffuse reflection spectroscopy (DRS) and nitrogen adsorption. Based on the electron microscopy observations of the products obtained under different experimental conditions, a possible growth mechanism which involves Ostwald ripening and self-assembly process was proposed. It was found the solvent plays a crucial role in the formation of the BiOCOOH hierarchical nanostructures. The optical properties, Brunauer-Emmett-Teller (BET) surface areas and photocatalytic activities on the degradation of Rhodamine B (RhB) of the different BiOCOOH hierarchitectures were also evaluated. The results indicated that the synthesized sponge-like BiOCOOH hierarchical nanostructures possessed favorable recycling characteristics, and exhibited the highest photocatalytic activity compared with other BiOCOOH hierarchitectures, because of its largest surface area (30.28 m(2) g(-1)) and large band gap (3.46 eV). This work not only provides new strategies for the controllable synthesis of other novel hierarchical materials, but also develops new promising photocatalysts for degrading organic pollutants and explores significant potential applications in environment pollution.
Reduced graphene oxide modified flower-like BiOCOOH architectures with enhanced photocatalytic activity
[J].
Two novel bi-based borate photocatalysts: crystal structure, electronic structure, photoelectrochemical properties, and photocatalytic activity under simulated solar light irradiation
[J].
Enhanced visible light photocatalytic activity of BiOI/BiOCOOH composites synthesized via ion exchange strategy
[J].
Bi2O3 Hierarchical nanostructures: controllable synthesis, growth mechanism, and their application in photocatalysis
[J].By introducing VO(3)(-) into the reaction system, uniform hierarchical nanostructures of Bi(2)O(3) have been successfully synthesized by a template-free aqueous method at 60-80 degrees C for 6 h. The as-prepared hierarchitectures are composed of 2D nanosheets, which intercross with each other. Based on the electron microscope observations, the growth of such hierarchitectures has been proposed as an Ostwald ripening process followed by self-assembly. The nucleation, growth, and self-assembly of Bi(2)O(3) nanosheets could be readily tuned, which brought different morphologies and microstructures to the final products. Pore-size distribution analysis revealed that both mesopores and macropores existed in the product. UV-vis spectroscopy was employed to estimate the band gap energies of the hierarchical nanostructures. The photocatalytic activities of as-prepared Bi(2)O(3) hierarchitectures were 6-10 times higher than that of the commercial sample, which was evaluated by the degradation of RhB dye under visible light irradiation (lambda>420 nm).
Synthesis of hierarchical rippled Bi2O3 nanobelts for supercapacitor applications
[J].Hierarchical rippled Bi(2)O(3) nanobelts were successfully synthesized by an electrodeposition route and tested as promising materials for supercapacitor applications.
Visible light photocatalytic properties of metastable γ-Bi2O3 with different morphologies
[J].
Enhanced photocatalytic activity of α-Bi2O3 with high electron-hole mobility by codoping approach: A first-principles study
[J].
Enhanced photocatalytic degradation of methyl orange by gamma Bi2O3 and its kinetics
[J].
Sonochemical synthesis of nanocrystallite Bi2O3 as a visible-light-driven photocatalyst
[J].
Nanowires of α- and β-Bi2O3: phase-selective synthesis and application in photocatalysis
[J].This paper is focused on the first selective synthesis of alpha-and beta-Bi2O3 nanowires by an oxidative metal vapour transport deposition technique. The nanowires growth conditions have been systematically investigated, providing insights into the mechanism of nanowires formation. We make a critical comparison of photocatalytic properties of the alpha- and beta-Bi2O3 nanowires by the photo-degradation of Orange G under visible light. The beta-Bi2O3 nanowires have shown the highest photo-catalytic activity under visible light of all the tested samples of Degussa TiO2 P25 and alpha-/beta-Bi2O3 nanowires, which can be interpreted by the narrowest band gap of the beta-Bi2O3 nanowires for the most effective harvest of the visible light as well as their smallest size for the most facile transfer of photo-excited electrons and the consequent most effective electron-hole separation.
Comparative study on photocatalytic performances of crystalline α- and β-Bi2O3 nanoparticles under visible light
[J].
Mechanism of 2, 4-dinitrophenol photocatalytic degradation by ζ-Bi2O3/Bi2MoO6 composites under solar and visible light irradiation
[J].
Facile preparation of heterostructured Bi2O3/Bi2MoO6 hollow microspheres with enhanced visible-light-driven photocatalytic and antimicrobial activity
[J].
In situ one-step combustion synthesis of Bi2O3/Bi2WO6 heterojunctions with notable visible light photocatalytic activities
[J].
Preparation and visible light photocatalytic activity of Bi2O3/Bi2WO6 heterojunction photocatalysts
[J].
Photoreduction of Cr(VI) in water using Bi2O3-ZrO2 nanocomposite under visible light irradiation
[J].4-Fe3O4 was synthesized using a solvothermal method and adopted as a photoreduction catalyst to removal of Cr(VI) in water under visible light irradiation. The physical and chemical properties of BiVO4-Fe3O4 were characterized by UV-vis-DRS, SEM, XRD, FTIR, and BET. The results demonstrated that the band gap for the obtained material is 1.74 eV with an average size of 15 nm and a specific surface area of 55.16 m2/g. A high photocatalytic performance was observed on the photoreduction of Cr(VI) and the removal efficiency was increased in the lower pH condition. The ascending catalyst dosages made the promotion effect, while the increase of Cr(VI) concentration contributed the inhibition for the reduction performance. The structural characteristics of the selected hole scavengers (ethanol, isopropanol, formic acid, citric acid, and oxalic acid) showed the various effects on the reactions due to the amounts of α-OH. For the optimal condition, 79.37% Cr(VI) was removed. Based on the excellent reusability of BiVO4-Fe3O4, this study demonstrated a potential method for the economic-friendly removal of high-valence metals with easier separation in the water.]]>
Role of Bi2O3 content on physical, optical and vibrational studies in Bi2O3-ZnO-B2O3 glasses
[J].
Band alignment and enhanced photocatalytic activation for α-Bi2O3/BiOCl (001) core-shell heterojunction
[J]. J
The influence of fluoride on the physicochemical properties of anodic oxide films formed on titanium surfaces
[J].The influence of fluoride (and its concentration) on the electrochemical and semiconducting properties of anodic oxide films formed on titanium surfaces was investigated by performing electrochemical measurements (potentiodynamic/pontiostatic polarization, open circuit potential (OCP), and capacitance measurements) for a titanium/oxide film/solution interface system in fluoride-containing 1.0 M HClO(4) solution. On the basis of the Mott-Schottky analysis, and with taking into account both the surface reactions (or, say, the specifically chemical adsorption) of fluoride ions at the oxide film surface and the migration/intercalation of fluoride ions into the oxide film, the changes in the electrochemical behavior of titanium measured in this work (e.g., the blocked anodic oxygen evolution, the increased anodic steady-state current density, the positively shifted flat band potential, and the positively shifted film breakdown potential) were interpreted by the changes in the surface and the bulk physicochemical properties (e.g., the surface charges, surface state density, doping concentration, and the interfacial potential drops) of the anodic films grown on titanium. The fluoride concentrations tested in this work can be divided into three groups according to their effect on the electrochemical behavior of the oxide films: < or =0.001 M, 0.001-0.01 M, and >0.01 M. By tracing the changes of the OCP of the passivated titanium in fluoride-containing solutions, the deleterious/depassive effect of fluoride ions on the titanium oxide films was examined and evaluated with the parameter of the film breakdown time. It was also shown that the films anodically formed on titanium at higher potentials (>2.5 V) exhibited significantly higher stability against the fluoride attack than that either formed at lower potentials (<2.5 V) or formed natively in the air.
Interfacial functionalization of TiO2 with smart polymers: ph-controlled switching of photocurrent direction
[J].
Heterogeneous p-n junction CdS/Cu2O nanorod arrays: synthesis and superior visible-light-driven photoelectrochemical performance for hydrogen evolution
[J].2O nanorod arrays have been fabricated by using a facile successive ionic-layer adsorption and reaction process to grow Cu2O nanoparticles on the surface of ordered CdS nanorod arrays. The heterogeneous p-n junction nanorod arrays exhibit superior photoelectrochemical performance for hydrogen (H2) generation and high stability under visible-light irradiation. The highest photocurrent density achieved by heterogeneous nanorod array photoelectrode is 4.2 mA cm-2 in a sacrificial Na2S and Na2SO3 mixture electrolyte solution at 0 V versus Ag/AgCl, which is 4 times higher than that of a pure CdS nanorod array photoelectrode. In addition, the heterogeneous nanorod array photoelectrode achieves an incident photon conversion efficiency value of 40.5% at 470 nm. The photocatalytic hydrogen generation rate of the heterogeneous nanorod array photoelectrode reaches up to 161.2 μmol h-1, around 3-fold increase compared to that of a bare CdS photoelectrode. Furthermore, the heterogeneous p-n junction CdS/Cu2O nanorod arrays show an excellent stability under long light illumination of 7200 s. The improved photoelectrochemical performance, photocatalytic activity, and excellent stability of the heterogeneous nanorod array photoelectrode resulted from the efficient separation of photoinduced electron-hole pairs, which is achieved by the synergistic effects of CdS, Cu2O, p-n junction, and an inner electric field in the photoelectrode. The present work provides a new strategy to fabricate a heterogeneous photoelectrode. This facile strategy is expected to be utilized to fabricate electrodes of other materials for highly efficient solar-driven water splitting application.]]>