ZICs是一种不对称电容器,使用电池电极材料可提高其能量密度。但是与锌离子电池(ZIBs)相比,ZICs的能量密度还可进一步提高。根据“短板效应”,电容电极材料的容量是影响其能量密度的关键因素。目前常见的电容电极是炭材料[12~15],研发具有高比容量的电容电极材料,对于开发高比能量的ZICs有重要的意义。金属有机骨架(MOFs)材料是一种超高孔隙率和巨大比表面积的多孔材料,有丰富的微/介孔结构。微/介孔结构的比表面积较大,其孔尺寸可调和有金属活性中心,是超级电容器极有前景的电极材料[16]。MOFs衍生层状双金属氢氧化物(LDH)具有复杂的组分和多种金属间的协同作用,其电化学活性远高于单一金属氢氧化物[17]。目前限制LDH用于电容电极材料的主要影响因素,是其较低的电导率和活性物质易团聚。碳材料如石墨烯(rGO)、碳纳米管等,其电导率较高和成本较低,将其与LDH复合可制备高性能复合电极材料[18,19]。有人用液相法合成的Ni-Al LDH/rGO复合材料,其比电容(629.8 F·g-1 at 1 A·g-1)比LDH 材料(517.8 F·g-1 at 1 A·g-1)的高[8]。但是,MOFs衍生复合材料用于ZICs使用锌离子水溶液为电解液时二价锌离子在水合离子尺寸、电荷携带量、扩散动力学等方面与H+、Na+、K+等一价离子有很大的差异。同时,对水系ZICs储能机制还有待深入认识。MOFs衍生复合电极材料中的导电炭黑和粘结剂使其在充放电过程中电极物相易受干扰。本文合成具有自支撑特性的MOFs衍生C/Ni-Co LDH/rGO网状复合材料,用这种材料作电容电极组装水系锌离子电池(ZICs)并研究其性能。
1 实验方法
1.1 实验用试剂和设备仪器
实验用试剂:六水合硝酸钴、2-甲基咪唑、甲醇、无水乙醇和硝酸镍(均为分析纯)。
实验用设备仪器:电子天平(FA2004),电热鼓风干燥箱(DGG-9030BD),高速离心机(HC-3018),管式炉(SK-1200),冷冻干燥机(FD-1A-50),电化学工作站 (CHI660E)。
1.2 MOFs衍生C/LDH/rGO网状复合材料和rGO电极材料的制备
将2 mmol CO(NO3)2·6H2O(1.164 g)溶到50 mL甲醇溶液中,然后加入溶有8 mmol 2-甲基咪唑(1.314 g)的50 mL甲醇溶液,用磁力搅拌器搅拌0.5 h后静置24 h。反应结束后收集沉淀并用无水乙醇离心洗涤,然后将产物在60 ℃恒温干燥箱中干燥12 h制备出ZIF-67单体。将ZIF-67单体放入400 ℃管式炉中在N2气氛中碳化2 h,得到MOFs衍生C/ZIF-67。升温速率为2 ℃·min-1。
将制得的MOFs衍生C/ZIF-67样品溶解在50 mL乙醇溶液中,超声1 h后再溶解180 mg的Ni(NO3)2·6H2O,磁力搅拌0.5 h。然后进行回流操作,将温度升高到90 ℃反应1 h。反应完成后用无水乙醇充分洗涤沉淀,然后将其放入60 ℃烘箱中干燥12 h,得到MOFs衍生C/Ni-Co LDH。
将用Hummers法制备的氧化石墨粉末[20]分散在水中,超声波处理2 h后得到氧化石墨烯悬浮液(GO,2 mg/mL)。将MOFs衍生C/Ni Co LDH粒子与GO水溶液在170 ℃水热反应5 h,反应完成后取出产物并将其在真空冻干机里冻干48 h,得到MOFs衍生C/LDH/rGO网状复合材料。
将50 mL的GO水溶液在170 ℃水热反应5 h,反应完成后取出产物并将其在真空冻干机里冻干48 h,得到rGO电极材料。
1.3 以MOFs衍生C/LDH/rGO网状复合材料为正极的锌离子电容器的组装和性能测试
以MOFs衍生C/LDH/rGO网状复合材料为正极材料、锌箔为负极、0.5 mol/L硫酸锌为电解液,组装锌离子电容器。为了比较,以rGO为正极材料制备锌离子电容器。根据恒流充放电曲线(CP)比电容(F·g-1)为
式中C是比容量,I是电流,V是电位窗口,t是放电时间,m是正极的质量。
2 结果和讨论
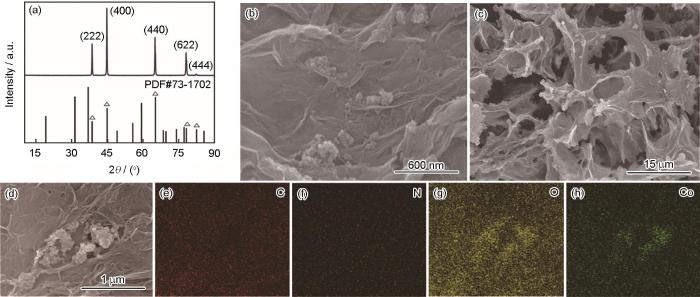
图1为MOFs衍生C/LDH/rGO网状复合材料的制备流程,图2a为其XRD谱。谱中38.3°、44.8°、65.3°、78.1°、88°处的衍射峰分别对应PDF#73-1702卡片(222) (400) (440) (622) (444)晶面,其它成分,如石墨烯、MOFs衍生碳为无定型的,在XRD谱中没有衍射峰[21]。图2b~d给出了MOFs衍生复合材料不同放大倍数的SEM照片,可见这种复合材料呈现网络状结构。这可能是氧化石墨烯的水热过程中自组装成了片层结构,在片层表面包裹一些颗粒状MOFs衍生C/Ni-Co LDH粒子。图2e~h给出了MOFs衍生复合材料的能谱。可以看出,这种复合材料含有元素C、N、O、Co等元素,均匀地分散在复合材料中,元素Ni的含量较低观测不到。
图1
图1
MOFs衍生C/LDH/rGO网状复合材料的制备流程
Fig.1
Preparation flow chart of MOFs derived C/LDH/rGO network composite materials
图2
图2
MOFs衍生C/LDH/rGO网状复合材料的XRD谱、SEM图和能谱图
Fig.2
XRD (a), SEM (b~d) and EDS mapping images (e~h) of MOFs derived C/LDH/rGO network composite materials
可用XPS检测复合材料中的元素和元素的化学环境。图3a为MOFs衍生复合材料的XPS全谱,谱中的附表为复合材料中元素的原子分数,还可见复合材料中主要有Co、O、C、N、Ni五种元素。图3b给出了C 1s的XPS谱,谱中有四种不同类型分别位于282.8、283.15、284.5和287.4 eV的碳峰:C―C、C=C、C―O和C=O[22];图3c给出了Co 2p的XPS谱,780.4 eV和784.2 eV是热解反应后氧化过程中产生的Co2+和Co3+的峰,表明在复合材料中有Co3+与Co2+ [23];在O 1s的XPS谱(图3d)中282.9 eV、283.16 eV处出现两个峰,分别对应M―O―M的晶格氧和M―O―H的羟基氧。
图3
图3
MOFs衍生C/LDH/rGO网状复合材料的XPS谱图
Fig.3
XPS spectra of MOFs derived C/LDH/rGO network composite materials
(a) full spectrum, (b) C 1s spectrum, (c) Co 2p spectrum, (d) O 1s spectrum
图4a为用MOFs衍生C/LDH/rGO网状复合材料组装的锌离子电容器的反应过程模拟,图4b给出了这种锌离子电容器在不同扫描速度下的CV图。可以看出,四条曲线均出现氧化还原峰,表明电极材料发生了赝电容反应。从图4a可见,电压提高时溶液中的阴离子SO
图4
图4
用MOFs衍生C/LDH/rGO网状复合材料组装成的锌离子电容器
Fig.4
Zinc ion capacitor assembled by MOFs derived C /LDH /rGO network composite materials
(a) Reaction process simulation diagram, (b) CV curves under different scanning rate, (c) Capacitance contribution CV curve at a scanning rate of 5 mV/s, (d) The proportion of surface-controlled capacitance contribution and diffusion-controlled contribution at different scanning rates, (e) CP curves at different current densities, (f) The relationship between electrode specific capacity and current densities
和不同扫描速率的CV曲线可计算电容贡献 [25]。两个方程中的I(mA)为固定电位下的电流,v(mV/s)为扫描速率,k1v和k2v1∕2分别为电容贡献和扩散贡献。
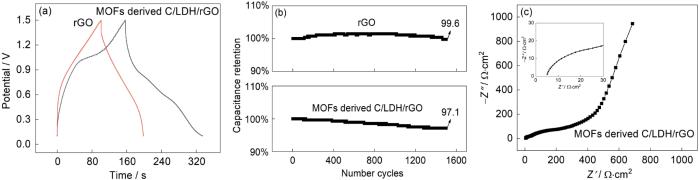
图5
图5
用MOFs衍生C/LDH/rGO网状复合材料和rGO分别组装的锌离子电容器性能的对比
Fig.5
Comparison of zinc ion capacitors assembled by MOFs derived C/LDH/rGO network composite materials and rGO
(a) CP curves of two devices (current density of 1 A/g), (b) Cycle life diagram of two devices, (c) EIS curves of zinc ion capacitors assembled by MOFs derived C/LDH/rGO network composite materials
3 结论
用液相法结合煅烧可制备MOFs衍生C/ZIF-67,再用质子刻蚀可制备MOFs衍生C/Ni-Co LDH粒子,用水热辅助冻干法可制备MOFs衍生C/LDH/rGO网状复合材料。用该材料组装的锌离子电容器循环1500次后其电容保留率仍高达97.1%。其原因是:MOFs衍生C/LDH/rGO网状复合材料的网状结构具有自支撑特性,能为电解液离子提供更多的传输通道和赝电容活性位点,其电容量可达248 F·g-1。
参考文献
Thickness-independent capacitance of vertically aligned liquid-crystalline MXenes
[J].
A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode
[J].
Molecular understanding of charge storage and charging dynamics in supercapacitors with MOF electrodes and ionic liquid electrolytes
[J].We performed constant-potential molecular dynamics simulations to analyse the double-layer structure and capacitive performance of supercapacitors composed of conductive metal-organic framework (MOF) electrodes and ionic liquids. The molecular modelling clarifies how ions transport and reside inside polarized porous MOFs, and then predicts the corresponding potential-dependent capacitance in characteristic shapes. The transmission line model was adopted to characterize the charging dynamics, which further allowed evaluation of the capacitive performance of this class of supercapacitors at the macroscale from the simulation-obtained data at the nanoscale. These 'computational microscopy' results were supported by macroscopic electrochemical measurements. Such a combined nanoscale-to-macroscale investigation demonstrates the potential of MOF supercapacitors for achieving unprecedentedly high volumetric energy and power densities. It gives molecular insights into preferred structures of MOFs for accomplishing consistent performance with optimal energy-power balance, providing a blueprint for future characterization and design of these new supercapacitor systems.
Preparation and electrochemical properties of hollow FeS2/NiS2/Ni3S2@NC cube composites
[J].
中空FeS2/NiS2/Ni3S2@NC立方体复合材料的制备及其电化学性能
[J].
An ultrafast rechargeable aluminium-ion battery
[J].
Materials for electrochemical capacitors
[J].Electrochemical capacitors, also called supercapacitors, store energy using either ion adsorption (electrochemical double layer capacitors) or fast surface redox reactions (pseudo-capacitors). They can complement or replace batteries in electrical energy storage and harvesting applications, when high power delivery or uptake is needed. A notable improvement in performance has been achieved through recent advances in understanding charge storage mechanisms and the development of advanced nanostructured materials. The discovery that ion desolvation occurs in pores smaller than the solvated ions has led to higher capacitance for electrochemical double layer capacitors using carbon electrodes with subnanometre pores, and opened the door to designing high-energy density devices using a variety of electrolytes. Combination of pseudo-capacitive nanomaterials, including oxides, nitrides and polymers, with the latest generation of nanostructured lithium electrodes has brought the energy density of electrochemical capacitors closer to that of batteries. The use of carbon nanotubes has further advanced micro-electrochemical capacitors, enabling flexible and adaptable devices to be made. Mathematical modelling and simulation will be the key to success in designing tomorrow's high-energy and high-power devices.
Review of hybrid ion capacitors: from aqueous to lithium to sodium
[J].
Challenges in the development of advanced Li-ion batteries: a review
[J].
Recent developments and future prospects for zinc‐ion hybrid capacitors: a review
[J].
Recent advances in aqueous zinc-ion batteries
[J].
Issues and opportunities facing aqueous zinc-ion batteries
[J].
2D metal Zn nanostructure electrodes for high‐performance Zn ion supercapacitors
[J].
Carbon-based supercapacitors produced by activation of graphene
[J].Supercapacitors, also called ultracapacitors or electrochemical capacitors, store electrical charge on high-surface-area conducting materials. Their widespread use is limited by their low energy storage density and relatively high effective series resistance. Using chemical activation of exfoliated graphite oxide, we synthesized a porous carbon with a Brunauer-Emmett-Teller surface area of up to 3100 square meters per gram, a high electrical conductivity, and a low oxygen and hydrogen content. This sp(2)-bonded carbon has a continuous three-dimensional network of highly curved, atom-thick walls that form primarily 0.6- to 5-nanometer-width pores. Two-electrode supercapacitor cells constructed with this carbon yielded high values of gravimetric capacitance and energy density with organic and ionic liquid electrolytes. The processes used to make this carbon are readily scalable to industrial levels.
Three-dimensional porous carbon framework coated with one-dimensional nanostructured polyaniline nanowires composite for high performance supercapacitors
[J].
Materials for electrochemical capacitors
[J].Electrochemical capacitors, also called supercapacitors, store energy using either ion adsorption (electrochemical double layer capacitors) or fast surface redox reactions (pseudo-capacitors). They can complement or replace batteries in electrical energy storage and harvesting applications, when high power delivery or uptake is needed. A notable improvement in performance has been achieved through recent advances in understanding charge storage mechanisms and the development of advanced nanostructured materials. The discovery that ion desolvation occurs in pores smaller than the solvated ions has led to higher capacitance for electrochemical double layer capacitors using carbon electrodes with subnanometre pores, and opened the door to designing high-energy density devices using a variety of electrolytes. Combination of pseudo-capacitive nanomaterials, including oxides, nitrides and polymers, with the latest generation of nanostructured lithium electrodes has brought the energy density of electrochemical capacitors closer to that of batteries. The use of carbon nanotubes has further advanced micro-electrochemical capacitors, enabling flexible and adaptable devices to be made. Mathematical modelling and simulation will be the key to success in designing tomorrow's high-energy and high-power devices.
Covalent graphene-MOF hybrids for high-performance asymmetric supercapacitors
[J].
In Situ Growth of layered bimetallic ZnCo hydroxide nanosheets for high-performance all-solid-state pseudocapacitor
[J].
Kirkendall growth and ostwald ripening induced hierarchical morphology of Ni-Co LDH/MMoSx (M = Co, Ni, and Zn) heteronanostructures as advanced electrode materials for asymmetric solid-state supercapacitors
[J].
Rationally designed ultrathin Ni-Al layered double hydroxide and graphene heterostructure for high-performance asymmetric supercapacitor
[J].
Preparation of graphitic oxide
[J].
of MOF derivatives@Synthesis3D graphene hybrid materials towards high-performance electrode material for supercapacitors
[J].
A simple one-step approach for preparing flexible rGO-MnO2 electrode material
[J].
A metal-organic framework template derived hierarchical Mo-doped LDHs@ MOF-Se core-shell array electrode for supercapacitors
[J].
Covalent graphene‐MOF hybrids for high‐performance asymmetric supercapacitors
[J].
Ordered mesoporous α-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors
[J].Capacitive energy storage is distinguished from other types of electrochemical energy storage by short charging times and the ability to deliver significantly more power than batteries. A key limitation to this technology is its low energy density and for this reason there is considerable interest in exploring pseudocapacitive materials where faradaic mechanisms offer increased levels of energy storage. Here we show that the capacitive charge-storage properties of mesoporous films of iso-oriented alpha-MoO(3) are superior to those of either mesoporous amorphous material or non-porous crystalline MoO(3). Whereas both crystalline and amorphous mesoporous materials show redox pseudocapacitance, the iso-oriented layered crystalline domains enable lithium ions to be inserted into the van der Waals gaps of the alpha-MoO(3). We propose that this extra contribution arises from an intercalation pseudocapacitance, which occurs on the same timescale as redox pseudocapacitance. The result is increased charge-storage capacity without compromising charge/discharge kinetics in mesoporous crystalline MoO(3).