膜层析技术,是一个新兴且潜力巨大的领域,有广阔的应用前景。膜层析技术在生物制药的纯化环节有重要的作用。膜层析技术的操作简便,有高流速、低传质扩散效应、高载量、低非特异性吸附、经济性和良好的可扩展性等优势。在膜层析技术领域,再生纤维素膜有机械稳定性高、具有生物相容性和生物可降解性等优势[1]。
在再生纤维素膜表面接枝PGMA使这种聚合物的侧链结构富含环氧基团,从而使其具有高度的反应活性。与多种核酸、蛋白质、氨基酸及其他含有活性基团的分子发生共价反应,可在纤维素膜中引入多种功能基团,从而实现对特定目标分子的高选择性捕获和分离。这种功能化策略拓宽了纤维素膜的应用范围,还能显著提高其在生物分离和纯化的性能。例如:进行二乙胺反应可制备阴离子交换膜[16],与4-巯基苯甲酸、正磷酸和硫酸等化合物反应可制备阳离子交换膜[17]。这两种膜均对牛血清白蛋白(BSA)和免疫球蛋白G(IgG)有高pH值依赖性静态容量[18,19],显示出其在生物分离和纯化过程中的优越性能。这些创新性的功能化策略为纤维素膜在分离纯化等领域的广泛应用提供了坚实的基础,进一步巩固其在膜技术领域的领先地位。
本文应用ATRP技术进行聚甲基丙烯酸缩水甘油酯(PGMA)在再生纤维素膜表面的高效接枝,研究引发剂投加量、反应温度、单体及催化剂用量等参数对接枝率的影响。
1 实验方法
1.1 实验用材料
实验用材料有:N,N-二甲基甲酰胺(DMF)、4-二甲氨基吡啶(DMAP)、2-溴异丁酰溴(BIBBr)、三乙胺(TEA)、溴化亚铜(CuBr)、甲基丙烯酸缩水甘油酯(GMA)、N,N,N’,N”,N”-五甲基二乙烯基三胺(PMEDTA)、以及抗坏血酸(AsAc),均为分析纯。再生纤维素膜的孔径为0.45 μm、尺寸为50 mm、厚度160~190 μm。
1.2 固定引发剂
将一定量的DMAP和BIBBr溶于(Schlenk反应瓶中的)20 mL DMF溶液中并通入氮气,将120 mg再生纤维素膜放入30 ℃反应液中并加入TEA中和反应生成的酸。在此条件下反应2 h后将膜取出,用乙醇索提6 h,然后超声水洗(3次,每次10 min)。清洗后将其放入60 ℃干燥箱干燥6 h。在固定引发剂过程中各组分的用量列于表1。
表1 固定引发剂过程中各组分的用量
Table 1
| Sample number | Temperature / oC | DMAP / mmol | BIBBr / mmol | TEA / mL |
|---|---|---|---|---|
| RC-BiB(I1) | 30 | 4.40 | 8.91 | 1.00 |
| RC-BiB(I2) | 13.37 | |||
| RC-BiB(I3) | 17.83 |
1.3 GMA接枝
将一定量的CuBr、GMA、PMEDTA溶于(Schlenk反应瓶中的) 20 mL DMF,通入氮气保护后稳定在一定温度。然后将修饰后的再生纤维素膜放入反应液中,反应3 h后将膜取出,乙醇索提6 h后超声水洗(3次,每次10 min)。清洗后将其放入60 ℃干燥箱干燥6 h。在GMA接枝过程中各组分的用量列于表2。
表2 GMA接枝过程中反应温度和各组分用量
Table 2
| Sample number | Temperature / oC | GMA / mmol | CuBr / mmol | PMEDTA/ mmol |
|---|---|---|---|---|
| RC-BiB(I2N1) | 50 | 17.83 | 0.09 | 0.40 |
| RC-BiB(I2N2) | 60 | 17.83 | 0.09 | |
| RC-BiB(I2N3) | 70 | 17.83 | 0.09 | |
| RC-BiB(I2N4) | 60 | 13.37 | 0.09 | |
| RC-BiB(I2N5) | 60 | 22.28 | 0.09 | |
| RC-BiB(I2N6) | 60 | 17.83 | 0.13 | |
| RC-BiB(I2N7) | 60 | 17.83 | 0.18 |
1.4 用两步接枝法制备PGMA接枝再生纤维素膜
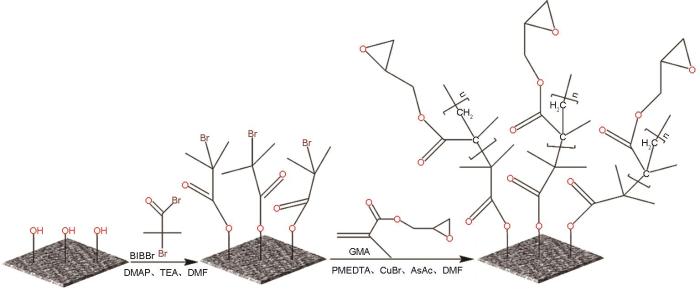
制备PGMA接枝再生纤维素膜过程,在图1中给出。使溴异丁酰溴(BIBBr)引发剂与再生纤维素膜表面丰富羟基进行取代反应,将BIBBr分子固定在膜表面。为了高效接枝,须使用过量的BIBBr。为此,控制BIBBr的量分别为羟基当量的2倍、3倍及4倍,制备出具有不同取代度的样品,以期通过调节引发剂在膜表面的接枝位点密度实现对再生纤维素膜表面PGMA接枝率的精细调控。
图1
图1
基于ATRP技术的再生纤维素膜表面接枝PGMA的技术路线
Fig.1
Technical roadmap for grafting PGMA onto the surface of regenerated cellulose membrane based on ATRP technology
1.5 测定环氧值和接枝率
将改性后的再生纤维素膜置于容积为15 mL的离心管中并加入8 mL的硫代硫酸钠溶液和2~3滴酚酞,将离心管置于30 ℃油浴中搅拌30 min。将膜取出后在室温条件下用移液枪逐滴加入浓度为CHCI S的盐酸,溶液由粉色转为无色后保持30 s,记录消耗的盐酸量VHCl S。
则环氧值为
接枝率为
其中CHCI S为盐酸的浓度,VHCl S为消耗盐酸量,M1为改性后再生纤维素膜的质量,W1为改性前再生纤维素膜的质量;W2为改性后再生纤维素膜的质量。
1.6 表征再生纤维素膜微观结构及表面官能团
用日立SU-8220型冷场发射扫描电子显微镜(SEM)观测再生纤维素膜的微观形貌,并结合SEM内置的能量色散X射线光谱分析(EDS)功能分析再生纤维素膜表面的元素。用傅立叶变换红外光谱仪(FTIR)测定再生纤维素膜的红外光谱。用空气做背景,每个样品扫描4次,扫描范围为500 cm-1~4000 cm-1,分辨率为4 cm-1。
2 实验结果
2.1 样品的傅里叶变换红外光谱和能量散射X射线光谱
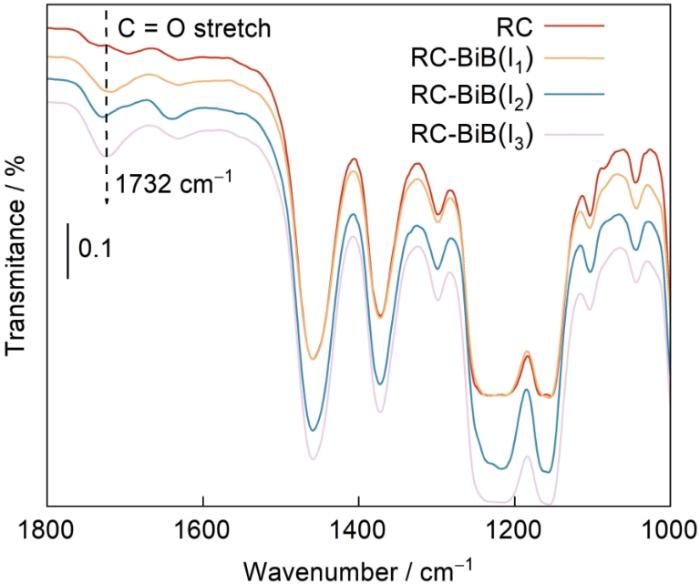
图2给出了样品的傅里叶变换红外光谱(FTIR)。可以看出:与原始样品的光谱对比,在改性后样品红外光谱中的1732 cm-¹处出现了显著的吸收峰。此峰对应2-溴异丁酸基团中羰基的伸缩振动,表明BIBBr已经接枝到再生纤维素膜的表面。随着BIBBr用量的增加(从2倍至4倍羟基当量),上述特征峰的强度呈现出递增趋势。这一结果证实:调整BIBBr的投加量即能调控纤维素膜表面羟基的取代程度,意味着不同取代度影响膜的表面性质和后续接枝的反应活性。
图2
图2
RC、RC- BiB、RC- BiB和RC- BiB的FTIR光谱
Fig.2
FTIR spectra of RC and RC-BiB (I1), RC-BiB (I2) and RC-BiB(I3)
图3给出了RC-BiB样品的SEM照片。可以看出,在接枝处理过程中,高度保留了再生纤维素膜的原有纤维结构。EDS表明,溴元素均匀地覆盖了膜纤维表面,证明了引发基团接枝的有效性。
图3
图3
引发剂固定再生纤维素膜(RC-BiB(I2))的SEM照片和EDS图像
Fig.3
SEM and EDS images of regenerated cellulose membrane (RC-BiB(I2)) fixed with initiator (a) SEM image, (b) EDS image of Br element
2.2 反应温度、单体GMA以及催化剂CuBr用量对PGMA接枝的影响
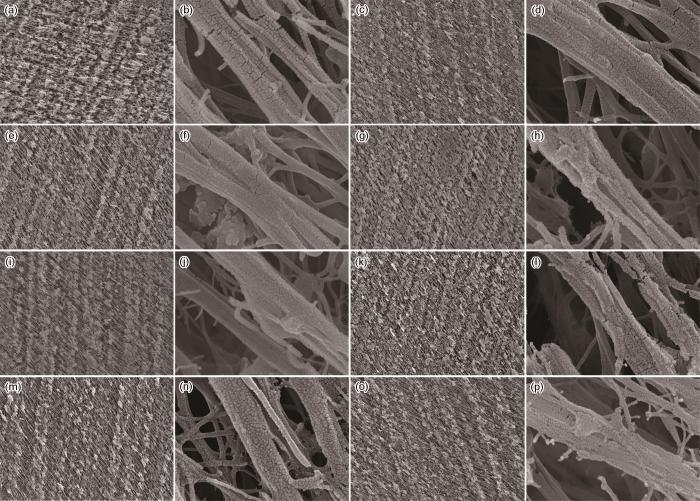
以制备出的RC-BiB(I2)为底物,用ATRP技术对再生纤维素膜进行PGMA接枝改性。进行三因素三水平的正交实验,研究了反应温度、单体GMA及催化剂CuBr用量对接枝效果的影响。图4给出了接枝PGMA前后再生纤维素膜的SEM照片。对比高倍率SEM照片可见,PGMA接枝后的再生纤维素膜表面明显地覆盖了一层颗粒状物质,使膜的表面更加粗糙,表明接枝反应对膜表面形态有显著的影响。低倍率SEM照片表明,PGMA接枝使膜表面更加致密,还良好地保留了膜内原有的孔隙结构,对保持膜材通量等性能极为重要。
图4
图4
接枝PGMA前后再生纤维素膜的SEM照片
Fig.4
SEM images of regenerated cellulose membrane before and after grafting with PGMA (a, b) RC sample, (c, d) RC-BiB(I2N1) sample, (e, f) RC-BiB(I2N2) sample, (g, h) RC-BiB(I2N3) sample, (i, j) RC-BiB(I2N4) sample, (k, l) RC-BiB(I2N5) sample, (m, n) RC-BiB(I2N6) sample, (o, p) RC-BiB(I2N7) sample
图5给出了接枝PGMA前后再生纤维素膜的FTIR光谱。谱中898 cm-1处的吸收峰为C-O的面外弯曲振动峰,是再生纤维素的特征峰(样品RC)。C-O的面外弯曲振动峰使本应出现在910 cm-1的环氧基团对称伸缩振动峰出现在902 cm-¹(样品RC-BiB(I2N2),RC-BiB(I2N5)和RC-BiB(I2N7)。出现在1726 cm-¹处的强而尖锐的羰基伸缩振动峰进一步表明,PGMA分子结构中特有的官能团已接枝到再生纤维素膜上。
图5
图5
接枝PGMA前后再生纤维素膜的FTIR光谱
Fig.5
FTIR spectra of regenerated cellulose membrane before (a) and after (b) grafting with PGMA
3 讨论
表3列出了样品RC-BiB(I2N1)、RC-BiB(I2N2)和RC-BiB(I2N3)的环氧值和接枝率。可以看出:反应温度从50 ℃提高到60 ℃,改性纤维素膜的环氧值和接枝率分别由2.19 umol/g和2.03%显著增至5.79 umol/g和4.99%。这一变化表明,适当提高反应温度能促进ATRP反应的进行和加速自由基的生成和传递,进而提高单体在纤维素链上的接枝效率,使环氧基团的数量及接枝率提高。但是,反应温度进一步提高到70 ℃,环氧值和接枝率轻微下降。这表明:一方面,过高的温度可能加速链转移和链终止反应而使自由基寿命缩短,不利于接枝反应的持续进行;另一方面,过高的温度还引发单体或聚合物链的降解以及催化剂活性的降低或失活,使接枝效果降低。同时,过高的温度可能加剧副反应(例如使自由基间的偶合终止),进一步减少可用于接枝的自由基数量[20]。
表3 PGMA改性再生纤维素膜样品的环氧值和接枝率
Table 3
| RC-BiB(I2N1) | RC-BiB(I2N2) | RC-BiB(I2N3) | RC-BiB(I2N4) | RC-BiB(I2N5) | RC-BiB(I2N6) | RC-BiB(I2N7) | |
|---|---|---|---|---|---|---|---|
| Epoxy value / μmol·g-1 | 2.19 | 5.37 | 5.38 | 2.45 | 5.42 | 8.57 | 12.63 |
| Percent grafting / % | 2.03 | 4.86 | 4.87 | 2.29 | 4.91 | 7.13 | 10.51 |
Epoxy value standard deviation | 0.03 | 0.03 | 0.05 | 0.27 | 0.02 | 0.02 | 0.18 |
Percent grafting standard deviation | 0.05 | 0.03 | 0.09 | 0.03 | 0.06 | 0.13 | 0.25 |
对样品RC-BiB(I2N2)、RC-BiB(I2N4)和RC-BiB(I2N5)的环氧值和接枝率数据的分析表明,适度增加GMA单体的用量能在一定程度上优化接枝效率。GMA单体用量由13.37 mmol (4倍羟基当量)增至17.83 mmol (5倍羟基当量),改性纤维素膜的环氧值和接枝率分别由2.45 umol/g和2.29%大幅增至5.37 umol/g和4.96%;GMA单体用量继续增至22.28 mmol (6倍羟基当量),虽然环氧值有所增大和接枝率有所提高,但是幅度不大。这表明,GMA单体用量超过某一阈值后(在本实验中约为17.83 mmol (5倍羟基当量)),继续增加其用量对提高环氧值和接枝率的作用有限。其原因是:首先,随着GMA单体用量的增加反应体系中GMA与基体材料(如聚合物)之间的碰撞频率提高,从而促进了接枝反应的发生。但是,单体浓度达到一定水平后,反应体系中的活性位点逐渐饱和或空间位阻效应的加剧,使新增的单体分子难以参与接枝反应,从而限制了接枝效率的进一步提高。其次,过高的单体浓度还可能引发均聚反应和消耗部分单体,从而削弱单体用量增加的效果[21]。
ATRP的机理是,利用过渡金属配合物作为催化剂参与氧化还原反应,从而控制聚合过程。因此,选择在ATRP反应体系中催化剂的用量至关重要。样品RC-BiB(I2N5)、RC-BiB(I2N5)和RC-BiB(I2N7)的环氧值和接枝率表明:增加催化剂CuBr的使用量,使环氧值和接枝率均极为显著的上升。其原因是,催化剂用量的增加产生了较多的活性位点,使更多的过渡金属配合物参与自由基的生成和转移。这些增加的活性位点不仅提高了自由基的生成速率,还通过捕捉和稳定自由基减少了自由基之间的偶合使反应终止,从而有利于链的增长。
4 结论
利用ATRP技术可将PGMA接枝到再生纤维素膜上。PGMA接枝层在膜纤维表面形成的粗糙颗粒层使膜致密化,膜内原有的孔隙结构对于保持膜材通量等关键性能至关重要。
参考文献
Ultrasound in cellulose-based hydrogel for biomedical use: from extraction to preparation
[J].
Graft modification of cellulose: methods, properties and applications
[J].
Grafting of regenerated cellulose films with fibrous polymer and modified into phosphate and sulfate groups: application for removal of a model azo-dye
[J].
Surface‐initiated atom transfer radical polymerization of regenerated cellulose membranes with thermo‐responsive properties
[J].
Double stimuli-responsive membranes grafted with block copolymer by ATRP method
[J].
Surface modification of regenerated cellulose membrane based on thiolactone chemistry-a novel platform for mixed mode membrane adsorbers
[J].
Fabrication of dual-responsive cellulose-based membrane via simplified surface-initiated ATRP
[J].
Choline phosphate functionalized cellulose membrane: a potential hemostatic dressing based on a unique bioadhesion mechanism
[J].Wound dressings are a key component in provision of optimal conditions for bleeding control and wound healing. For absorbent dressings, electrostatic interactions are frequently utilized as one of the mechanisms driving dressing adhesion. Herein, a choline phosphate functionalized biocompatible cellulose membrane that can efficiently arrest human red blood cells was developed to have potential application in wound dressing. The bioadhesion is based on the unique multivalent electrostatic interaction between the head groups of phosphatidyl choline based lipids on the cell membrane and its inverse orientation but virtually identical structure, choline phosphate, coupled to the cellulose membrane. For functionalization, the cellulose membrane was decorated with polymer brushes bearing multiple choline phosphate groups via surface-initiator atom transfer radical polymerization followed by click chemistry. The modified cellulose membranes were characterized by ATR-FTIR and the molecular weight and the grafting density of polymer brushes grafted from the cellulose membrane surface were thoroughly evaluated by calibrated force-distance measurements with atomic force microscopy (AFM). This new method provides an approach to estimating polymer brush parameters on rough surfaces of unknown surface area based on the dependence of brush thickness on brush density and polymer molecular weight for a calibration set of brushes. The dependence of binding of human red blood cells (RBCs) to the cellulose membrane surface on the number density of choline phosphate groups (e.g. molecular weight) and the grafting density were investigated using this AFM-based approach. Bound RBCs showed "pseudopodia"-like membrane projections under scanning electron microscopy where cells contacted the microfibers of the cellulose, distorting the RBC shape, reflecting the multivalent interactions between the RBCs and the choline phosphate-doped cellulose membrane. We believe this efficient strategy provides a promising approach to blood conservation and trauma management.Uncontrolled bleeding can dramatically affect morbidity and mortality. Absorptive wound dressings provide either adherent or non-adherent layers to control bleeding. Our new adherent material is based on a universal adhesion reaction between cell membrane phosphatidyl choline (PC) headgroups and cellulose membranes (CM) decorated with polymer brushes carrying a CP group per monomer. The CP-PC multivalent interactions provide adherence to cut tissue margins and blood cells, blocking bleeding. We here demonstrate the strong specific binding of red cells to CM-CP but not CM-PC membranes and determine the requisite brush molecular weight and surface concentration via a new approach using atomic force microscopy, applicable to rough surfaces. We believe this strategy provides a promising approach to blood conservation and trauma management.Copyright © 2016. Published by Elsevier Ltd.
Electrochemically mediated atom transfer radical polymerization (eATRP)
[J].
Kinetics of atom transfer radical polymerization
[J].
Atom transfer radical polymerization: from mechanisms to applications
[J].
A new protocol for ash wood modification: synthesis of hydrophobic and antibacterial brushes from the wood surface
[J].
Modification of wood-based materials by atom transfer radical polymerization methods
[J].
Hydrophobic modification of fir wood surface via low ppm ATRP strategy
[J].
Grafting polymers from cellulose nanocrystals via surface‐initiated atom transfer radical polymerization
[J].
Using diethylamine as crosslinking agent for getting polyepichlorohydrin-based composite membrane with high tensile strength and good chemical stability
[J].
Development of multimodal membrane adsorbers for antibody purification using atom transfer radical polymerization
[J].
Preparation of high-capacity, weak anion-exchange membranes by surface-initiated atom transfer radical polymerization of poly (glycidyl methacrylate) and subsequent derivatization with diethylamine
[J].
A new multimodal membrane adsorber for monoclonal antibody purifications
[J].
Cu-mediated butadiene ATRP
[J].
Effects of solvent and monomer on the kinetics of radical generation in atom transfer radical polymerization
[J].
Toward green atom transfer radical polymerization: current status and future challenges
[J].
Following principles of green chemistry: low ppm photo-ATRP of DMAEMA in water/ethanol mixture
[J].
Ultralow ppm seATRP synthesis of PEO-b-PBA copolymers
[J].