CFA的改性方法包括机械研磨法、酸改性法、碱改性法、盐改性法、高温热改性法和复合改性法等。用盐酸、硫酸、硝酸和氢氟酸等改性剂可增加CFA表面正电荷增强对负离子的吸附,酸溶液生成的Al3+和Fe3+与负离子发生絮凝沉淀也能提高其吸附性能。Li等[15]研究了三种酸改性CFA对磷酸盐的吸附,发现用1 mol/L HCl + 1 mol/L H2SO4对磷酸盐的去除率达到98%。余荣台等[16]发现,酸改性CFA(不同浓度的硫酸、盐酸、硫酸+盐酸混合酸)可提高其对废水中磷酸盐的吸附,其吸附等温线符合Langmuir模型。碱改性是利用碱与CFA中的SiO2、Al2O3腐蚀CFA的玻璃体表面,生成的大量孔结构和表面羟基结构可提高CFA的孔隙率和增大比表面积,碱浸溶解硅铝氧化物可增加表面羟基点位而有利于对阳离子金属的吸附[17~19]。Nguyen等[20]用2-MBT (巯基苯并噻唑)和SDS (十二烷基硫酸钠)为表面活性剂经1 mol/L NaOH溶液改性粉煤灰,研究了未改性粉煤灰(UCFA)和改性粉煤灰(MCFA)对Cd2+和Hg2+的吸附行为,发现MCFA的吸附离子量高于UCFA。魏丽丹[21]等用NaOH改性的CFA吸附硫化物,发现10 g的CFA对硫化物的吸附率达到48%~64%。本文用酸碱混合改性法制备改性CFA吸附材料,研究其对染料的吸附及其机理。
1 实验方法
1.1 实验用试剂和仪器
实验用试剂:工业级粉煤灰(CFA),盐酸(HCl),氢氧化钠(NaOH),溴化钾,染料:碱性品红、孔雀石绿、刚果红和甲基紫。
实验用仪器设备:FA2004型分析天平;JY1002型电子天平;KH-250E型超声波清洗器;SHA-C型水浴振荡器;EVOLUTION 201型紫外可见光分光光度计;SHZ-D(III)型循环水真空泵;Quanta200型扫描电镜;Autosorb-iQ-C(BET)全自动物理化学吸附仪;Nicolet is50型FT-IR红外光谱仪;D/max2500型X射线衍射仪以及PHI 5000 Versaprobe III型X射线光电子能谱仪
1.2 改性粉煤灰吸附剂MCFA的制备
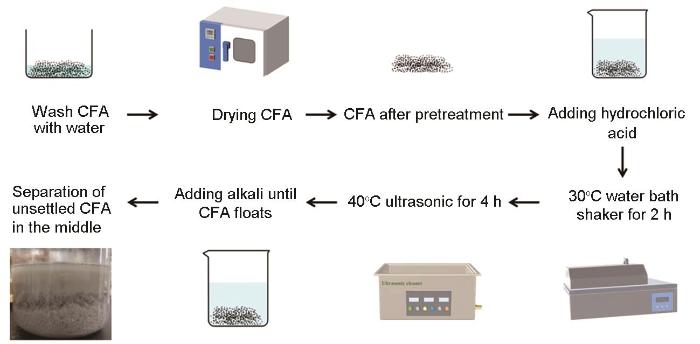
制备吸附剂的流程如图1所示。用蒸馏水将浓盐酸稀释配制成质量分数为5%的盐酸改性剂800 mL,加入40 g(水洗后在120℃烘干)未改性粉煤灰(UCFA),密封后放置在30℃的水浴摇床震荡2 h,然后用超声波清洗器中在40℃、110 r/min的条件下超声处理4 h。最后用(预先制备的)800 mL的NaOH溶液(浓度为1 mol/L)将粉煤灰溶液的pH值调至11,使CFA在烧杯内絮凝。烧杯底部的沉降颗粒为废料,大部分为未发生反应的粉煤灰。将烧杯中部的材料即改性粉煤灰静置4 h,稳定后去除上清液得到改性粉煤灰(MCFA)。
图1
图1
HCl-NaOH法改性粉煤灰吸附材料的制备流程
Fig.1
Preparation process of fly ash adsorption material modified by HCl-NaOH method
1.3 结构和性能表征
用Quanta200型扫描电镜观察改性前后粉煤灰样品的表面结构和形态。用Autosorb-iQ-C(BET)全自动物理化学吸附仪在77.35 K氮气气氛中测试样品的吸附性能。使用Nicolet is50型FT-IR红外光谱仪和溴化钾压片法分析改性前后的粉煤灰样品和负载染料的MCFA,测试样品与溴化钾的混合比例为1∶100。使用D/max2500型X射线衍射仪测试改性前后粉煤灰的XRD谱。扫描速度为8 (°)/min,扫描范围10°~80°,步宽2θ = 0.02°。使用PHI 5000 Versaprobe III型X射线光电子能谱仪测试改性前后粉煤灰中C、O元素的XPS谱。
1.4 吸附性能的测定
实验用吸附质为碱性品红、孔雀石绿、刚果红以及甲基紫4种染料,其结构式列于表1。
表1 吸附用染料的基本参数
Table 1
| Dye | Molecular weight | Structural formula | Wavelength / nm |
|---|---|---|---|
| Basic violet | 337.85 | 543.88 | |
| Basic green | 364.92 | 564.63 | |
| Congo red | 696.68 | 495.25 | |
| Methyl violet | 379.93 | 584.94 |
用于吸附4种染料的吸附剂MCFA的剂量,分别为2、4、6、8和10 mL。将5种不同剂量的吸附剂分别加到具塞锥形瓶中体积为200 mL、浓度为100 mg/L的4种染液(碱性品红、孔雀石绿、刚果红、甲基紫)中,再将其放在水浴振荡器中在110 r/min、30℃条件下振荡30 min,然后用真空泵抽滤。将抽滤后的溶液依次加入到比色管中定容,摇晃比色管后放置5 min,在对染料最大吸收的波长(λmax)处用紫外可见光分光光度计测吸附前后各滤液的吸光度以计算脱色率和吸附量[22]。
吸附量(mg/g)为
脱色率为
其中C0为吸附前的染料溶液浓度(mg/L),Q为MCFA对染料的吸附量(mg/g),C1为吸附后的染料溶液浓度(mg/L),V为染料溶液的体积(L),m为改性粉煤灰的质量(g)。
1.5 等温吸附实验
将碱性品红、孔雀石绿、刚果红、甲基紫4种染料均配制成浓度分别为20、40、60、80、100、130、160、190、200、250 mg/L的染液,然后将2 mL的MCFA依次加到不同浓度的每种染液中,将其在水浴振荡器中在110 r/min、30℃的条件下振荡吸附2 h。用真空泵抽滤后将滤液放在比色管中,将其摇晃均匀后用紫外可见光分光光度计测试染料最大吸收波长处(λmax)的吸光度,用于计算吸附量。
1.6 Langmuir模型和Freundlich模型线性拟合
Langmuir等温吸附模型
描述在吸附剂表面发生的单分子层吸附,其中qmax为表面上全部覆盖单分子层吸附质的吸附量(mg/g),qe为平衡时吸附量(mg/g),KL为吸附系数(吸附平衡常数),Ce为平衡浓度(mg/L)。
Freundlich吸附等温模型
假定吸附是多分子层的。其中n为与吸附强度或有利吸附程度相关的特征常数,qe为平衡时吸附量(mg/g),KF为Freundlich吸附系数,Ce为平衡浓度(mg/L)。
1.7 吸附动力学实验
将2 mL的MCFA依次加到容量为250 mL的具塞锥形瓶中,再分别将200 mL浓度为100 mg/L的碱性品红、孔雀石绿、刚果红、甲基紫染液加入其中。将具塞锥形瓶放到水浴振荡器中,在110 r/min、30℃的条件下进行振荡吸附。将吸附不同的时间(30、60、90、120、150、240、300、360、420和480 s)后的每种染液取出,用真空泵抽滤后取滤液放于比色管中定容,摇晃均匀后用紫外可见光分光光度计测其吸光度以计算吸附量。
用准一级动力学模型和准二级动力学模型拟合实验数据。根据吸附动力学研究吸附平衡的速率,根据模型拟合分析吸附剂的吸附机理。用于固液吸附体系常用的模型,有准一级动力学模型和准二级动力学模型[23]。准一级动力学模型可表示为
其中K1为一级吸附速率常数,t为吸附时间(s),q为在吸附时间为t时吸附剂对染料的吸附量(mg/g),qe为吸附平衡时的吸附量(mg/g)。
准二级动力学模型基于固相吸附容量,可表示为
式中:K2为准二级模型的速率常数,t为吸附时间(s),q为在吸附时间为t时吸附剂对染料的吸附量(mg/g),qe为吸附平衡时的吸附量(mg/g)。
吸附动力学研究吸附材料对目标吸附物质的吸附量与吸附时间之间的关系,吸附模型分为准一级动力学模型和准二级动力学模型。对吸附数据进行动力学模型拟合,可揭示吸附机理,确定吸附符合物理吸附或是化学吸附。
1.8 染料pH值的影响
在容量为250 mL的锥形瓶中配制pH值分别为3、4、5、7、9、11的碱性品红、孔雀石绿、刚果红、甲基紫染液各200 mL,将2 mL的MCFA依次加到具塞锥形瓶中,然后将这24个具塞锥形瓶放在水浴振荡器中在110 r/min和30℃条件下振荡30 min。用真空泵抽滤后将抽滤的溶液依次加到比色管中并且定容,摇晃均匀后用紫外可见光分光光度计测其吸光度以计算吸附效率。
1.9 CFA和MCFA的吸附原理
根据CFA形成的燃烧条件,部分气体逸出产生开放性孔穴使表面呈蜂窝状;部分未逸出的气体裹在颗粒内形成封闭性孔穴,内部也呈蜂窝状。这些封闭性孔穴在酸的作用下形成大量微细小孔,增加了比表面积和提高了孔隙率。碱破坏了CFA颗粒表面的坚硬外壳而使玻璃体表面的可溶性物质与碱性氧化物反应生成凝胶物质,使CFA的活性提高[24]。
在用盐酸溶液浸渍CFA的过程中,CFA主要成分中的Al2O3和部分氧化物与盐酸反应生成Al3+和极少量的Fe3+。Al3+和Fe3+不仅使染液废水中悬浮胶粒的电位下降,使微粒可能发生碰撞聚结而失去稳定性从而被CFA吸附,还有一定的絮凝沉降作用。同时,酸还与CFA孔隙中的杂质反应将杂质溶解,从而疏通了孔隙、增大了表面空洞和凹槽量的作用,进而增大了CFA的比表面积和提高了孔隙率,从而使其吸附性能提高。
在用NaOH溶液调节酸改性CFA溶液的pH值的过程中,碱进一步侵蚀CFA的表面,Al3+与NaOH反应生成Al(OH)3,CFA主要成分中的Al2O3和SiO2会与碱溶液反应生成SiO
2 结果和讨论
2.1 CFA和MCFA样品表面的微观形貌
图2
2.2 改性对样品孔径和比表面积的影响
使用BET检测了样品的孔径及比表面积,探究了改性的影响。图3给出了CFA和MCFA的氮气吸附-解吸等温线和BJH的孔径分布曲线。可以看出,CFA和MCFA的氮气吸附解吸量分别为4.527和42.3872 cm3·g-1。根据IUPAC的分类,CFA的吸附等温线属于第三类,吸附质与吸附剂之间的相互作用较弱;MCFA的吸附等温线属于IV型吸附等温线并伴有H3型迟滞回线,是介孔材料吸附的典型特征。这表明,改性后的吸附剂其孔径分布不均匀,在吸附过程中发生的是多分子层吸附[26]。相对压力P/P0较低时吸附缓慢,相对压力为0.4~1.0吸附曲线陡然上升,滞回环的出现表明发生了毛细管冷凝,是均匀且窄孔分布材料的典型特征。图3中的子图给出了CFA和MCFA的孔径分布,可见其孔径分别为3.061和4.900 nm (表2)。
图3
图3
CFA和MCFA样品的孔径分布和氮气吸脱附曲线
Fig.3
Pore size distribution and nitrogen adsorption desorption curves of CFA and MCFA samples
表2 CFA和MCFA的比表面积和孔径尺寸
Table 2
| Samples | Surface area / m2·g-1 | N2 quantity amount / cm3·g-1 | Correlation coefficient (r) | Diameter / nm |
|---|---|---|---|---|
| CFA | 5.2 | 4.527 | 0.998700 | 3.061 |
| MCFA | 32.0 | 42.3872 | 0.999927 | 4.900 |
表2给出的CFA和MCFA的BET数据参数,表明MCFA吸附材料性能优异。可以看出,改性后的粉煤灰其比表面积从5.2 m2/g增大到32.0 m2/g。
2.3 CFA和MCFA的分子结构和化学组成
红外光谱仪可用来测定物质的红外光谱以分析其分子结构和化学组成。分子振动对光产生吸收,在红外光的透射下不同官能团的振动频率产生的吸收峰不同。CFA的主要成分是SiO2和Al2O3[27]。图4给出了CFA、MCFA和负载染料的MCFA的红外光谱,可见556 cm-1处的峰是反应CFA材料中的Si-O-Al的对称伸缩振动峰,负载染料的MCFA在1089 cm-1附近显示出复杂的吸收带,是由染料分子中的C-N键(BV、BG、MV三种染料中存在)和O-S键(CR染料中存在)的拉伸振动引起的,反应出染料已经负载在MCFA上。1452 cm-1处的特征峰为Si-O-Si的分对称伸缩振动峰,3100 cm-1附近的是碳氢键的伸缩振动峰,4种染料中的苯环使负载了染料吸附剂的吸收峰强度明显提高。在3430和1624 cm-1处的峰是水羟基的伸缩振动峰及弯曲振动峰。在MCFA和负载染料的MCFA的红外谱图中,吸收峰的强度提高且宽度增大。这表明,改性后的材料中-OH数目增多。这些含氧基团的增加使其表面极性增强[28],从而使MCFA吸附剂对染料分子的氢键能力提高。
图4
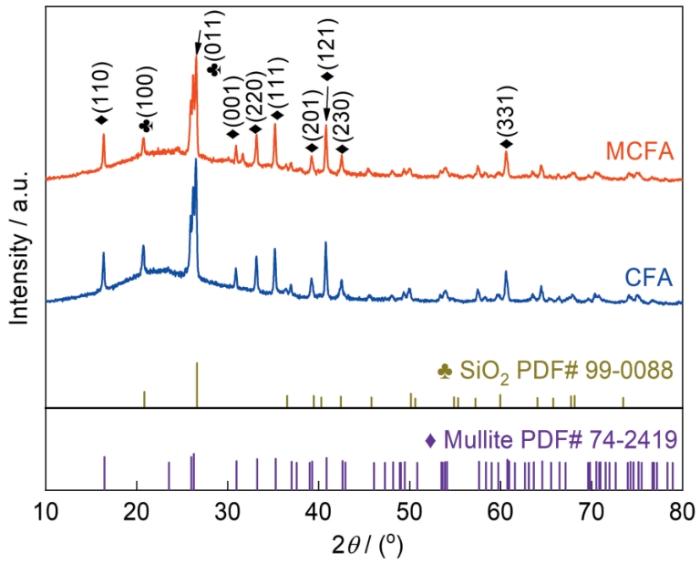
2.4 CFA和MCFA的物相和晶体结构
图5给出了CFA和MCFA的X射线衍射谱。可以看出,CFA的谱中2θ为16.4°、30.9°、33.2°、35.2°、39.2°、40.8°、42.6°和60.7°处出现的峰与莫来石(Al2.3Si0.7O4.85)的标准卡片PDF#74-2419的衍射峰一致,对应的晶面为(110)、(001)、(220)、(111)、(201)、(121)、(230)、(331);在2θ为20.8°和26.5°处出现的峰与石英(SiO2)的标准卡片PDF#99-0088的衍射峰一致,对应的晶面为(100)、(011)。根据对CFA和MCFA的衍射峰的分析,改性前的CFA其物相组成是SiO2和Al2O3。改性后的MCFA特征峰没有发生明显偏移,表明改性保证了CFA的化学稳定且有良好的扩容效果[29]。同时,CFA的晶格强度较高,而MCFA的特征峰减弱且结构变成无定形的。其原因是,在物理和化学作用下结晶(石英和莫来石)结构受到破坏,使结晶度有所降低。改性后的MCFA非结晶性大大提高,可能是比表面积增加的主要原因,有利于提高其吸附性能[30]。
图5
2.5 CFA和MCFA的元素和化学态
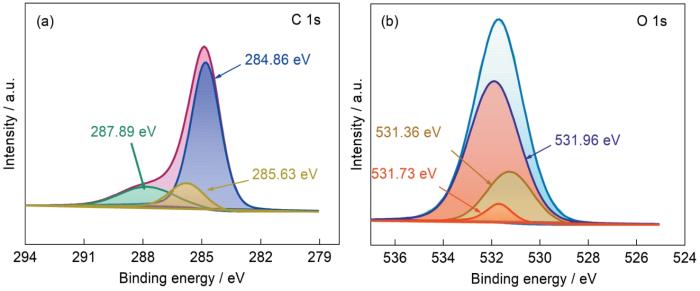
用X射线光电子能谱(XPS)可定性或半定量地分析CFA和MCFA的元素及化学态[31]。由图6可见,CFA主要由O、C、Al、Si元素组成,只含有少量Fe、Ca等金属元素。MCFA表面Mn元素的比例增大,表明在酸碱改性过程中释放了CFA内部的活性位点,使MCFA的吸附能力提高。图7和图8中的C1s图谱表明,结合能从改性前的289.47、287.83、285.42和284.59 eV变为改性后的287.89、285.63和284.86 eV。其原因是,在酸性条件下碳表面生成了一定数量的含氧基团羰基、羟基和羧基[32]。CFA的O1s谱图表明,改性前后结合能也有较小的变化,531.9 eV附近的峰是SiO2和Al2O3晶体中的氧原子。在图8的O1s谱图中,新的峰在536 eV附近的较高能带处合并,属于羰基、硅羟基、羧基等极性基团中的氧原子[33]。XPS分析结果表明,混合改性后MCFA的结构发生了变化,含氧基团增多。这些极性基团增强了染料分子和吸附剂之间的氢键和范德华力,使MCFA成为对染料的良好吸附剂。
图6
图7
图8
2.6 MCFA的剂量对模拟印染废水吸附性能的影响
图9给出了MCFA的剂量对去除4种染料能力的影响。可以看出,随着MCFA剂量的增加4种染液的脱色率呈上升趋势,对碱性品红、孔雀石绿以及甲基紫的脱色效果优于刚果红。其原因可能是,刚果红属于直接染料,其相对分子质量较大,具有线性平面结构,缔合性较高,水溶性较差[34]。随着MCFA用量的增加,碱性品红和孔雀石绿的脱色率先迅速提高然后缓慢提高。其原因是,随着吸附剂用量的增加吸附点位增多,脱色率增大。但是因为吸附点位的数量是有限的,继续增加MCFA用量脱色率的变化也不大。随着改性MCFA的增加对4种染液的吸附量下降,因为吸附量与吸附剂的质量成反比,与染液浓度的变化量呈正比。因此,吸附量不能简单地依靠吸附剂用量的增加。综合考虑后期的分离和经济成本以及CFA是一种固体废物,其投加量过大反而污染环境。吸附模拟染料废水的投加量,以2 mL的MCFA为宜。
图9
图9
不同剂量的MCFA对染料的吸附性能
Fig.9
Adsorption properties of dyes with different doses of MCFA
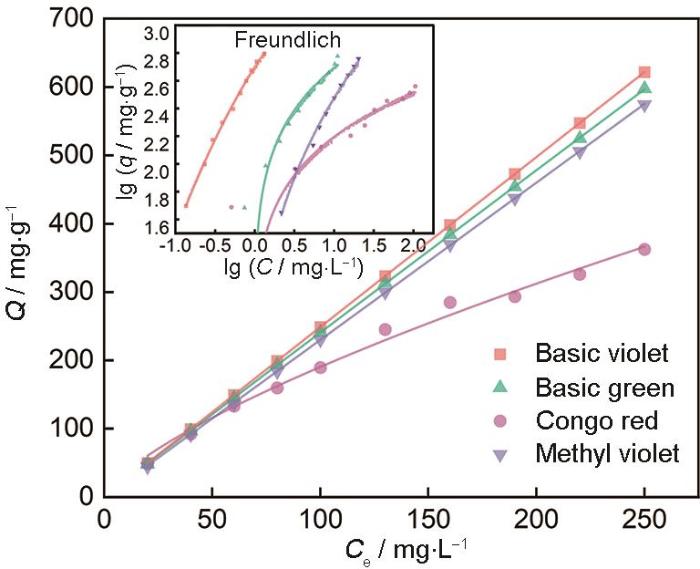
2.7 用等温吸附方程拟合吸附数据
吸附等温线描述吸附剂与被吸附分子间的作用,对于固液体系常用的是Langmuir和Freundlich 吸附等温模型。图10给出了染料的初始浓度对MCFA吸附效果的影响。吸附等温学是在一定温度和浓度梯度下对染料的吸附,适用于定性分析[35]。为了更深入地探讨改性粉煤灰的吸附机理,用Langmuir等温方程和Freundlich等温方程对吸附数据(表3)进行线性拟合。由图10可见,对于各个染料的吸附行为Freundlich等温吸附模型显然比Langmuir有更高的拟合度(R2 > 0.99)。MCFA对不同浓度的染料的吸附性能不同,Freundlich等温模型的拟合结果基本上都呈线性关系,即吸附量与染料浓度成正比关系。在同一浓度时刻,MCFA对碱性品红的吸附量高于其他三种染料。
图10
图10
染料的浓度对其吸附效果的影响
Fig.10
Influence of concentrations on the adsorption effect of four dyes
表3 MCFA吸附4种染液的等温学拟合参数
Table 3
| Model | Parameters | Basic violet | Basic green | Congo red | Methyl violet |
|---|---|---|---|---|---|
| Langmuir | KL / L·g-1 | 0.0003 | 0.0005 | 0.0026 | 0.0004 |
| qm / mg·g-1 | 7.67 | 27 | 15.115 | 101.25 | |
| R2 | 0.2778 | 0.8499 | 0.9654 | 0.2911 | |
| Freundlich | KF / mg·g-1 | 512.7433 | 72.3936 | 61.6027 | 21.8424 |
| nf | 0.9015 | 1.1242 | 2.6652 | 0.8997 | |
| R2 | 0.9908 | 0.9903 | 0.9902 | 0.9909 |
2.8 用准一级动力学模型与准二级动力学模型线性拟合吸附数据
图11
图11
4种染液的吸附时间对吸附效果的影响
Fig.11
Influence of time on adsorption effect of four dyes
为了研究对4种染液的吸附机理,将以上数据用准一级动力学模型与准二级动力学模型进行线性拟合,结果列于表4。
表4 MCFA吸附4种染液的动力学拟合参数
Table 4
| Model | Parameters | Basic violet | Basic green | Congo red | Methyl violet |
|---|---|---|---|---|---|
| Pseudo-first-order | K1 / min-1 | 5.520 × 10-2 | 2.223 × 10-1 | 2.670 × 10-1 | 1.386 × 10-1 |
| qe / mg·g-1 | 3.4697 | 1.8104 | 2.7114 | 1.4374 | |
| R2 | 0.6420 | 0.2461 | 0.9577 | 0.8848 | |
| Pseudo-second-order | K2 / g·(mg·min)-1 | 1.006 × 10-3 | 1.335 × 10-3 | 8.590 | 2.469 × 10-4 |
| qe / mg·g-1 | 250.00 | 250.00 | 263.16 | 256.41 | |
| R2 | 0.9999 | 0.9999 | 0.9809 | 0.9980 |
可以看出,用准二级动力学比准一级动力学更好地描述对4种染料的吸附行为,表明改性粉煤灰表面含氧基团和染料分子极性部分之间的氢键结合作用在吸附过程中起重要作用[37]。同时,准二级动力学模型被认为代表吸附速率与两种反应物(包括吸附质和吸附剂)浓度的线性关系,因此吸附剂用量是一个重要的因素。准二级动力学认为吸附剂的吸附速率主要受化学吸附机理控制,表明MCFA的吸附机理以化学吸附为主,其中氢键结合作用是主要因素。
2.9 染液的pH值对MCFA吸附性能的影响
图12给出了MCFA对不同pH值的4种染料的吸附量。可以看出,MCFA对4种染料的吸附容量随着染液pH值的增大而增大,对pH = 9的染料吸附效果最优。其原因是,在碱性条件下OH-将HCl的H+从上述染料分子上剥离,粉煤灰中的Al3+和Fe3+替代了原本染料分子末端的H+,使得吸附效果明显提高。pH值较小的染料,其分子中磺酸基的离解度较小,大部分以-SO3H的形式存在。因此,染料分子之间有很强的氢键,很容易形成聚集体。聚集体形成后其尺寸和空间位阻尺寸增加,移动能力降低,与粉煤灰的结合能力降低,因此不容易吸附在MCFA表面。随着pH值的增大染料分子中磺酸基的离解度提高,染料分子转变为阴离子,它们之间的排斥力增加而使聚集体解聚。
图12
图12
在pH值为3~11条件下MCFA对4种染液的吸附量
Fig.12
Adsorption capacity of MCFA for four dyes at pH 3~11
但是,pH值过高即在强碱环境下MCFA与染料分子之间的静电排斥增大。过碱环境影响粉煤灰表面的基团,吸附溶液中有大量的OH-,且染料分子因电离产生大量的负电荷,从而使脱色率降低。因此,在与实际的印染废水pH值(pH~9)接近弱碱环境,MCFA表现出最大的吸附能力。
3 结论
(1) MCFA的优异吸附性能源于其多孔结构和比表面积的增大,且能与染料分子更好的相互作用。改性后粉煤灰的形貌发生了很大的变化,表面更加粗糙多孔、凹凸不平,比表面积大幅度地增大,含氧基团的增加和活性的提高使其吸附性能提高。MCFA的结晶度(Si-O-Si和Si-OH)较低,但是表面积更大,使其对染料吸附能力更高。
(2) 改性粉煤灰对碱性品红、孔雀石绿、刚果红、甲基紫染液的吸附过程都符合准二级动力学模型,化学吸附机理为吸附速率的主要控制因素,并且改性粉煤灰对这4种染料的等温吸附行为更符合Freundlich等温吸附模型。改性粉煤灰对染料的吸附行为是一种多层吸附过程。
参考文献
Research progress and prospect of resource utilization of fly ash in China
[J].
中国粉煤灰的资源化利用研究进展与前景
[J].针对我国燃煤电厂逐年增加的粉煤灰堆积造成的日益突出的土地和环境污染问题,论文总结了我国粉煤灰的物理化学性质、矿物学及环境地球化学特征,综合论述了我国粉煤灰在建筑工程(水泥、混凝土、筑路、建筑物建造)、农业(生产肥料、改良土壤等)、环境保护(废水、废气治理)、陶瓷生产、深度分离等低附加值应用以及沸石合成、稀有金属提取、地质聚合物合成、泡沫玻璃制备等高附加值应用领域的综合利用现状及研究进展,探讨了粉煤灰资源化利用存在的问题与发展前景.未来我国粉煤灰的资源化利用在聚焦于高附加值利用技术的突破和推广应用的同时,应高度重视粉煤灰的环境污染问题.
Research progress in the comprehensive utilization of fly ash
[J].
粉煤灰综合利用研究进展
[J].
Fly ash-based adsorbent for adsorption of heavy metals and dyes from aqueous solution: a review
[J].
Research progress of printing and dyeing wastewater treatment
[J].
印染废水处理研究进展
[J].
Performance assessment of five adsorbents based on fly ash for removal of cadmium ions
[J].
Multicycle adsorption and desorption for recovery of U(VI) from aqueous solution using oxime modified zeolite-A
[J].
Excellent adsorption of Zn(II) using NaP zeolite adsorbent synthesized from coal fly ash via stage treatment
[J].
Progress and development of high value utilization for coal fly ash from power plant
[J].
电厂粉煤灰高值化利用现状与最新进展
[J].
High adsorption and high catalyst regeneration kinetics observed for Flyash-Fe3O4-Ag magnetic composite for efficient removal of industrial azo reactive dyes from aqueous solution via persulfate activation
[J].
Enhancing As(V) and As(III) adsorption performance of low alumina fly ash with ferric citrate modification: Role of FeSiO3 and monosodium citrate
[J].
Preparation of amino-functionalized fly ash based tobermorite for enhanced removal of Cr(VI)
[J].
Dyeing of cotton with reactive dyestuffs: the continuous reuse of textile wastewater effluent treated by Ultraviolet/Hydrogen peroxide homogeneous photocatalysis
[J].
Preparation and characterization of thermoplastic starch composites with fly ash modified by planetary ball milling
[J].
Experimental study on Cd(Ⅱ) and Cu(Ⅱ) of adsorbent prepared by mixing fly ash and domestic sludge
[J].
粉煤灰与生活污泥混合制备吸附剂对Cd(Ⅱ)和Cu(Ⅱ)的试验研究
[J].
Adsorption of phosphate by acid-modified fly ash and palygorskite in aqueous solution: Experimental and modeling
[J].
The mechanism of acid modification of coal fly ash for the removal of phosphate from wastewater
[J].
酸改性粉煤灰去除废水中磷酸盐的机理解析
[J].
Application of fly ash for adsorptive removal of malachite green from aqueous solutions
[J].
Modified fly ash and zeolites as an effective adsorbent for metal ions from aqueous solution
[J].
Effect of modification with nitrogen functional groups on structure and adsorption performence of biomass active carbon
[J].Nitrogen-doped bagasse biochar BAC-EDA was prepared via a two-step process, namely, the bagasse biochar was prepared with bagasse as raw material and phosphoric acid as modifying agent and then on which nitrogen-containing functional groups were further coupled with ethylenediamine as dressing agent. The pore structure of BAC and BAC-EDA was characterized by means of BET, BJH and t-plot methods based on the experimental data of liquid nitrogen adsorption/desorption. Meanwhile acidic groups were characterized by FTIR method. Results show that pores of biochar BAC and BAC-EDA are mainly of mesopores with a small amount of micropores. There existed oxygen-containing acidic functional groups on the surface the nitrogen-doped bagasse biochar BAC-EDA, such as carboxyl groups, lactone groups and amino nitrogen-containing functional groups etc. The proportions of the micro-mesopores of the biomass carbon are constant before and after modification, which indicating that the introduction of the functional groups is realized simultaneously on the surface of both the micro- and meso-pores. Adsorption experiments show that the maximum adsorption amount of the modified BAC-EDA is 137 mg·g-1, much higher than that of the plain BAC 73 mg·g-1, indicating that the nitric acidoxidation and ethylenediamine modification effectively improve the adsorption capacity of biomass biochar. The Langmuir model can better describe the adsorption of Hg(Ⅱ) by BAC-EDA, further illustrating the homogeneity of the active sites of the modified carbon. Besides, the temperature is conducive to adsorption, showing that the process is a spontaneous endothermic process.
介孔蔗渣碳的氮官能化改性及其对Hg2+吸附的影响
[J].以甘蔗渣为原料、磷酸作为改性剂制备了生物质碳BAC,用硝酸氧化和乙二胺对其修饰后制备出多胺介孔蔗渣基生物质碳BAC-EDA。采用低温液氮吸附/脱附,根据BET、BJH、t-plot、FTIR分析改性前后蔗渣碳BAC、BAC-EDA的孔结构和表面化学官能团,研究了改性对重金属汞吸附的影响。结果表明,生物质碳BAC和BAC-EDA由介孔和少量微孔组成,在改性后的多胺介孔蔗渣基生物质碳表面新增了羧基、内酯基等含氧酸性官能团以及氨基含氮官能团,而且改性前后生物质碳的微介孔所占比例恒定。这些结果表明,官能团的引入是在微介孔上同时进行的。吸附试验结果表明,改性后的蔗渣碳BAC-EDA的最大吸附量为137 mg·g<sup>-1</sup>,远高于未改性的蔗渣碳AC的吸附量73 mg·g<sup>-1</sup>,说明硝酸氧化和乙二胺改性提高了生物质碳的吸附性能。使用Langmuir模型能更好地描述汞在BAC-EDA上的吸附,进一步说明改性后碳材料活性位点的均一性。同时,温度升高有利于吸附,说明这个过程是一个自发吸热过程。
Using modified fly ash for removal of heavy metal ions from aqueous solution
[J].
Study on the adsorption performance of modified fly ash on sulfides in water
[J].
改性粉煤灰对水中硫化物吸附性能研究
[J].
Competitive adsorption of methylene blue and Pb (II) ions on the nano-magnetic activated carbon and alumina
[J].
A study on removal characteristics of heavy metals from aqueous solution by fly ash
[J].The purpose of this study was to investigate the possibility of the utilization of coal fly ash as a low cost adsorbent. Batch experiments were conducted to evaluate the removal of heavy metals from aqueous solutions by fly ash under various conditions of metal concentration, pH and fly ash dosage. The heavy metals used in this study were zinc, lead, cadmium and copper. Adsorption studies were done at various pH values (3-10) at 25 degrees C and at heavy metal concentrations of 10-400 mg/L using fly ash concentrations of 10, 20 and 40 g/L, respectively. Experiments were also conducted without fly ash to determine the extent of heavy metal removal by precipitation. Kinetic experiments were also performed and the zeta-potentials of the fly ash particles were measured at various pH's. The adsorption data was described by the Freundlich adsorption model. The test results using real wastewater indicated that fly ash could be used as a cheap adsorbent for the removal of heavy metals in aqueous solutions if not strongly acidic.
Effects of different heavy metals on fly ash-based geopolymer
[J].Fly ash based geopolymer were prepared with fly ash as raw material and several compounds of heavy metals as additives. Then the effect of the type, content and chemical form of the heavy metal on the compressive strength, reaction products, and pore structure of the prepared geopolymers was investigated. The results show that the incorporation of Pb2+、Cr3+ and Cu2+ has great effect on the late compressive strength and results in formation of reinhardbraunsite in the solidified body. Moreover, the Pb2+ reduces the total pore volume of the solidified body, while Cr3+ and Cu2+ increase it. The content of the heavy metal compounds should be controlled within a reasonable range. When the content of the heavy metal compounds is relatively small, the total pore volume is small and the solidified body is much compact. Besides, the different chemical forms of chromium have different effect on the stability of the geopolymers. The single addition of chromium oxide or elemental Cr may reduce the total pore volume of the geopolymers, whereas the average pore size increases obviously for the geopolymers with addition of a variety of other heavy metal compounds.
不同重金属对粉煤灰基地聚合物的影响作用
[J].以粉煤灰为主要原材料制备粉煤灰基地聚合物,研究了掺入的重金属的种类、掺量、化学形态对地聚合物体系的抗压强度、反应产物、孔结构的影响。结果表明:Pb<sup>2+</sup>、Cr<sup>3+</sup>、Cu<sup>2+</sup>的掺入对固化体后期的抗压强度影响较大,在反应产物中出现了重硅钙石,含Pb<sup>2+</sup>固化体的总孔体积减小,而含Cr<sup>3+</sup>和Cu<sup>2+</sup>固化体的总孔体积增大;重金属的掺量应该控制在合理的范围内。当重金属的掺量较小时其总孔体积较小,体系较为致密;铬掺入的形式不同对地聚合物体系稳定性的影响也不同,且单掺铬的氧化物或单质可使固化体体系的总孔体积减小,而掺入多种重金属时固化体的平均孔径明显增大。
Environmental, physical and structural characterisation of geopolymer matrixes synthesised from coal (co-)combustion fly ashes
[J].
Adsorptive treatment of coking wastewater using raw coal fly ash: Adsorption kinetic, thermodynamics and regeneration by Fenton process
[J].Raw coal fly ash (RCFA) was used directly as adsorbent to treat coking wastewater. The results show that the RCFA can introduce COD (14, 4, and 11 mg L at pH = 1.0, 7.0, and 14.0, respectively) into water due to the dissolution of reductive components from RCFA, this can be avoided by washing (6 times) using distilled water. The concentration of leached metal elements in wastewater is lower than the standard in GB18918-2002, China. The adsorption process accords with the pseudo-second order adsorption kinetic model and Langmuir thermodynamics model better than other ones, and it belongs to a physical and exothermic process. More loading of RCFA (10-60 mg L) means less adsorption capacity, and 40 g L is the optimal value. The variation of regeneration rate of SRCFA for the first time (RR) with regeneration time accords with the behavior of hyperbolic function (1RR=0.986+53.913t), and the [HO], [Fe] and regeneration temperature can affect the RR at the manner of exponential function ( [Formula: see text], [Formula: see text], and RR=2.064·e). At the optimal regeneration condition of SRCFA ([HO] = 5 mM, [Fe] = 8 mM and temperature = 293 K), the RR can reach 87.1% after 270 min. The stability of RCFA shows two different stages, i.e., within the first 4 regenerations the RR increases from 87.1% to 89.7% and then decreases gradually and always. This variation trend can be confirmed by the results of SEM and BET tests.Copyright © 2018 Elsevier Ltd. All rights reserved.
Study on technology and adsorption properties of modified fly ash by microwave chemical method
[J].
微波化学法改性粉煤灰的工艺及吸附性能研究
[J].
Magnetic chitosan biopolymer as a versatile adsorbent for simultaneous and synergistic removal of different sorts of dyestuffs from simulated wastewater
[J].
Potential of removing Cd(II) and Pb(II) from contaminated water using a newly modified fly ash
[J].
Zn-VOx-Co nanosheets with amorphous/crystalline heterostructure for highly efficient hydrogen evolution reaction
[J].
Study on fly ash as catalyst carrier
[J].
以粉煤灰作为催化剂载体的研究
[J].
Research progress on preparation of adsorbent based on fly ash and its application in the removal of heavy metals in water
[J].
粉煤灰基吸附剂去除水中重金属的研究进展
[J].
Physical, chemical, and geotechnical properties of coal fly ash: A global review
[J].
Removal of Pb(II), Cd(II) and Hg(II) from aqueous solution by mercapto-modified coal gangue
[J].A low-cost mercapto-modified coal gangue (CG-SH) was fabricated by modification of coal gangue (CG) with (3-mercaptopropyl) trimethoxysilane. The structure and composition for as-prepared CG-SH were characterized by using X-ray diffraction (XRD), Fourier transfer infrared spectroscopy (FTIR), scanning electron microscopy (SEM) and X-ray fluorescence (XRF). Results indicated that larger amounts of mercapto-groups (-SH) was successfully introduced onto CG, which followed by acted as active sites for the removal of heavy metal cations, such as Pb(II), Cd(II) and Hg(II). The factors that affected the adsorption equilibrium as well as the removal efficiency, i.e., contact time, initial concentration, pH and temperature, were investigated in detail. The adsorption isotherms for Pb(II), Cd(II) and Hg(II) were well fitted with Langmuir model. The maximum adsorption capacity of CG-SH for Pb(II), Cd(II) and Hg(II) were calculated to be 332.8, 110.4 and 179.2 mg g, respectively. The adsorption for Pb(II), Cd(II) and Hg(II) on CG-SH could be well described by pseudo-second-order kinetic model. And thermodynamic analysis suggests that the adsorption process for Pb(II) is exothermal, while that for Cd(II) and Hg(II) are endothermal. The results suggest CG-SH have great potential to be used as efficient absorbent for the removal of heavy metal cations from water.Copyright © 2018 Elsevier Ltd. All rights reserved.
Synthesis of magnetic zeolites from recycled fly ash for adsorption of methylene blue
[J].
Heavy metal adsorption with zeolites: The role of hierarchical pore architecture
[J].Zeolites have been widely used for heavy metal removal due to their high cation exchange capacity and surface sorption properties. However, the empirically determined adsorption capacity trend of Pb2+ > Cu2+ > Ni2+ was not well understood. Herein, we discovered that the adsorption of Ni2+ by zeolites was greatly influenced by hierarchical pore geometry, while the adsorption of Pb2+ or Cu2+ was dependent on cation exchange determined by the framework composition. This difference in adsorption mechanisms is manifested through the systematic study of heavy metal adsorption using low-silica LTA and FAU zeolites with different mesopore architectures and compositions. Synthesizing mesoporous zeolite A of MLTA-P using proline mesoporogen considerably enhanced the Ni2+ adsorption capacity to more than twice of that of conventional zeolite A synthesized without organics, while the adsorption capacities for Cu2+ and Pb2+ were almost unaltered at similar to 170 and 510 mg/g. The study on the effect of zeolite synthesis time and adsorbate pH value revealed the significant influence of zeolite crystallinity, surface hydroxyl group, and hierarchical pore architecture on the Ni2+ uptake. The MLTA-P zeolite showed higher stability against pH variation in acid range and remarkably enhanced uptake in alkaline conditions, reaching 218 mg/g at a pH of 11. The full characterization of the adsorbent by scanning electron microscopy and X-ray photoelectron spectroscopy indicated the involvement of the surface reaction forming Ni phyllosilicate nanosheet species during the nickel metal removal process in which transport limitation played a crucial role. The uptake kinetics and isotherms could be perfectly reflected by a pseudo-secondorder rate equation and the Langmuir model, confirming the nature of chemisorption.
Study on isotherm, kinetics, and thermodynamics of adsorption of crystal violet dye by calcium oxide modified fly ash
[J].