二维层状ZnNiAl-LDH 负载氧化亚铜光催化剂的制备及其降解性能
郭智楠 1 , 赵强 , 1 , 2 , 李淑英 1 , 3 , 王俊丽 1 , 3 , 许琳 1 , 尚建鹏 1 , 2 , 郭永 1 , 2
1.山西大同大学化学与化工学院 大同 037009
2.山西省清洁能源材料联合实验室 大同 037009
3.煤基生态碳汇技术教育部工程研究中心 大同 037009
Preparation and Degradation Performance of Composite Photocatalyst of Two-Dimensional Layered ZnNiAl-LDH/ Cuprous Oxide Particles
GUO Zhinan 1 , ZHAO Qiang , 1 , 2 , LI Shuying 1 , 3 , WANG Junli 1 , 3 , XU Lin 1 , SHANG Jianpeng 1 , 2 , GUO Yong 1 , 2
1.School of Chemistry and Chemical Engineering, Shanxi Datong University, Datong 037009, China
2.Engineering Research Center of Coal-based Ecological Carbon Sequestration Technology of the Ministry of Education, Datong 037009, China
3.Shanxi Province Union laboratory of Clean Energy Materials, Shanxi Datong University, Datong 037009, China
通讯作者: 赵 强,教授,zhaoqiangtyla@126.com ,研究方向为光电降解污染物
收稿日期: 2023-07-03
修回日期: 2024-01-20
基金资助:
国家自然科学基金 (21908135 )山西省自然科学基金 (201901D111308,201901D211435,201801D221057 )山西省留学回国人员科技活动项目择优资助 (2019-20 )山西大同大学博士科研启动基金 (2018-B-01,2020-B-02 )山西大同大学研究生创新基金 (21CX22,22CX17 )山西省大学生创新创业训练计划项目 (20220807,2016172 )
Corresponding authors: ZHAO Qiang, Tel: 15934234565 , E-mail:zhaoqiangtyla@126.com
Received: 2023-07-03
Revised: 2024-01-20
Fund supported:
National Natural Science Foundation of China (21908135 )Natural Science Foundation of Shanxi Province (201901D111308,201901D211435,201801D221057 )Overseas Students Science and Technology Activities Project Merit Funding of Shanxi Province (2019-20 )PhD Research Startup Foundation of Shanxi Datong Univeisity (2018-B-01,2020-B-02 )Postgraduate Education Innovation Project of Shanxi Datong University (21CX22,22CX17 )Shanxi Province Innovation and Entrepreneurship Training Program for College Students (20220807,2016172 )
摘要
用沉淀法将ZnNiAl-LDH掺杂在Cu2 O中制备出一种可见光光催化剂。用这种催化剂可见光降解四环素(TC),研究其光催化性能。结果表明,这种ZnNiAl-LDH/Cu2 O的催化活性比纯Cu2 O的更高,掺杂7%ZnNiAl-LDH的ZnNiAl-LDH/Cu2 O光催化剂其降解活性最优,在50 min内能将TC降解89.6%。这表明,ZnNiAl-LDH/Cu2 O催化剂对TC的光催化降解活性较高。7%ZnNiAl-LDH/Cu2 O具有较高光降解效率的原因是,在Cu2 O与ZnNiAl-LDH之间高效的界面电荷转移和协同作用提高了光生电子空穴对的分离效率。
关键词:
无机非金属材料 沉淀法 Cu2 O ZnNiAl-LDH 四环素 光降解性能
Abstract
A highly active and stable visible light photocatalyst of composite ZnNiAl-LDH/Cu2 O was successfully prepared by using co-precipitation method to depositing two-dimensional layered ZnNiAl-ZnNiAl-LDH on Cu2 O particles. The photocatalytic activity of the prepared composite catalyst was evaluated by the degradation of tetracycline (TC) under visible light. It is found that the developed ZnNiAl-LDH/Cu2 O exhibited higher activity than the pure Cu2 O, while the ZnNiAl-LDH/Cu2 O photocatalyst doped with 7% of ZnNiAl-LDH exhibited the highest photodegradation activity, by which 89.6% of TC was decomposed within 50 minutes. The ZnNiAl-LDH/Cu2 O photocatalyst present considerably high photocatalytic degradation activities on TC. The high photodegradation efficiency of 7%ZnNiAl-LDH/Cu2 O could be ascribed to the efficient interfacial charge transfer at the composite and the synergistic effect between Cu2 O and ZnNiAl-LDH, which resulted in the enhanced separation efficiency of photogenerated electron-hole pairs.
Keywords:
inorganic non-metallic materials precipitation method cuprous oxide ZnNiAl-LDH tetracycline photodegradation property
本文引用格式
郭智楠, 赵强, 李淑英, 王俊丽, 许琳, 尚建鹏, 郭永. 二维层状ZnNiAl-LDH 负载氧化亚铜光催化剂的制备及其降解性能 [J]. 材料研究学报 , 2024, 38(6): 423-429 DOI:10.11901/1005.3093.2023.328
GUO Zhinan, ZHAO Qiang, LI Shuying, WANG Junli, XU Lin, SHANG Jianpeng, GUO Yong. Preparation and Degradation Performance of Composite Photocatalyst of Two-Dimensional Layered ZnNiAl-LDH/ Cuprous Oxide Particles [J]. Chinese Journal of Materials Research 10.11901/1005.3093.2023.328
目前,环境和能源问题已经成为影响人类生存的难题,急待解决。因此,开发清洁的环境净化技术和无污染、可持续的能源迫在眉睫。
光催化技术有重要的应用价值[1 ,2 ] 。光催化的基本原理是:在光照下纳米半导体材料产生的具有较强氧化活性的基团能降解水体中的有机物[3 ] 。太阳光取之不尽用之不竭[4 ] ,节能又绿色环保。因此,研究太阳光催化有重要的意义。
纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] 。在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] 。这表明,Cu2 O光催化剂有广阔的应用前景[8 ] 。但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] 。为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合。
ZnNiAl-LDH二维层状三金属氢氧化物是一种比表面积较大的多孔材料,有较强的吸附能力和优良的导电性能[22 ] 。纳米级金属氧化物的氧化能力比普通粒径的材料强,因此其催化性能更高。但是,这类材料放入水中使用后很难回收且容易团聚,其光生电子-空穴对还容易复合。将其与LDHs材料复合,可增大催化剂的比表面积并使光生电子迁移到LDHs材料表面与氧分子反应生成超氧自由基(·O2 - ) 。光生空穴与表面羟基离子或水反应产生的羟基自由基(·OH)都有很强的氧化活性,能促进光生电子-空穴对的分离而使其催化性能大大提高。因此,LDHs是一种很适合作为纳米级光催化剂载体的材料[23 ] 。将纳米ZnO负载在LDHs材料上,ZnO能均匀地分布在LDHs材料的周围而不容易团聚,可提高其催化性能和易于回收[24 ] 。鉴于此,本文将Cu2 O负载在不同含量的ZnNiAl-LDH上制备ZnNiAl-LDH/Cu2 O复合光催化剂,研究其在模拟太阳光照条件下对四环素(TC)的降解性能。
1 实验方法
1.1 实验用试剂和仪器
实验用试剂:无水乙醇,氯化铜,氢氧化钠,盐酸羟胺,ZnNiAl-LDH以及四环素,均为分析纯。
实验用仪器:电子天平,DF-101S集热式恒温加热磁力搅拌器,HC-2518高速离心机,SB-5200 DTDN超声波清洗机,DGX-9073B-1电热恒温鼓风干燥箱, 氙灯光源系统,可见分光光度计。
1.2 催化剂的制备
Cu2 O催化剂的制备:将83.4 mL H2 O、5 mL CuCl2 (0.5 mol/L)和20 mL C2 H5 OH依次置于500 mL的烧杯中,在40℃搅拌30 min后滴加30 mL C2 H5 OH (3~4 s/d)和9 mL NaOH (1.0 mol/L、6~7 s/d)。然后迅速加入9.8 mL NH2 OH·HCl (0.5 mol/L)搅拌10 min,静置3 h后高速离心,用体积比为1∶1的乙醇水溶液充分洗涤沉淀,将其在40℃真空干燥24 h即得Cu2 O催化剂。
7%ZnNiAl-LDH/Cu2 O的制备:将Cu2 O(0.092 g)、ZnNiAl-LDH (0.0065 g)和无水乙醇(10 mL)置于试剂瓶中超声60 min,然后在40℃真空干燥24 h,即得到7%ZnNiAl-LDH/Cu2 O复合光催化剂。用相同的方法制备1%、3%、5%、10%ZnNiAl-LDH/Cu2 O复合光催化剂。
1.3 光催化剂性能的表征
用日本理学D/max 2500型粉末X射线衍射(XRD)仪表征光催化剂的物相。用扫描电子显微镜(SEM,TESCAN MAIA 3 LMH),透射电子显微镜(FEI-G20)和高分辨率透射电镜(HRTEM,JEOL,JEM-29999FMII apparatus)观察了样品的形貌。用ASAP-2020 PLUS HD88比表面孔径分析仪测试样品的N2 吸附-脱附等温线。用X射线光电子能谱(XPS,ESCACAB 2S0Xi,Fermo Fisher Scientific)分析样品的元素组成。在可见光下,在含有Na2 SO4 (0.2 mol/L)的溶液中测试光电流响应,在含有KCl (0.1 mol/L)和[Fe(CN)6 ]3-/4- (2.5 mmol/L)的溶液中测试电化学阻抗谱(EIS)。
将100 mg 光催化剂置于100 mL的夹层石英杯中,加入90 mL TC(30 mg/L)溶液后搅拌进行暗反应30 min。选择λ max 为373 nm测TC的吸光度A0 ,待暗反应结束取4 mL试样将其离心5 min后测试吸光度。然后在光照下进行反应,每隔10 min测一次吸光度以计算四环素质量浓度和降解率。
2 结果和讨论
2.1 催化剂的物相和形貌
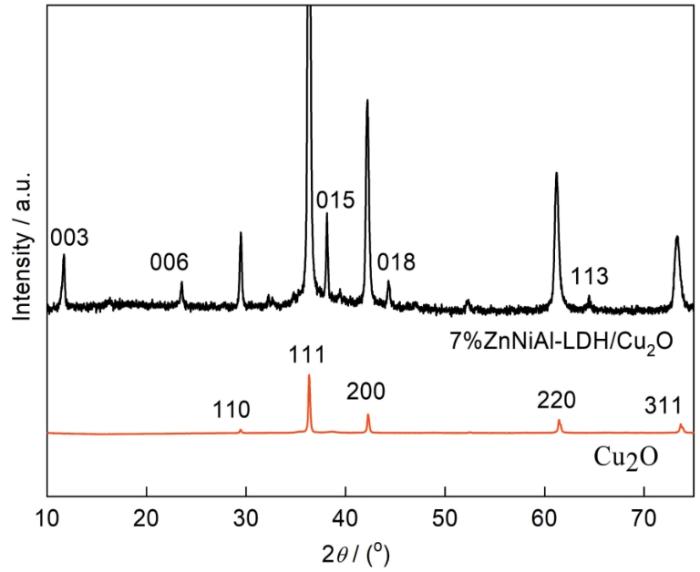
图1 给出了光催化剂的XRD谱。谱中位于2θ 为29.3°、36.2°、42.1°、61.3°和73.4°的衍射峰对应Cu2 O的(110),(111),(200),(220)和(311)晶面,位于2θ 为11.4°、23.7°、36.5°、42.5°和61.3°的峰对应ZnNiAl-LDH的(003)、(006)、(015)、(018)、(113)晶面。这表明,在制备过程中ZnNiAl-LDH的添加并没有影响Cu2 O十二面体晶型的形成。
图1
图1
Cu2 O和7%ZnNiAl-LDH/Cu2 O的XRD谱
Fig.1
XRD patterns of Cu2 O and 7%ZnNiAl/Cu2 O composite
图2 a、b给出了7%ZnNiAl-LDH/Cu2 O光催化剂的扫描电镜照片,可见氧化亚铜具有菱方十二面体结构,ZnNiAl-LDH具有层状结构,ZnNiAl-LDH较为均匀地分布在Cu2 O周围。
图2
图2
7%ZnNiAl-LDH/Cu2 O的SEM照片
Fig.2
SEM images of 7%ZnNiAl-LDH/Cu2 O composite
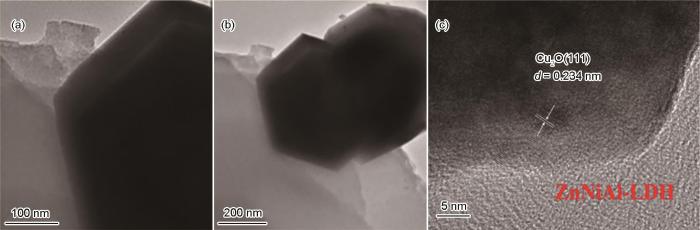
图3 给出了7%ZnNiAl-LDH/Cu2 O光催化剂的TEM和HRTEM表征图像。从图3 a、b可见Cu2 O具有菱方十二面体结构,ZnNiAl-LDH具有层状结构,ZnNiAl-LDH比较均匀地分布在Cu2 O的周围。从图3 c可观察到Cu2 O的(111)晶面,晶面间距为0.234 nm。
图3
图3
7%ZnNiAl-LDH/Cu2 O的TEM照片和HRTEM图像
Fig.3
TEM images of 7%ZnNiAl-LDH/Cu2 O composite (a, b) and HRTEM image of 7%ZnNiAl-LDH/Cu2 O composite (c)
2.2 催化剂的组成和光吸收性能
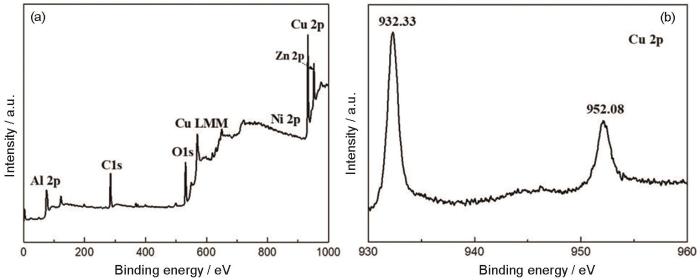
图4 给出了催化剂的XPS谱。图4 a给出了7%ZnNiAl-LDH/Cu2 O光催化剂的XPS总谱,图4 b给出了Cu 2p的XPS谱,谱中位于932.33 eV和952.08 eV的峰对应Cu 2p3/2和Cu 2p1/2。在932.33 eV和952.08 eV处出现的特征峰,表明Cu2 O存在于光催化剂中。
图4
图4
7%ZnNiAl-LDH/Cu2 O的XPS谱
Fig.4
XPS analysis for 7%ZnNiAl-LDH/Cu2 O composite (a) survey spectrum; (b) Cu 2p XPS spectrum
图5 a给出了纯Cu2 O和7%ZnNiAl-LDH/Cu2 O的紫外-可见漫反射吸收光谱(UV-Vis DRS)。可以看出,两种催化剂样品在380 nm~700 nm范围内均有光吸收。由图5 a可见,Cu2 O和7%ZnNiAl-LDH/Cu2 O光吸收边缘都位于630 nm,表明ZnNiAl-LDH的加入没有显著改变7%ZnNiAl-LDH/Cu2 O的光吸收性能。根据图5 b可计算出Cu2 O和7%ZnNiAl-LDH/Cu2 O的禁带宽度分别为1.98 eV、1.91 eV。这表明,ZnNiAl-LDH与Cu2 O复合降低了禁带宽度,提高了对可见光的吸收性能。
图5
图5
Cu2 O和7% ZnNiAl-LDH/Cu2 O的UV-vis DRS谱和(αhν )2 与光子能量(hν )的关系
Fig.5
UV-vis DRS spectra (a) and optical absorption edges (b) of Cu2 O and 7%ZnNiAl-LDH/Cu2 O composite
2.3 催化剂的比表面积和孔结构
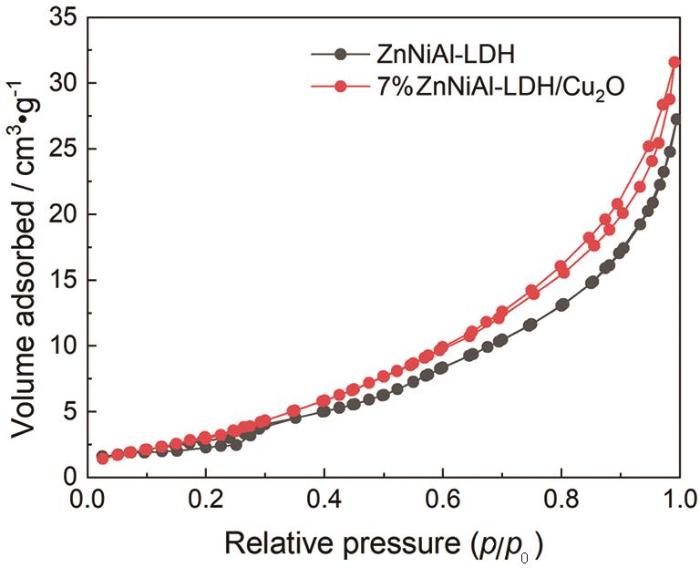
表1 和图6 给出了催化剂的比表面积和孔结构分析。由表1 可见,ZnNiAl-LDH的加入提高了光催化剂的比表面积,表明复合后的催化材料表面的活性位点更多,使其光催化降解性能提高。
图6
图6
ZnNiAl-LDH和7% ZnNiAl-LDH/Cu2 O氮气吸附-脱附等温线
Fig.6
Nitrogen adsorptiondesorption isotherms of ZnNiAl-LDH and 7% ZnNiAl-LDH/Cu2 O
2.4 光电流响应谱和电化学阻抗谱
图7 给出了Cu2 O,7%ZnNiAl-LDH/Cu2 O和ZnNiAl-LDH样品的瞬时光电流响应和电化学阻抗。图7 a给出了催化剂样品的光电流响应谱,可见ZnNiAl-LDH样品对光电流信号没有明显的响应,而Cu2 O和7%ZnNiAl-LDH/Cu2 O样品均表现出明显的光电流信号,表明ZnNiAl-LDH的加入提高了催化剂的光催化活性。同时,7%ZnNiAl-LDH/Cu2 O样品的光电流强度明显高于纯Cu2 O的光电流强度,表明ZnNiAl-LDH的加入提高了导电性和分离光生电子和空穴的性能。图7 b给出了Cu2 O,7%ZnNiAl-LDH/Cu2 O和ZnNiAl-LDH样品在氙灯照射下的电化学阻抗谱。可以看出,7%ZnNiAl-LDH/Cu2 O复合材料的圆半径小于Cu2 O,表明这种复合材料的界面电荷电子转移速率比Cu2 O的大,ZnNiAl-LDH/Cu2 O复合材料比Cu2 O的光致载流子的生成和转移能力更强。
图7
图7
催化剂样品的光电流响应曲线和电化学阻抗谱图
Fig.7
Photocurrent time dependence curves (a) and EIS Nyquist plots of catalyts (b)
2.5 光催化剂的光降解性能和光催化机理
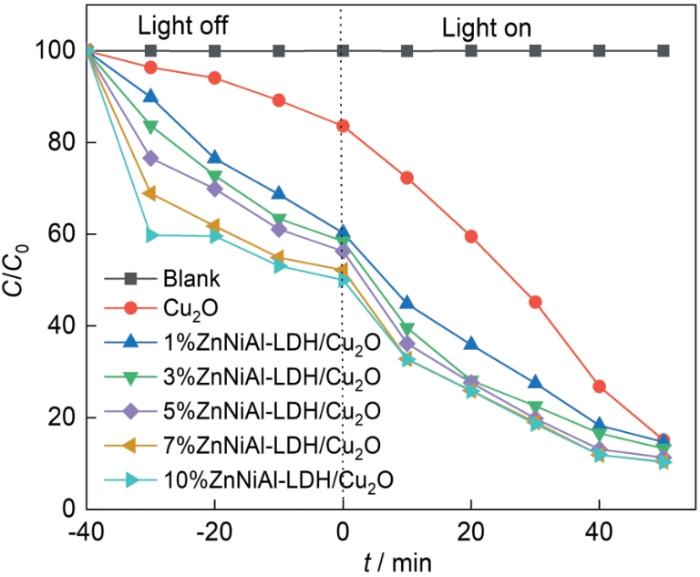
图8 中的7条折线分别为四环素的自降解、Cu2 O对四环素溶液的降解以及不同比例ZnNiAl-LDH/Cu2 O对四环素溶液降解的变化曲线。可以看出,四环素几乎不发生自降解,ZnNiAl-LDH/Cu2 O复合材料的降解性能比纯Cu2 O的高。其中含10%ZnNiAl-LDH的光降解率最高,50 min的光降解率为89.7%,含7%ZnNiAl-LDH的复合材料的光降解率为89.6%,与其几乎相同。这表明,7%ZnNiAl-LDH/Cu2 O复合光催化剂的光降解性能最佳。
图8
图8
Cu2 O和不同比例ZnNiAl-LDH/Cu2 O光催化剂的光催化性能曲线
Fig.8
Photocatalytics degradation curves for decomposition of TC with different photocatalysts
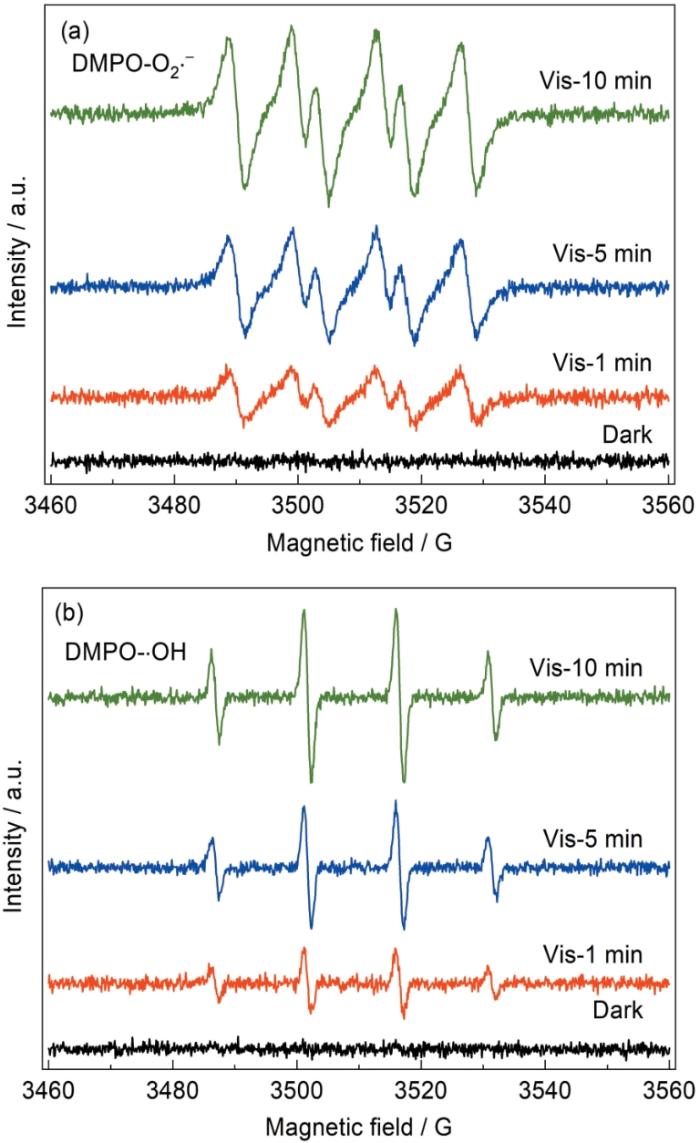
进行EPR自由基捕获实验,以揭示7%ZnNiAl-LDH/Cu2 O的光催化机理。由图9 可见,在黑暗条件下可观察到EPR信号,而在可见光条件下可观察到明显的DMPO-·O2 - - 的特征信号,表明主要的活性物质是⋅ 2 - ⋅ - 。
图9
图9
7%ZnNiAl-LDH/Cu2 O在甲醇体系(DMPO-·O2 - ) 和水体系(DEPO-·OH)辐射5 min后的DMPO自旋捕获EPR谱
Fig.9
DMPO spin-trapping EPR spectra of 7%ZnNiAl-LDH/Cu2 O composite with irradiation for 5 min in methanol dispersion (for DMPO-·O2 - ) (a) and aqueous dispersion (for DEPO-·OH) (b)
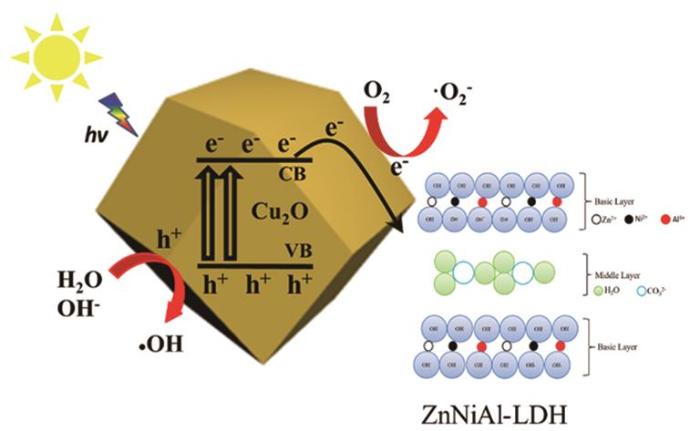
图10 给出了ZnNiAl-LDH/Cu2 O在可见光照射下的光催化反应机理。由图10 可见,在可见光照射下纳米Cu2 O价带(VB)上的电子得到能量跃迁到导带(CB)上产生了电子,然后迅速从纳米Cu2 O的表面转移到ZnNiAl-LDH的表面,将吸附在其表面的O2 还原为⋅ 2 - 2 O表面的H2 O或OH- 氧化生成⋅ ⋅ 2 - ⋅ 2 O在促进光生空穴和光生电子分离的同时还抑制了Cu2 O内部载流子的复合和重组,从而大大提高了催化剂的光催化活性。
图10
图10
可见光下ZnNiAl-LDH/Cu2 O光催化反应机理
Fig.10
Proposed mechanism of the ZnNiAl-LDH/Cu2 O photocatalyst under visible light irradiation
3 结论
(1) 将不同含量的ZnNiAl-LDH掺杂在Cu2 O中制备的ZnNiAl-LDH/Cu2 O复合材料,其光降解性能比纯Cu2 O的性能高。
(2) 7%ZnNiAl-LDH/Cu2 O复合光催化剂的光降解性能最优,用50 min可降解89.6%的TC。与ZnNiAl-LDH掺杂能显著抑制Cu2 O的光腐蚀,具有良好的可重复性。7%ZnNiAl-LDH/Cu2 O的优异光降解效率,归因于加入的ZnNiAl-LDH使Cu2 O的比表面积增大和改善了催化剂中电子-空穴对的分离效果,从而使光催化的性能提高。
参考文献
View Option
[1]
Zheng W Can L Kazunari D Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting
[J]. Chem. Soc. Rev , 2018 , 48 (7 ): 2109
[本文引用: 1]
[2]
Peng Z T Gao Y R Yao C et al Preparation and photocatalytic activity of Fe/Yb Co-doped titanium dioxide hollow sphere
[J]. Chin. J. Mater. Res. , 2021 , 35 (2 ): 135
DOI
[本文引用: 1]
Composites of Fe/Yb co-doped TiO2 hollow spheres (Fe/Yb-TiO2HS) were prepared by template method and then characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FT-IR) and thermogravimetric analysis (TG). The photocatalytic performance of the composites was assessed with a simulated wastewater of 20 mg/L methyl orange solution under radiation of visible light. The results show that the degradation efficiency of doped titanium dioxide hollow spheres for the methyl orange could be significantly improved. The composite doped with 0.1% Fe and 1% Yb presents the optimal photocatalytic performance with degradation efficiency up to 92.57% for the methyl orange.
彭子童 , 郜艳荣 , 姚 楚 等 铁/镱掺杂二氧化钛空心球的制备及其光催化性能
[J]. 材料研究学报 , 2021 , 35 (2 ): 135
DOI
[本文引用: 1]
用模板法制备铁/镱共掺杂二氧化钛空心球(Fe/Yb-TiO<sub>2</sub>HS),使用扫描电子显微镜(SEM)观察和X射线衍射(XRD)、X射线光电子能谱(XPS)、傅里叶红外光谱(FT-IR)、热失重(TG)等测试方法对对其进行了表征。使用浓度为20 mg/L的甲基橙溶液模拟废水,研究了铁/镱共掺杂二氧化钛空心球的催化性能。结果表明:铁/镱共掺杂的二氧化钛空心球对甲基橙的降解效果较好,铁掺杂量为0.1%和镱掺杂量为1%的催化剂其光催化性能达到92.57%。
[3]
Schwitzgebel J Ekerdt J G Gerischer H et al Role of the oxygen molecule and of the photogenerated electron in TiO2 -photocatalyzed air oxidation reactions
[J]. J. Phys. Chem. C , 1995 , 99 (15 ):5633
[本文引用: 1]
[4]
Yu Y Bao H B Comparative research on solar photovoltaic market deployment policies
[J]. Sci. Technol. Manag. Res. , 2012 , 32 (15 ): 21
[本文引用: 1]
余 杨 , 包海波 太阳能光伏市场应用政策的国别比较研究
[J]. 科技管理研究 , 2012 , 32 (15 ): 21
[本文引用: 1]
[5]
Matsumoto H Hasuo M Kono S et al Revived interest on yellow-exciton series in Cu2 O-an experimental aspect
[J]. Solid State Commun , 1996 , 97 (2 ): 125
[本文引用: 1]
[6]
Ma H M Zhu Z L Status and prospect of heterogeneous photocatalysis technology of semiconductor
[J]. Environ. Prot. Sci. , 2006 , (01 ): 28
[本文引用: 1]
马红梅 , 朱志良 半导体多相光催化技术研究现状及发展趋势
[J]. 环境保护科学 , 2006 , (01 ): 28
[本文引用: 1]
[7]
Wang H J Li Z Heterogenous photocatalytic oxidation technology for semiconductors
[J]. Modern Chemical Industry , 2002 (2 ): 56
[本文引用: 1]
王红娟 , 李 忠 半导体多相光催化氧化技术
[J]. 现代化工 , 2002 , (2 ): 56
[本文引用: 1]
[8]
Wei M Z Huo J Z Lun N et al A novel semiconductor photocatalyst—nano cuprous oxide
[J]. Materials Reports , 2007 , (6 ): 130
[本文引用: 1]
魏明真 , 霍建振 , 伦 宁 等 一种新型的半导体光催化剂——纳米氧化亚铜
[J]. 材料导报 , 2007 , (6 ): 130
[本文引用: 1]
[9]
Long D Zhou J L Shi H M et al Research progress on the improved performance of cuprous oxide photocatalyst and its enhancement mechanism
[J]. Chem. Ind. Eng. Prog. , 2019 , 38 (6 ):2756
[本文引用: 1]
龙 丹 , 周俊伶 , 时洪民 等 氧化亚铜光催化剂性能提升及增强机制的研究进展
[J]. 化工进展 , 2019 , 38 (6 ): 2756
[本文引用: 1]
[10]
Jongh P Vanmaekelbergh D Kelly J J Cu2 O: a catalyst for the photochemical decomposition of water?
[J]. Chem Commun , 1999 , 0 (12 ): 1069
[本文引用: 1]
[11]
Toe C Y Zheng P Z Wu H et al Photocorrosion of cuprous oxide in hydrogen production: rationalising self‐oxidation or self‐reduction
[J]. Angew. Chem. , 2018 , 130 (41 ): 13801
[本文引用: 1]
[12]
Karthikeyan S Kumar S Durndell L et al Size-dependent visible light photocatalytic performance of Cu2 O nanocubes
[J]. C. & Chem , 2018 , 10 (16 ): 3554
[本文引用: 1]
[13]
Wu C Pd-Cu2 O and Ag-Cu2 O hybrid concave nanomaterials for an effective synergistic catalyst
[J]. Angew. Chem , 2013 , 52 (42 ):9023
[本文引用: 1]
[14]
Siripala W Ivanovskaya A Jaramillo T F et al A Cu2 O/TiO2 heterojunction thin film cathode for photoelectrocatalysis
[J]. Sol. Energy Mater Sol.Cells , 2003 , 77 (3 ): 229
[本文引用: 1]
[15]
Xie L Wang P Li Z F et al Hydrothermal synthesis and photocatalytic activity of CuO/ZnO composite photocatalyst
[J]. Chin. J. Mater. Res. , 2019 , 33 (10 ): 728
DOI
[本文引用: 1]
Nanocomposites of CuO/ZnO were synthesized with cetyltrimethylammonium bromide as a growth regulator by one-step hydrothermal method. The catalyst was characterized by X-ray diffractometry (XRD), scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), fluorescence spectrometer (FL) and UV-Vis spectrometer (UV-Vis). The photocatalytic effect of the composite photocatalyst with different ratios of CuO to ZnO on the degradation efficiency of methyl orange under ultraviolet light irradiation, and the cyclic stability of the composite photocatalyst were investigated. The results show that CuO/ZnO photocatalysts are mainly composed of CuO nanoparticles and ZnO nanosheets. The proper amount of CuO can effectively adjust the light absorption performance of ZnO and enhance the efficiency of ultraviolet photocatalysis. Excess CuO (?7%) has inhibitory effect on ZnO ultraviolet catalytic efficiency. CuO/ZnO has good stability in the photocatalytic process.
谢 亮 , 王 平 , 李之锋 等 CuO/ZnO复合光催化剂的制备和性能
[J]. 材料研究学报 , 2019 , 33 (10 ): 728
[本文引用: 1]
[16]
Wu Z Cheung G Wang J Wavelength dependent photochemical charge transfer at the Cu2 O-BiVO4 particle interface-evidence for tandem excitation
[J]. Chem. Commun , 2018 , 54 : 9023
[本文引用: 1]
[17]
Guangliang C Chuanhai X Pinghua Z et al In situ electrodeposition of a Cu2 O/SnO2 periodical heterostructure film for photosensor applications
[J]. Phys. chem. chem. phys , 2016 , 18 (16 ): 10918
DOI
PMID
[本文引用: 1]
Heterostructure materials with a strictly periodic arrangement in hundreds of microns based on tunneling modulation are ideal candidates for micro-nanodevice applications. In this paper, we propose a Cu2O/SnO2 periodical heterostructure film, which is prepared by electrochemical deposition in a quasi-2D ultra-thin liquid layer. The surface morphology and the component of the film were analyzed by scanning electron microscopy (SEM), scanning probe microscopy (SPM) and transmission electron microscopy (TEM). The influences of frequency and amplitude of periodic deposition potential on the morphology and regular distribution of the interface were studied. The photoresponsivity of this material was researched, and the response behaviors for different illumination conditions were recorded carefully. Based on the tunneling modulation mechanism, it exhibits reasonable photoresponsivity to UV light.
[18]
Song J Rodenbough P Xu W Reduction of nano Cu2 O: crystallite-size dependent and the effect of nano-ceria support
[J]. J Phys Chem C , 2015 , 119 (31 ): 17667
[本文引用: 1]
[19]
Wang B An W Liu L et al Novel Cu2 S quantum dots coupled flower-like BiOBr for efficient photocatalytic hydrogen production under visible light
[J]. Rsc Adv , 2015 , 5 (5 ): 3224
[本文引用: 1]
[20]
Wang L Zhao F Han Q et al Spontaneous formation of Cu2 O-g-C3 N4 core-shell nanowires for photocurrent and humidity responses
[J]. Nanoscale , 2015 , 7 (21 ): 9694
[本文引用: 1]
[21]
Qin Y L Yang Y Zhao P Y et al Microstructures and photocatalytic properties of BiOCl-RGO nanocomposites prepared by two-step hydrothermal method
[J]. Chin. J. Mater. Res. , 2020 , 34 (02 ): 92
[本文引用: 1]
秦艳利 , 杨 艳 , 赵鹏羽 等 两步水热法制备BiOCl-RGO纳米复合材料及其光催化性能
[J]. 材料研究学报 , 2020 , 34 (02 ): 92
[本文引用: 1]
[22]
Huang Z J Frabication of layered double hydroxides (LDHs) composites and their photocatalytic properties study
[D]. Guangzhou : South China University of Technology , 2014
[本文引用: 1]
黄柱坚 层状双氢氧化物(LDHs)复合材料的构建及光催化性能研究
[D]. 广州 : 华南理工大学 , 2014
[本文引用: 1]
[23]
Kulamani P Minarva S Lagnamayee M Incorporation of Fe3+ into Mg/Al layered double hydroxide framework: effects on textural properties and photocatalytic activity for H2 generation
[J]. J. Mater. Chem. A , 2012 , 22 (15 ):7350
[本文引用: 1]
[24]
Zhi Y Li Y Zhang Q et al ZnO nanoparticles immobilized on flaky layered double hydroxides as photocatalysts with enhanced adsorptivity for removal of acid red G
[J]. Langmuir , 2010 , 26 (19 ):15546
DOI
PMID
[本文引用: 1]
Flaky layered double hydroxides (FLDH) composed of cross-linked nanoflakes were prepared by the reconstruction of their oxides in alkali solution. The effect of reconstruction temperatures on the physicochemical properties was investigated. FLDH with a specific surface area of as high as 217 m(2)/g was obtained at a reconstruction temperature of 6 °C, and its derived flaky mixed metal oxides (FMMO) had a specific surface area of 249 m(2)/g. The ZnO nanoparticles were homogeneously deposited on the surface of the FLDH by coprecipitation. After calcination at 500 °C for 2 h, the ZnO-coated FLDH was transformed into ZnO-coated flaky mixed metal oxides (FMMO). The powders were characterized by X-ray diffraction, field-emission scanning electron microscopy, transmission electron microscope, N(2) adsorption-desorption isotherm, UV-vis diffuse reflectance spectroscopy, and Fourier transform infrared spectroscopy. In the presence of FLDH as a support, the ZnO nanoparticles were of about 10 nm in size and showed higher photocatalytic decomposition of acid red G than bare ZnO powder prepared under similar experimental conditions. It should be noted that the ZnO-coated FMMO combined excellent adsorption with photocatalytic activity. The flaky structure of mixed metal oxides appears to play important roles in the adsorption and photodecomposition process.
Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting
1
2018
... 光催化技术有重要的应用价值[1 ,2 ] .光催化的基本原理是:在光照下纳米半导体材料产生的具有较强氧化活性的基团能降解水体中的有机物[3 ] .太阳光取之不尽用之不竭[4 ] ,节能又绿色环保.因此,研究太阳光催化有重要的意义. ...
Preparation and photocatalytic activity of Fe/Yb Co-doped titanium dioxide hollow sphere
1
2021
... 光催化技术有重要的应用价值[1 ,2 ] .光催化的基本原理是:在光照下纳米半导体材料产生的具有较强氧化活性的基团能降解水体中的有机物[3 ] .太阳光取之不尽用之不竭[4 ] ,节能又绿色环保.因此,研究太阳光催化有重要的意义. ...
铁/镱掺杂二氧化钛空心球的制备及其光催化性能
1
2021
... 光催化技术有重要的应用价值[1 ,2 ] .光催化的基本原理是:在光照下纳米半导体材料产生的具有较强氧化活性的基团能降解水体中的有机物[3 ] .太阳光取之不尽用之不竭[4 ] ,节能又绿色环保.因此,研究太阳光催化有重要的意义. ...
Role of the oxygen molecule and of the photogenerated electron in TiO2 -photocatalyzed air oxidation reactions
1
1995
... 光催化技术有重要的应用价值[1 ,2 ] .光催化的基本原理是:在光照下纳米半导体材料产生的具有较强氧化活性的基团能降解水体中的有机物[3 ] .太阳光取之不尽用之不竭[4 ] ,节能又绿色环保.因此,研究太阳光催化有重要的意义. ...
Comparative research on solar photovoltaic market deployment policies
1
2012
... 光催化技术有重要的应用价值[1 ,2 ] .光催化的基本原理是:在光照下纳米半导体材料产生的具有较强氧化活性的基团能降解水体中的有机物[3 ] .太阳光取之不尽用之不竭[4 ] ,节能又绿色环保.因此,研究太阳光催化有重要的意义. ...
太阳能光伏市场应用政策的国别比较研究
1
2012
... 光催化技术有重要的应用价值[1 ,2 ] .光催化的基本原理是:在光照下纳米半导体材料产生的具有较强氧化活性的基团能降解水体中的有机物[3 ] .太阳光取之不尽用之不竭[4 ] ,节能又绿色环保.因此,研究太阳光催化有重要的意义. ...
Revived interest on yellow-exciton series in Cu2 O-an experimental aspect
1
1996
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
Status and prospect of heterogeneous photocatalysis technology of semiconductor
1
2006
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
半导体多相光催化技术研究现状及发展趋势
1
2006
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
Heterogenous photocatalytic oxidation technology for semiconductors
1
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
半导体多相光催化氧化技术
1
2002
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
A novel semiconductor photocatalyst—nano cuprous oxide
1
2007
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
一种新型的半导体光催化剂——纳米氧化亚铜
1
2007
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
Research progress on the improved performance of cuprous oxide photocatalyst and its enhancement mechanism
1
2019
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
氧化亚铜光催化剂性能提升及增强机制的研究进展
1
2019
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
Cu2 O: a catalyst for the photochemical decomposition of water?
1
1999
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
Photocorrosion of cuprous oxide in hydrogen production: rationalising self‐oxidation or self‐reduction
1
2018
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
Size-dependent visible light photocatalytic performance of Cu2 O nanocubes
1
2018
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
Pd-Cu2 O and Ag-Cu2 O hybrid concave nanomaterials for an effective synergistic catalyst
1
2013
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
A Cu2 O/TiO2 heterojunction thin film cathode for photoelectrocatalysis
1
2003
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
Hydrothermal synthesis and photocatalytic activity of CuO/ZnO composite photocatalyst
1
2019
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
CuO/ZnO复合光催化剂的制备和性能
1
2019
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
Wavelength dependent photochemical charge transfer at the Cu2 O-BiVO4 particle interface-evidence for tandem excitation
1
2018
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
In situ electrodeposition of a Cu2 O/SnO2 periodical heterostructure film for photosensor applications
1
2016
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
Reduction of nano Cu2 O: crystallite-size dependent and the effect of nano-ceria support
1
2015
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
Novel Cu2 S quantum dots coupled flower-like BiOBr for efficient photocatalytic hydrogen production under visible light
1
2015
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
Spontaneous formation of Cu2 O-g-C3 N4 core-shell nanowires for photocurrent and humidity responses
1
2015
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
Microstructures and photocatalytic properties of BiOCl-RGO nanocomposites prepared by two-step hydrothermal method
1
2020
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
两步水热法制备BiOCl-RGO纳米复合材料及其光催化性能
1
2020
... 纳米氧化亚铜(Cu2 O)是一种典型的p型半导体光催化材料,其禁带宽度为2.0~2.2 eV对应的吸收波长在可见光范围内[5 ] .在可见光的照射下Cu2 O产生的电子-空穴对可将水体中的有机物氧化降解为稳定且无害的CO2 、H2 O或其它小分子[6 ] ,还能去除水体中的镉(Cd)、砷(As)、汞(Hg)等重金属离子[7 ] .这表明,Cu2 O光催化剂有广阔的应用前景[8 ] .但是,Cu2 O产生的光生电子-空穴对容易复合,投入水中使用后难以回收,且易发生光腐蚀[9 ] 和稳定性不高,使其应用受到限制[10 ] .为了解决这些问题,可将其与Au[11 ] 、Ag[12 ] 、Pt[13 ] 等贵金属以及TiO2 [14 ] 、ZnO[15 ,16 ] 、SnO2 [17 ] 、CeO2 [18 ] 、BiOBr[19 ] 、C3 N4 [20 ] 等金属氧化物或者碳量子点、碳纳米管、石墨烯[21 ] 等碳材料复合. ...
Frabication of layered double hydroxides (LDHs) composites and their photocatalytic properties study
1
2014
... ZnNiAl-LDH二维层状三金属氢氧化物是一种比表面积较大的多孔材料,有较强的吸附能力和优良的导电性能[22 ] .纳米级金属氧化物的氧化能力比普通粒径的材料强,因此其催化性能更高.但是,这类材料放入水中使用后很难回收且容易团聚,其光生电子-空穴对还容易复合.将其与LDHs材料复合,可增大催化剂的比表面积并使光生电子迁移到LDHs材料表面与氧分子反应生成超氧自由基(·O2 - ). 光生空穴与表面羟基离子或水反应产生的羟基自由基(·OH)都有很强的氧化活性,能促进光生电子-空穴对的分离而使其催化性能大大提高.因此,LDHs是一种很适合作为纳米级光催化剂载体的材料[23 ] .将纳米ZnO负载在LDHs材料上,ZnO能均匀地分布在LDHs材料的周围而不容易团聚,可提高其催化性能和易于回收[24 ] .鉴于此,本文将Cu2 O负载在不同含量的ZnNiAl-LDH上制备ZnNiAl-LDH/Cu2 O复合光催化剂,研究其在模拟太阳光照条件下对四环素(TC)的降解性能. ...
层状双氢氧化物(LDHs)复合材料的构建及光催化性能研究
1
2014
... ZnNiAl-LDH二维层状三金属氢氧化物是一种比表面积较大的多孔材料,有较强的吸附能力和优良的导电性能[22 ] .纳米级金属氧化物的氧化能力比普通粒径的材料强,因此其催化性能更高.但是,这类材料放入水中使用后很难回收且容易团聚,其光生电子-空穴对还容易复合.将其与LDHs材料复合,可增大催化剂的比表面积并使光生电子迁移到LDHs材料表面与氧分子反应生成超氧自由基(·O2 - ). 光生空穴与表面羟基离子或水反应产生的羟基自由基(·OH)都有很强的氧化活性,能促进光生电子-空穴对的分离而使其催化性能大大提高.因此,LDHs是一种很适合作为纳米级光催化剂载体的材料[23 ] .将纳米ZnO负载在LDHs材料上,ZnO能均匀地分布在LDHs材料的周围而不容易团聚,可提高其催化性能和易于回收[24 ] .鉴于此,本文将Cu2 O负载在不同含量的ZnNiAl-LDH上制备ZnNiAl-LDH/Cu2 O复合光催化剂,研究其在模拟太阳光照条件下对四环素(TC)的降解性能. ...
Incorporation of Fe3+ into Mg/Al layered double hydroxide framework: effects on textural properties and photocatalytic activity for H2 generation
1
2012
... ZnNiAl-LDH二维层状三金属氢氧化物是一种比表面积较大的多孔材料,有较强的吸附能力和优良的导电性能[22 ] .纳米级金属氧化物的氧化能力比普通粒径的材料强,因此其催化性能更高.但是,这类材料放入水中使用后很难回收且容易团聚,其光生电子-空穴对还容易复合.将其与LDHs材料复合,可增大催化剂的比表面积并使光生电子迁移到LDHs材料表面与氧分子反应生成超氧自由基(·O2 - ). 光生空穴与表面羟基离子或水反应产生的羟基自由基(·OH)都有很强的氧化活性,能促进光生电子-空穴对的分离而使其催化性能大大提高.因此,LDHs是一种很适合作为纳米级光催化剂载体的材料[23 ] .将纳米ZnO负载在LDHs材料上,ZnO能均匀地分布在LDHs材料的周围而不容易团聚,可提高其催化性能和易于回收[24 ] .鉴于此,本文将Cu2 O负载在不同含量的ZnNiAl-LDH上制备ZnNiAl-LDH/Cu2 O复合光催化剂,研究其在模拟太阳光照条件下对四环素(TC)的降解性能. ...
ZnO nanoparticles immobilized on flaky layered double hydroxides as photocatalysts with enhanced adsorptivity for removal of acid red G
1
2010
... ZnNiAl-LDH二维层状三金属氢氧化物是一种比表面积较大的多孔材料,有较强的吸附能力和优良的导电性能[22 ] .纳米级金属氧化物的氧化能力比普通粒径的材料强,因此其催化性能更高.但是,这类材料放入水中使用后很难回收且容易团聚,其光生电子-空穴对还容易复合.将其与LDHs材料复合,可增大催化剂的比表面积并使光生电子迁移到LDHs材料表面与氧分子反应生成超氧自由基(·O2 - ). 光生空穴与表面羟基离子或水反应产生的羟基自由基(·OH)都有很强的氧化活性,能促进光生电子-空穴对的分离而使其催化性能大大提高.因此,LDHs是一种很适合作为纳米级光催化剂载体的材料[23 ] .将纳米ZnO负载在LDHs材料上,ZnO能均匀地分布在LDHs材料的周围而不容易团聚,可提高其催化性能和易于回收[24 ] .鉴于此,本文将Cu2 O负载在不同含量的ZnNiAl-LDH上制备ZnNiAl-LDH/Cu2 O复合光催化剂,研究其在模拟太阳光照条件下对四环素(TC)的降解性能. ...